Abstract
Lower extremity fatigue has been associated with decline in postural stability, alteration of normal walking patterns and increased fall risk. Effects of lower extremity fatigue on amount of movement variability as assessed by linear variability such as standard deviation and root mean square is well known but there is lack of information about how fatigue influences nonlinear temporal structure of variability in healthy human gait. In this study ten subjects (5 males and 5 females) were asked to perform treadmill walking for three minutes with an Inertial Measurement Unit (IMU) sensor affixed at their trunk level, thereafter the participants conducted squatting exercises and fatigue was induced as per standard fatigue protocol. The participants were asked to walk again on treadmill at their preferred walking speed for three minutes. The signals derived from the inertial sensor were used to compute stride interval time series (SIT) and signal magnitude difference (SMD) time series signals. These SIT and SMD signals were analyzed for non-linear variability such as complexity (approximate entropy and multiscale entropy) and Detrended Fluctuation Analysis (DFA). It was found that that there was significantly higher complexity in SMD signals due to fatigue inducement (p=0.04). Similarly, it was also found that fatigue significantly decreased fractal properties of SMD signals (p=0.013). In conclusion, lower extremity localized muscle fatigue influences magnitude of kinematic variability and induced anti-persistence in the trunk kinematics. In future, more work is needed to understand how kinematic variability in angular velocities due to fatigue may affect fall risk in healthy adults.
Keywords: Lower extremity fatigue, complexity, inertial sensors
INTRODUCTION
Localized muscle fatigue is one of the potential risk factor for slip-induced falls [1] as muscle fatigue adversely affects proprioception [2–4], movement coordination and muscle reaction times [5] leading to postural instability [6] and gait changes [1, 7]. Human locomotion is programmed in the central nervous system (CNS) and is further adapted by proprioceptive feedback [8]. During stance phase of the gait cycle, proprioceptive input from extensor muscles and mechanoreceptors in the sole of foot provide load information [9] to the CNS. Fatigue in lower extremity muscles (around hip joint) may increase irregular firing of motor neurons during walking [10–12]. As such, fatiguing of the muscles around a joint may not only inhibit the joint’s neuromuscular feedback but also disturb synergism between joint proprioception and muscular function leading to instability and gait changes [13–21].
Effects of fatigue on linear variability are well known [22, 23]. However, there is paucity in knowledge about the effects of fatigue on non-linear variability during walking. Complexity is a regularity statistic from nonlinear dynamics and has recently shown great promise in detecting subtle changes in motor control system [24]. Quantifying the complexity of physiologic time series with/and without localized muscle fatigue is of considerable interest. Fatigue in lower extremity musculature is also well associated with decline in postural stability, motor performance and alters normal walking patterns in human subjects but its effects on gait complexity have not been evaluated. To our knowledge this is the first study which looks into effects of fatigue on non-linear dynamics of kinematic time series in healthy young individuals during treadmill walking.
METHODS
Participants
Ten healthy young adults (five males and five females) participated in this study. The participants mean age was 29 ± 9 years, height was in the range of 173 ± 10 cm, and weight was 72 ± 11 kg. All participants were healthy, independent and non-sedentary and, were formally screened for major musculoskeletal, cardiovascular, and neurological disorders by a research coordinator during initial participant contact. Exclusion criteria of this study were factors that could interfere with gait, such as medication use, presence of neuromuscular disease and, balance and vision disorders. Informed consent was approved by the Institutional Review Board (IRB) of Virginia Tech and was signed by all participants prior to the study.
Participants were instructed not to perform any strenuous exercise 48 h prior to the experiment. All experiments were conducted between 11:00 am and 3:00 pm, and this was conducted to control the circadian effects of fatigue. Walking trials were conducted both prior and after the fatigue inducement. Three Inertial Measurement Units (IMU’s) were affixed on the participants, both at right and left shanks and the other at the sternum level using velcro straps and surgical tapes (Figure 1).
Figure 1.

Attachment of IMU sensors at sternum level using Velcro strap.
