Abstract
RNA binding proteins (RBPs) play key roles in RNA dynamics, including subcellular localization, translational efficiency and metabolism. Cold-inducible RNA binding protein (CIRP) is a stress-induced protein that was initially described as a DNA damage-induced transcript (A18 hnRNP), as well as a cold-shock domain containing cold-stress response protein (CIRBP) that alters the translational efficiency of its target messenger RNAs (mRNAs). This review summarizes recent work on the roles of CIRP in the context of inflammation and cancer. The function of CIRP in cancer appeared to be solely driven though its functions as an RBP that targeted cancer-associated mRNAs, but it is increasingly clear that CIRP also modulates inflammation. Several recent studies highlight roles for CIRP in immune responses, ranging from sepsis to wound healing and tumor-promoting inflammation. While modulating inflammation is an established role for RBPs that target cytokine mRNAs, CIRP appears to modulate inflammation by several different mechanisms. CIRP has been found in serum, where it binds the TLR4-MD2 complex, acting as a Damage-associated molecular pattern (DAMP). CIRP activates the NF-κB pathway, increasing phosphorylation of Iκκ and IκBα, and stabilizes mRNAs encoding pro-inflammatory cytokines. While CIRP promotes higher levels of pro-inflammatory cytokines in certain cancers, it also decreases inflammation to accelerate wound healing. This dichotomy suggests that the influence of CIRP on inflammation is context dependent and highlights the importance of detailing the mechanisms by which CIRP modulates inflammation.
Graphical abstract
Introduction
RNA binding proteins (RBPs) play key roles in RNA dynamics, including subcellular localization, translational efficiency and metabolism (Gerstberger, Hafner, & Tuschl, 2014; Lukong, Chang, Khandjian, & Richard, 2008). As these diverse roles suggest, RBPs have been identified as key molecules in many diseases, including neurodegenerative disorders, cardiovascular disease, genetic disease, developmental disorders and several cancers (Gerstberger et al., 2014).
Cold-inducible RNA binding protein (CIRP, also known as CIRBP and A18 hnRNP) belongs to the glycine-rich RNA-binding protein family, which possesses an RNA recognition motif (RRM), and a carboxyl-terminal domain containing several RGG motifs (Nishiyama et al., 1997) CIRP is expressed in wide variety of tissues and cells and can be induced in response to cellular stress, translocating from the nucleus to the cytosol (De Leeuw et al., 2007; C. Yang & Carrier, 2001). In the cytosol, CIRP binds the 3′ untranslated regions (UTRs) of target mRNAs using its RNA-recognition motif (RRM) and can increase or suppress their translation (Fornace, Alamo, & Hollander, 1988; Nishiyama et al., 1997; Sheikh et al., 1997). Originally described as a DNA damage-induced transcript (Fornace et al., 1988), and named heterogeneous nuclear ribonucleoprotein A18 (A18 hnRNP) (Sheikh et al., 1997), CIRP was later characterized as a cold-stress response protein. Upon moderate cold stress, CIRP is expressed and binds to poly(U) polypyrimidine tracks at the 3′ ends of introns as well as to 5′ and 3′ regions of mRNAs (Wilusz, Feig, & Shenk, 1988). Its binding has been suggested to be important for 3′end cleavage and polyadenylation, as well as for regulating translation of specific mRNAs helping the cell to adapt to cold stress (Lleonart, 2010). CIRP's initial roles as a tumor suppressor was established during hypothermic stress and DNA damage (Nishiyama et al., 1997; Sheikh et al., 1997).
Recent studies have implicated CIRP in human disease, including several types of cancer, as well as a modulator of inflammation (Brochu et al., 2013; L. Chen, Ran, Xie, Xu, & Zhou, 2016; Juan et al., 2016; Qiang et al., 2013; Ren et al., 2014; Sakurai et al., 2014; Yoo et al., 2016; Zhu, Bührer, & Wellmann, 2016). In this review, we summarize what is known about the role of CIRP in human cancers, where it has been implicated in tumor suppression and promotion, as well as its emerging role in inflammation in human disease, including its role in cancer-related inflammation.