Fatigue Inducement
A custom built Biodex (Biodex System 3 Dynamometer, Shirley, New York, USA) attachment for the shoulders was used to assess maximum voluntary isokinetic exertions (MVE) during squatting (Figure 2). The Biodex attachment was designed to measure combined torque from the ankles, knees and hips through vertical motion/force exerted via shoulders. Although MVEs were performed using the shoulder attachment with a dynamometer during squat protocol, fatigue was induced by holding 5% of their body weight in front of themselves by both hands while squatting repeatedly at 22 repetitions per minute. An exercise set was set for 5 minutes and was followed by measurement of three MVEs using dynamometer. Experimenters did not instruct participants to take break between the exercise sets, but it was kept on participant’s choice to start their next new exercise set as soon as they felt they were ready for it. The exercise sets continued until the participants reached 60% of their baseline MVE; this was categorized as fatigued state (time taken by participants was 52±7 minutes to reach this state).
Figure 2.
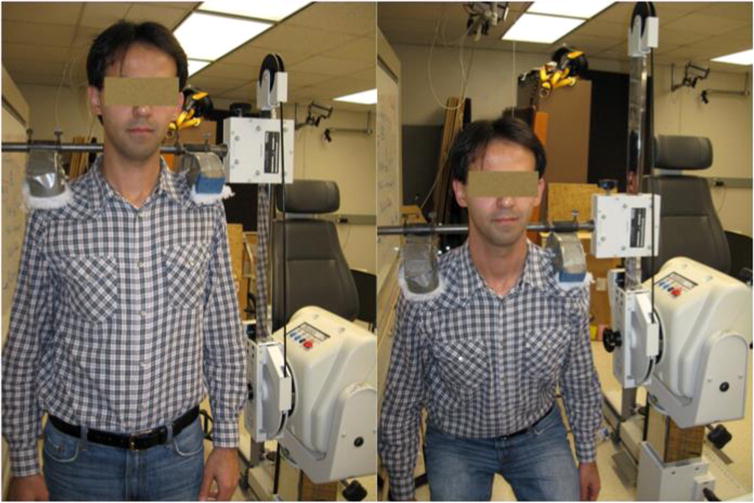
Customized Biodex attachment for measurement of maximum voluntary exertions.
Instrumentation
The IMU node consisted of MMA7261QT tri-axial accelerometers and IDG-300 (x and y plane gyroscope) and ADXRS300, z-plane uniaxial gyroscope aggregated in the Technology-Enabled Medical Precision Observation (TEMPO) platform which was manufactured in collaboration with the research team of the University of Virginia [25, 26]. The data acquisition was carried out using a Bluetooth adapter and laptop through a custom built program in LabView (LabView 2009, National Instruments Corporation, Austin, TX). Data was acquired with sampling frequency of 120 Hz. This frequency is largely sufficient for human movement analysis in daily activities, which occurs, in low bandwidth [0.8–5Hz] [27]. The data was processed using custom software written in MATLAB (MATLAB version 6.5.1, 2003, computer software, The MathWorks Inc., Natick, Massachusetts). Protocol: Participants were asked to walk on treadmill for 5 minutes at their self-selected pace. After this normal walking treadmill walking trial, participant’s MVE were noted and fatigue was induced as per protocol provided above. Once it was determined that participants have reached 60% of their baseline MVE. Participants were asked to walk again on treadmill for 5 minutes. The treadmill speed was kept same as during the normal walking trial.
Stride Interval Time series (SIT): The temporal fluctuations in stride intervals time series has been widely used as a non-invasive technique to evaluate effects of neurological impairments on gait and its changes with aging and disease[28, 29]. A customized MATLAB algorithm was used to identify peaks from gyroscope signals from trunk mounted inertial sensor. The time difference from one peak to the other was considered as stride interval and all these consecutive intervals made up Stride Interval Time Series (SIT) (Figure 3).
Figure 3.
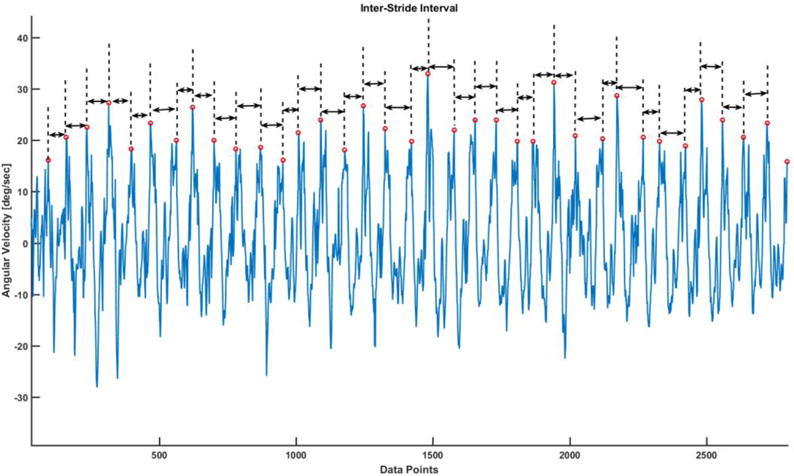
The time interval between consecutive peaks were used to compute stride interval time series.