CIRP as a Tumor Suppressor
One of the earliest reported functions of CIRP was suppression of mammalian cell growth in vitro in response to mild hypothermia (Nishiyama et al., 1997). In this study, CIRP overexpression in NIH3T3 cells slowed cell growth by prolonging the G1 phase of the cell cycle. These effects were abolished upon siRNA mediated knockdown of CIRP. In response to DNA damage, CIRP was upregulated and increased the translational efficiency of the mRNAs for thioredoxin (Trx-1), replication protein A (RPA2) and ATR, by binding both their 3′UTR and eukaryotic translation initiation factor 4 gamma (eIF4G) (R. Yang, Weber, & Carrier, 2006; R. Yang et al., 2010). Trx-1 quenches reactive oxygen species while RPA2 is involved in repair of damaged DNA. ATR signals cell cycle arrest and initiates response to DNA damage by recruiting RPA2 and other repair proteins. This ability to inhibit proliferation and protect cells from genotoxic damage during cellular stress in vitro is consistent with circumstantial evidence for a tumor suppressor role for CIRP in vivo.
In agreement with a role in suppressing proliferation, in normal endometrium CIRP expression inversely correlated with the proliferation marker Ki-67 during the menstrual cycle. CIRP expression was consistently highest in normal endometrium, variable in endometrial hyperplasias and significantly reduced in carcinomas (Hamid et al., 2003). This hints that loss of CIRP expression could play a role in endometrial carcinogenesis. Loss of CIRP expression is also implicated in the progression of benign ovarian cancer to malignancy. A large microarray analysis of benign and malignant human ovarian tumors identified CIRP as an upregulated gene in benign vs. malignant tumors. This finding was confirmed using CIRP-transfected ovarian cancer cells, where CIRP transfected cells slowed doubling time (Biade et al., 2006). A recent study in normal mouse mammary glands showed that CIRP overexpression impedes proliferation during mammary gland development (Lujan et al., 2016). Lastly, higher CIRP levels in breast tissue were part of the gene signature identified for parity, a condition with reduced lifetime risk for breast cancer for post-menopausal women (S. Peri et al., 2012). These studies suggest that CIRP may function as a tumor suppressor via suppressing proliferation, potentially via its function in the DNA damage response. CIRP's reported roles as a tumor suppressor are summarized in Table 1 and Fig. 1.
Table 1. Roles of CIRP as a Tumor Suppressor.
| Role | Possible Mechanisms/Conclusions | Experimental Model(s) | References |
|---|---|---|---|
| Slows cell growth via prolonging G1 phase | Possible interaction with G1 regulators | Mouse Fibroblasts (BALB/3T3 Cells) | (Nishiyama et al., 1997) |
| Inhibits proliferation through its function as a stress-induced RBP | Binds and increases translational efficiency of DNA damage response genes ATR, Trx-1, RPA2 via 3′ UTR binding | Human Rectal carcinoma cells (RKO cells) | (R. Yang et al., 2006; R. Yang et al., 2010) |
| Inversely correlates with proliferation/reduced in endometrial cancer compared to normal endometrium | Maintains normal endometrial function, aside from its role as a stress response protein | Human Normal Endometrium and Endometrial Carcinoma Tissues | (Hamid et al., 2003) |
| Slows doubling time in ovarian cancer cells/reduced in malignant compared to benign ovarian tumors | Prevents malignancy in ovarian tumors | Human Malignant and Benign Ovarian Tumors, Ovarian Cancer Cells | (Biade et al., 2006) |
| Decreases proliferation during mammary gland development | Halts proliferation when it is no longer needed during development | Transgenic mice expressing hCIRP in mammary glands | (Lujan et al., 2016) |
| Part of transcriptomic signature for parity | Breast differentiation leads to transcriptomic changes that decrease the lifetime risk for breast cancer in parous women | Human breast biopsies | (Suraj Peri et al., 2012) |
Figure 1.
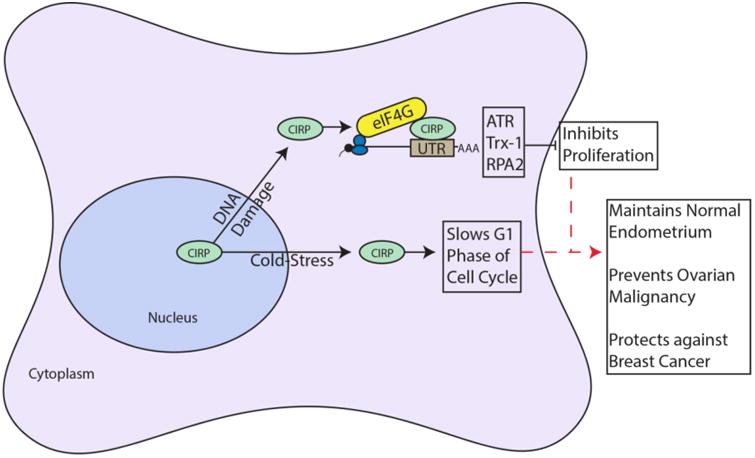
CIRP as a Tumor Suppressor. CIRP moves from the nucleus to the cytoplasm in response to stress such as DNA damage and cold-stress. In the cytoplasm, CIRP binds the 3′UTR of mRNAs encoding ATR, Trx-1 and RPA2, and interacts with eIF4G to increase translational efficiency of these mRNAs. The end result is inhibition of proliferation. In response to cold-stress, CIRP slows G1 phase of the cell cycle by an unknown mechanism. Red dashed arrows indicate potential links to suggested CIRP functions
CIRP as a Tumor Promoter
Other studies provide evidence for CIRP as a tumor promoter. CIRP displayed the ability to bypass replicative senescence in primary mouse embryonic fibroblasts through activation of the ERK1/2 signaling pathway (Artero-Castro et al., 2009). In this study, CIRP was overexpressed in subsets of prostate, breast, and colon cancer, correlative evidence for an in vivo role as a tumor promoter. Downregulation of CIRP enhanced chemosensitivity and impaired survival of prostate cancer cells (Zeng, Kulkarni, Inoue, & Getzenberg, 2009). CIRP downregulation was thought to mimic the molecular effects of heat stress on prostate cancer cells, which is known to boost the efficacy of chemotherapeutics. CIRP was overexpressed in multiple breast cancer cell lines, with its overexpression contributing to upregulation of cyclin E1, increasing proliferation and decreasing apoptosis (Guo, Wu, & Hartley, 2010). Cyclin E1 positively regulates the cell cycle and is a marker of poor prognosis in breast cancer (Gao, Ma, & Lu, 2013; Keyomarsi et al., 2002). Also through its function as an RBP, CIRP upregulated HIF-1α to promote tumor growth in ectopic mouse xenograft models of human breast cancer and melanoma (Chang, Parekh, Yang, Nguyen, & Carrier, 2016). As further evidence for a role in breast tumorigenesis, CIRP was identified as part of a serum autoantibody signature in breast cancer progression from ductal carcinoma in situ to invasive breast cancer (Mangé et al., 2012). This suggests that CIRP may be targeted by the immune system during breast cancer development.
In addition to its upregulation in carcinoma, CIRP was upregulated in pituitary corticotroph adenoma, where its expression correlated with Cushing's disease recurrence (Jian et al., 2016). CIRP overexpression in pituitary corticotroph cells increased cell proliferation as well as proopiomelanocortin transcription, a marker of aberrant pituitary function. Similar to studies with primary mouse embryonic fibroblasts, CIRP promoted proliferation via ERK-signaling, in this case by downregulating the CDK-inhibitor p21, and inducing cyclin D1. This study identified CIRP as a marker of poor prognosis in pituitary adenoma and as a possible marker for recurrence.