Signal Magnitude Difference Time series (SMD): The differences in peak heights of angular velocity signals are categorized as signal magnitude differences. These differences in magnitudes of angular velocity were used to construct a time series which was named as Signal Magnitude Difference (SMD) Time series. The total length of SMD time series is one less than the total number of strides walked by the subject.
RESULTS
It was found that SMD time series complexity increased significantly due to fatigue. ApEn of SMD signals in post-fatigue condition was significantly higher than in normal walking condition (p = 0.04) (figure 6). Similar results were found for multiscale entropy which also confirmed increase in SMD complexity due to fatigue (p=0.02). We found that there was significant decline in persistence of SMD signals (p=0.01) (figure 7).
Figure 6.
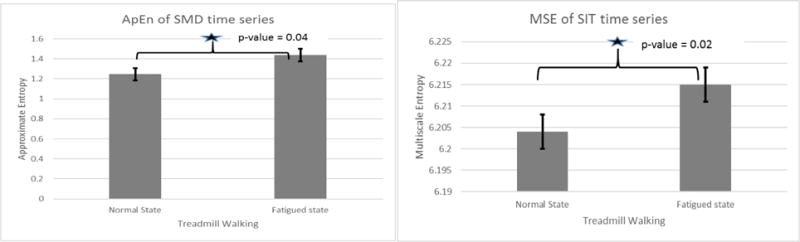
The SMD signal complexity from ApEn and Multiscale Entropy (MSE)
Figure 7.
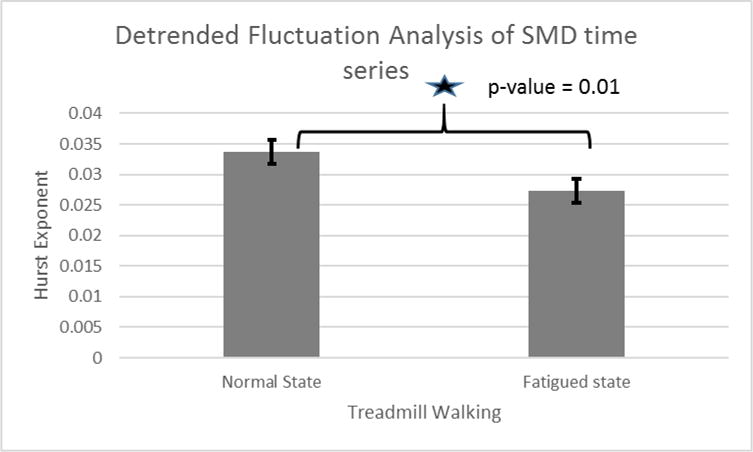
Hurst exponent for fatigue and normal walking in SMD signals
DISCUSSION
The present study investigates the effects of lower extremity fatigue on nonlinear variability during treadmill walking. Indeed, some authors have reported higher linear variability after inducement of fatigue[23] but the objective of this study was to assess effects of lower extremity fatigue on nonlinear variability of SIT and SMD time series signals. Our results show that there was significant increase in complexity of SMD time series signals due to fatigue. Thus it reveals that there is increase in unpredictability of velocities produced in fatigue state. However, we did not find any significant changes in complexity for SIT time series. Which infers that probably temporal structure of variability is not affected by lower extremity fatigue. Previously it has been reported that the reduced capacity to adapt to stress is attributed to the loss of complexity with aging and disease [30]. This reduced complexity [30] in elderly, is dependent on the nature of the intrinsic dynamics of the system and one’s ability for short time adaptive change, which is required to meet an immediate task demand is reduced [31]. Complexity is usually associated with adaptive capacity of motor control system. Thus it appears that trunk kinematics and motor control have increased adaptive capacity in generating trunk momentum during fatigued walking.
We also found that hurst exponent declined in SMD signals due to fatigue, which indicates that kinematics such as velocity produced during fatigued state have lost long range correlations. There is decline in fractal properties of SMD signals due to fatigue inducement in lower extremity. Previous research has shown that elderly with low fractal scaling are more likely to fall than those with high fractal scaling[32].
CONCLUSIONS
In conclusion, the results of our analysis of complexity due to lower extremity fatigue acknowledged our hypothesis that fatigue also influenced nonlinear variability during treadmill walking. Higher complexity revealed higher unpredictability in generating trunk momentums. We also found that lower extremity fatigue negatively influenced long range correlations in trunk velocity signals.
Figure 4.
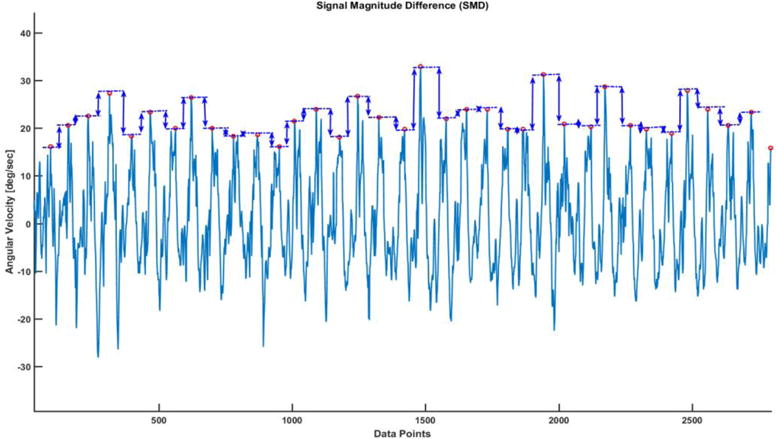
Schematic diagram showing how peak heights were used to compute SMD time series
Figure 5.
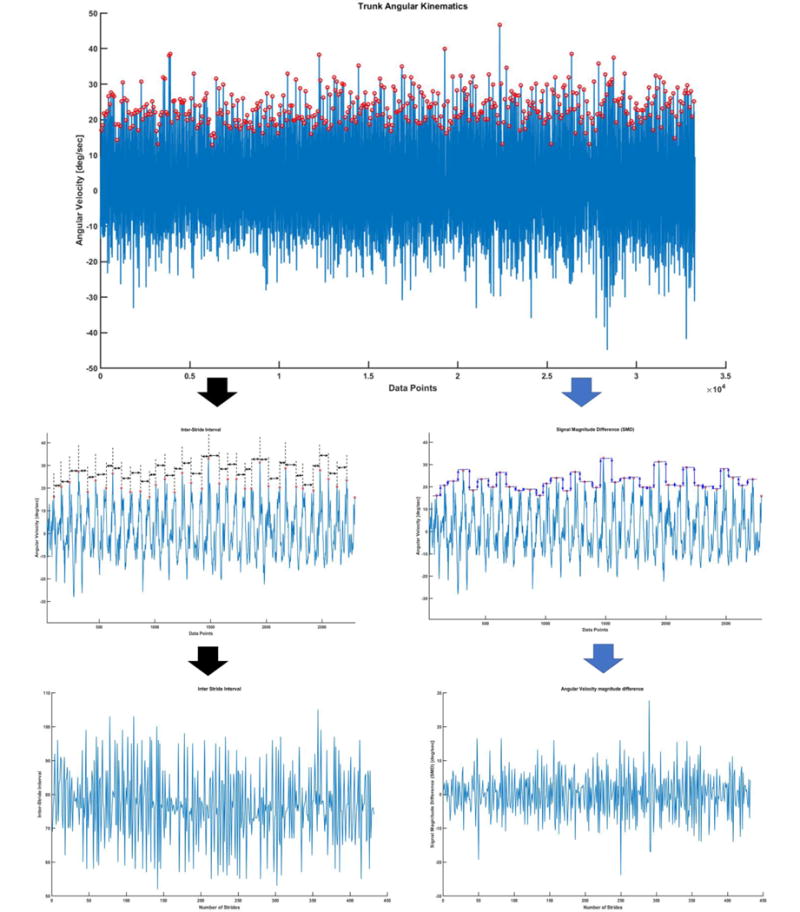
Schematic diagram of derivation of SID and SMD time series from angular velocity signals from trunk IMU during walking on treadmill.