Further evidence for CIRP's oncogenic function is its upregulation of telomerase activity in a temperature dependent fashion (mild hypothermia) in several human cell lines of different origins including HEK293T (embryonic kidney), HeLa (ovarian cancer), HTC75 (T lymphocytes), and U2OS (osteosarcoma) (Zhang et al., 2015). Telomerase is responsible for adding telomeric repeats to chromosomal ends and consists of the reverse transcriptase TERT and the RNA subunit TERC. Telomere shortening due to lack of or decreased telomerase activity causes cellular senescence, while aberrant activation of telomerase has been observed in >85% of human cancers (Akincilar, Unal, & Tergaonkar, 2016). CIRP regulated telomerase through its function as an RBP, binding to TERC mRNA to increase its stability. CIRP also regulated telomerase assembly via protein-protein interaction in Cajal bodies, increasing telomerase complex stability during assembly (Zhang et al., 2015). These studies show that CIRP can directly bypass senescence by boosting telomerase function, as well as indirectly by promoting signaling pathways that promote proliferation.
Recent mechanistic studies show that CIRP promotes epithelial to mesenchymal transition (EMT) (Lee et al., 2016) and inhibits apoptosis via ERK activation (Lee et al., 2015; Sakurai et al., 2006), both established hallmarks of cancer (Hanahan & Weinberg, 2011), by downregulating p53. TGF-β treatment of human lung carcinoma cells (A549 cells) overexpressing CIRP upregulated mesenchymal markers, downregulated epithelial markers, and increased migration and invasion. Knockdown of CIRP abrogated these effects as well as downregulated the EMT marker Snail, which suggests that CIRP likely upregulates Snail through activation of ERK and p38MAPK pathways. Table 2 summarizes CIRP's purported roles in promoting tumorigenesis.
Table 2. Roles of CIRP as a Tumor Promoter.
| Role | Possible Mechanisms/Conclusions | Model | References |
|---|---|---|---|
| Bypasses replicative senescence in vitro; overexpressed in breast, prostate, colon and pituitary cancers | Enhances translation via ribosomal protein S6 and 4E-BP1 interactions; increases ERK activation, cyclin D1 and proliferation; decreases p27 via Erk1/2 signaling pathway | Mouse Embryo Fibroblasts and Human Tumors | (Artero-Castro et al., 2009; Jian et al., 2016) |
| Downregulation impairs prostate cancer cell survival | Downregulation mimics heat stress and impairs cancer cell survival | Human Prostate Cancer Cell Lines (PC3 and LNCaP Cells) | (Zeng et al., 2009) |
| Upregulates HIF-1α in breast cancer and melanoma xenografts, cyclin E1 in breast cancer cells, and is part of autoantibody signature in breast cancer | Stabilizes HIF-1α and cyclin E1 mRNAs, increasing their translation | Human Breast Cancer cells (MCF7 Cells), Human Melanoma Cells (HEMa-LP) and Human Breast Tumors | (Chang et al., 2016; Guo et al., 2010; Mangé et al., 2012) |
| Knockdown results in decreased telomerase activity in vitro | Stabilizes TERC mRNA, increasing its translation; stabilizes telomerase complex assembly in Cajal bodies | Cancer Cell Lines (HTC75, HeLa, U2OS, HEK293) | (Zhang et al., 2015) |
| Upregulation decreases p53 levels and activity, decreasing apoptosis; induced EMT markers | Unknown mechanism of p53 regulation; function in EMT relies on its RRM motif | Lung Carcinoma Cells (A549); Hepatoma cells (HepG2, SK-HEP-1) | (Lee, Ahn, & Jang, 2015, 2016; Sakurai et al., 2006) |
Taken as a whole, these studies provide evidence for CIRP's role as an oncogene through its actions in various contexts, including bypassing replicative senescence, upregulating telomerase activity, promoting proliferation, inhibiting apoptosis, and promoting EMT (Fig. 2). Although not yet experimentally linked, these roles are likely interdependent. For example, CIRP's role in p53 regulation and EMT suggests that it could be affecting senescence and telomerase maintenance via its regulation of p53, as the link between p53, replicative senescence and telomerase maintenance has been established in many cancer types (Beauséjour et al., 2003; Z. Chen et al., 2005; Chin et al., 1999; Xue et al., 2007). Links between the known roles of CIRP in activating ERK signaling pathways and potential downstream effects on p53 have not been explored.
Figure 2.
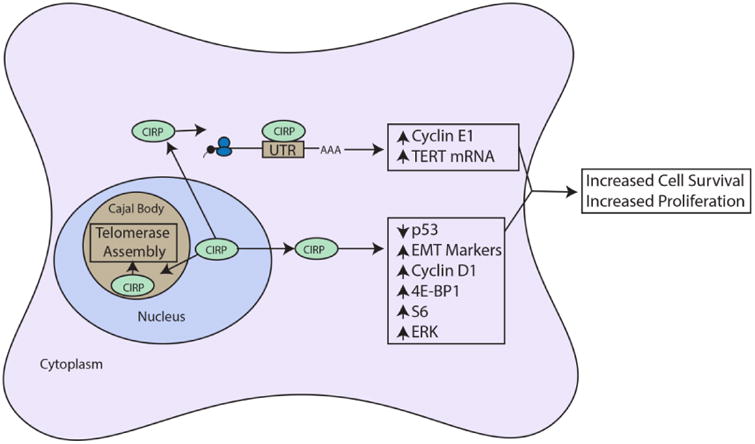
Roles of CIRP as a tumor promoter. CIRP moves from the nucleoplasm into Cajal bodies where it stabilizes hTERC and telomerase complex assembly via protein-protein interaction. CIRP also moves from the nucleus to the cytoplasm where it binds and stabilizes cyclin E1 mRNA, and binds hTERT mRNA, increasing both of these proteins. CIRP also downregulates p53 and upregulates EMT markers, cyclin D1, 4E-BP1, S6 and ERK1/2 by unknown mechanisms. The overall result is increased cell proliferation and cell survival. Arrows and boxes in black represent known roles from literature and red dashed arrows and boxes represent possible mechanisms or connections.
CIRP as a Mediator of Inflammation
Several recent studies highlight roles for CIRP in immune responses, ranging from sepsis and pulmonary inflammation to wound healing and tumor-promoting inflammation The first study to implicate CIRP in inflammation showed that it was upregulated in the serum of patients undergoing hemorrhagic shock and sepsis (Qiang et al., 2013). Patients with elevated levels of CIRP had a significantly higher mortality rate than patients without elevated CIRP levels. This finding was noteworthy because it was the first time CIRP was observed extracellularly. CIRP was also upregulated in the heart and liver of mice undergoing hemorrhagic shock and was released via lysosomal secretion; neutralizing antibodies against CIRP ameliorated the hemorrhagic effects. CIRP was shown to function as a DAMP in this study, as it bound to the TLR4-MD2 complex on professional antigen presenting cells (APCs) and stimulated release of pro-inflammatory cytokines TNFα and IL-6. Although this study first hinted that CIRP could serve as a marker for poor prognosis in sepsis and hemorrhagic shock, a more recent study corroborated and confirmed their findings (Y. Zhou et al., 2015). The mechanism of CIRP's extracellular localization was not explored in either of these studies, nor was its function as an RBP. Bolognese et al. recently established that CIRP also activates splenic T-cells via TLR-4 (Bolognese et al., 2016). CIRP stimulated both CD8+ and CD4+ T-cells and drove CD4+ to a Th1 type response profile, which could explain its abilities as a tumor suppressor in specific contexts (Bolognese et al., 2016). CIRP may also play a role in synovial inflammation, as it is upregulated in the serum and synovial fluid of patients with rheumatoid arthritis and is correlated with inflammatory disease activity, which implicates CIRP as a marker for chronic synovial inflammation (Yoo et al., 2016).
In a model for pulmonary cold-stress, CIRP mediated increased tissue injury, inflammation and increased mucus secretion through the TLR4/NF-κB pathway (L. Chen et al., 2016). These effects were further supported by the finding that CIRP was increased in both patients with chronic obstructive pulmonary disease (COPD) and mice exposed to cold air. Blocking of CIRP expression, TLR4 function, or NF-κB function, each attenuated an increase in inflammation, tissue damage and mucus secretion. CIRP can also increase mucus secretion and airway inflammation though a TLR4 independent mechanism. Using rats exposed to either cigarette smoke or cold air, CIRP was increased in a TLR4-independent fashion (Juan et al., 2016). This study also showed that CIRP overexpression in normal human bronchial epithelial cells promoted the formation of stress granules and that CIRP bound to the 3′UTR of MUC5AC to increase its translational efficiency and thus increases mucus production and secretion. These studies show that CIRP can modulate airway inflammation by triggering TLR4, further establishing CIRP as a damage-associated molecular pattern (DAMP).