References
- 1.Parijat P, Lockhart TE. Effects of lower extremity muscle fatigue on the outcomes of slip-induced falls. Ergonomics. 2008 Dec;51(12):1873–84. doi: 10.1080/00140130802567087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skinner HB, Wyatt MP, Hodgdon JA, Conard DW, Barrack RL. Effect of fatigue on joint position sense of the knee. J Orthop Res. 1986;4(1):112–8. doi: 10.1002/jor.1100040115. [DOI] [PubMed] [Google Scholar]
- 3.Hiemstra LA, Lo IK, Fowler PJ. Effect of fatigue on knee proprioception: implications for dynamic stabilization. J Orthop Sports Phys Ther. 2001 Oct;31(10):598–605. doi: 10.2519/jospt.2001.31.10.598. [DOI] [PubMed] [Google Scholar]
- 4.Gear WS. Effect of different levels of localized muscle fatigue on knee position sense. Journal of Sports Science and Medicine. 2011 Dec;10(4):725–730. [PMC free article] [PubMed] [Google Scholar]
- 5.Hakkinen K, Komi PV. Effects of fatigue and recovery on electromyographic and isometric force- and relaxation-time characteristics of human skeletal muscle. Eur J Appl Physiol Occup Physiol. 1986;55(6):588–96. doi: 10.1007/BF00423202. [DOI] [PubMed] [Google Scholar]
- 6.Lin D, Nussbaum MA, Seol H, Singh NB, Madigan ML, Wojcik LA. Acute effects of localized muscle fatigue on postural control and patterns of recovery during upright stance: influence of fatigue location and age. Eur J Appl Physiol. 2009 Jun;106(3):425–34. doi: 10.1007/s00421-009-1026-5. [DOI] [PubMed] [Google Scholar]
- 7.Helbostad JL, Leirfall S, Moe-Nilssen R, Sletvold O. Physical fatigue affects gait characteristics in older persons. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2007 Sep;62(9):1010–1015. doi: 10.1093/gerona/62.9.1010. [DOI] [PubMed] [Google Scholar]
- 8.Dietz V. Proprioception and locomotor disorders. Nat Rev Neurosci. 2002 Oct;3(10):781–90. doi: 10.1038/nrn939. [DOI] [PubMed] [Google Scholar]
- 9.Dietz V, Duysens J. Significance of load receptor input during locomotion: a review. Gait Posture. 2000 Apr;11(2):102–10. doi: 10.1016/s0966-6362(99)00052-1. [DOI] [PubMed] [Google Scholar]
- 10.Gandevia SC, Macefield G, Burke D, McKenzie DK. Voluntary activation of human motor axons in the absence of muscle afferent feedback. The control of the deafferented hand. Brain. 1990 Oct;113(Pt 5):1563–81. doi: 10.1093/brain/113.5.1563. [DOI] [PubMed] [Google Scholar]
- 11.Garland SJ, Enoka RM, Serrano LP, Robinson GA. Behavior of motor units in human biceps brachii during a submaximal fatiguing contraction. J Appl Physiol. 1994 Jun;76(6):2411–9. doi: 10.1152/jappl.1994.76.6.2411. [DOI] [PubMed] [Google Scholar]
- 12.Gandevia SC. Neural control in human muscle fatigue: changes in muscle afferents, motoneurones and motor cortical drive [corrected] Acta Physiol Scand. 1998 Mar;162(3):275–83. doi: 10.1046/j.1365-201X.1998.0299f.x. [DOI] [PubMed] [Google Scholar]
- 13.Johnston RB, 3rd, Howard ME, Cawley PW, Losse GM. Effect of lower extremity muscular fatigue on motor control performance. Med Sci Sports Exerc. 1998 Dec;30(12):1703–7. doi: 10.1097/00005768-199812000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Aune AK, Nordsletten L, Skjeldal S, Madsen JE, Ekeland A. Hamstrings and gastrocnemius co-contraction protects the anterior cruciate ligament against failure: an in vivo study in the rat. J Orthop Res. 1995 Jan;13(1):147–50. doi: 10.1002/jor.1100130122. [DOI] [PubMed] [Google Scholar]
- 15.Baratta R, Solomonow M, Zhou BH, Letson D, Chuinard R, D’Ambrosia R. Muscular coactivation. The role of the antagonist musculature in maintaining knee stability. Am J Sports Med. 1988 Mar-Apr;16(2):113–22. doi: 10.1177/036354658801600205. [DOI] [PubMed] [Google Scholar]
- 16.Ferrell WR, Jr, Rosenberg JR, Baxendale RH, Halliday D, Wood L. Fourier analysis of the relation between the discharge of quadriceps motor units and periodic mechanical stimulation of cat knee joint receptors. Exp Physiol. 1990 Nov;75(6):739–50. doi: 10.1113/expphysiol.1990.sp003456. [DOI] [PubMed] [Google Scholar]
- 17.Johansson H, Sjolander P, Sojka P. A sensory role for the cruciate ligaments. Clin Orthop Relat Res, no. 1991 Jul;268:161–78. [PubMed] [Google Scholar]
- 18.Kennedy JC, Alexander IJ, Hayes KC. Nerve supply of the human knee and its functional importance. Am J Sports Med. 1982 Nov-Dec;10(6):329–35. doi: 10.1177/036354658201000601. [DOI] [PubMed] [Google Scholar]
- 19.Lephart SM, Henry TJ. Functional rehabilitation for the upper and lower extremity. Orthop Clin North Am. 1995 Jul;26(3):579–92. [PubMed] [Google Scholar]
- 20.Marshall KW, Tatton WG. Joint receptors modulate short and long latency muscle responses in the awake cat. Exp Brain Res. 1990;83(1):137–50. doi: 10.1007/BF00232202. [DOI] [PubMed] [Google Scholar]
- 21.Solomonow M, Baratta R, Zhou BH, Shoji H, Bose W, Beck C, D’Ambrosia R. The synergistic action of the anterior cruciate ligament and thigh muscles in maintaining joint stability. Am J Sports Med. 1987 May-Jun;15(3):207–13. doi: 10.1177/036354658701500302. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Lockhart TE, Soangra R. Classifying lower extremity muscle fatigue during walking using machine learning and inertial sensors. Ann Biomed Eng. 2014 Mar;42(3):600–12. doi: 10.1007/s10439-013-0917-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cortes N, Onate J, Morrison S. Differential effects of fatigue on movement variability. Gait Posture. 2014 Mar;39(3):888–93. doi: 10.1016/j.gaitpost.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavanaugh JT, Mercer VS, Stergiou N. Approximate entropy detects the effect of a secondary cognitive task on postural control in healthy young adults: a methodological report. Journal of NeuroEngineering and Rehabilitation. 2007;4(1):42. doi: 10.1186/1743-0003-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soangra R, Lockhart TE, Lach J, Abdel-Rahman EM. Effects of Hemodialysis Therapy on Sit-to-Walk Characteristics in End Stage Renal Disease Patients. Ann Biomed Eng. 2012 Dec 5; doi: 10.1007/s10439-012-0701-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barth AT, Hanson MA, Powell HC, Jr, Lach J. TEMPO 3.1: A Body Area Sensor Network Platform for Continuous Movement Assessment. 2009:71–76. [Google Scholar]
- 27.Bouten CVC, Koekkoek KTM, Verduin M, Kodde R, Janssen JD. A triaxial accelerometer and portable data processing unit for the assessment of daily physical activity. Ieee Transactions on Biomedical Engineering. 1997 Mar;44(3):136–147. doi: 10.1109/10.554760. [DOI] [PubMed] [Google Scholar]
- 28.Aziz W, Arif M. Complexity analysis of stride interval time series by threshold dependent symbolic entropy. Eur J Appl Physiol. 2006 Sep;98(1):30–40. doi: 10.1007/s00421-006-0226-5. [DOI] [PubMed] [Google Scholar]
- 29.Fasano A, Marmelat V, Torre K, Beek PJ, Daffertshofer A. Persistent Fluctuations in Stride Intervals under Fractal Auditory Stimulation. PLoS ONE. 2014;9(3):e91949. doi: 10.1371/journal.pone.0091949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipsitz LA, Goldberger AL. Loss of ‘complexity’ and aging. Potential applications of fractals and chaos theory to senescence. JAMA. 1992 Apr 1;267(13):1806–9. [PubMed] [Google Scholar]
- 31.Vaillancourt DE, Newell KM. Changing complexity in human behavior and physiology through aging and disease. Neurobiol Aging. 2002 Jan-Feb;23(1):1–11. doi: 10.1016/s0197-4580(01)00247-0. [DOI] [PubMed] [Google Scholar]
- 32.Herman T, Giladi N, Gurevich T, Hausdorff JM. Gait instability and fractal dynamics of older adults with a “cautious” gait: why do certain older adults walk fearfully? Gait Posture. 2005 Feb;21(2):178–85. doi: 10.1016/j.gaitpost.2004.01.014. [DOI] [PubMed] [Google Scholar]