Further elucidating CIRP's function in pulmonary inflammation, CIRP stimulated activation and assembly of the Nlrp3 inflammasome in mouse lung vascular endothelial cells (MLVECs) (W.-L. Yang et al., 2016). Treatment of MLVECs with CIRP led to increased caspase-1 and IL-1β and induction of pyroptosis, while intravenous CIRP injection into wild-type mice led to endothelial cell activation and significant lung damage. These findings provide a possible mechanism for lung damage under conditions of hemorrhagic shock and sepsis (Fein & Calalang-Colucci, 2000).
CIRP likely stimulates release of proinflammatory cytokines through activation of the NF-κB pathway (Brochu et al., 2013). Using UVC radiation to induce upregulation of CIRP in neonatal foreskin fibroblasts (NFhTrt cells), Brochu et al. screened for novel CIRP induced transcripts and identified IL-1β, IL-8 and TNFα mRNAs. These increases were mediated by the NF-κB pathway, as blockade of CIRP decreased Iκκ phosphorylation. Additional evidence for CIRP as a positive regulator of the NF-κB pathway was provided by Zhou et al. when they found that CIRP activated NF-κB in microglia, which caused neuroinflammation in vivo and apoptosis in neurons in vitro (M. Zhou, Yang, Ji, Qiang, & Wang, 2014). Although the direct mechanism is not known, these studies provide strong support for CIRP as a positive regulator of the NF-κB pathway. While CIRP can upregulate TNFα through the NF-κB pathway, TNFα has also been shown to be a negative regulator of CIRP through the non-canonical RelB NF-κB pathway, suggesting a negative feedback loop (M. Lopez et al., 2014; M. A. Lopez, Meier, Wong, & Fontana, 2016).
Although the studies above provide several contexts in which CIRP functions as a positive inflammatory modulator, CIRP has also been shown to decrease inflammation (Idrovo et al., 2016). In a study of CIRP in wound healing, wild-type and CIRP knockout mice were subjected to a full thickness wound. When measured post-injury, TNFα expression was increased 65-fold in CIRP knockout mice and only 16-fold in wild-type mice, which suggests CIRP could be decreasing wound-associated inflammation (Idrovo et al., 2016). In addition, during the initial (inflammatory) phase of wound healing, TNFα was significantly decreased in wild-type mice when compared to CIRP knockout mice. Wounds in CIRP knockout mice healed significantly faster with shorter initial inflammatory phases when compared to wild-type mice. The function of CIRP in inflammatory signaling appears to be context dependent (Table 3 and Fig. 3), as further illustrated by studies of CIRP-influenced inflammation in cancer, discussed in the next section.
Table 3. Roles of CIRP as a Mediator of Inflammation.
| Role | Possible Mechanisms/Conclusions | Model | References |
|---|---|---|---|
| Stimulates inflammation in sepsis and hemorrhagic shock; where it serves as a marker for poor prognosis | Secreted through lysosomal export and binds the TLR4/MD2 complex on APCs to stimulate release of TNFα and IL-6 | Human serum, mouse and rat models of sepsis and hemorrhagic shock | (Qiang et al., 2013; Y. Zhou et al., 2015) |
| Activates splenic T-cells during sepsis, contributing to T-cell dysregulation | Binds TLR4/MD2 complex on CD4+ and CD8+ T-cells to induce activation and Th1 hyperinflammatory response | Mice with induced sepsis | (Bolognese et al., 2016) |
| Mediates lung damage, inflammation and increased mucus secretion; elevated in COPD patients and cold-air exposed mice and rats | Increases inflammation and tissue damage by activation of TLR4/NFκB pathway; stabilizes MUC5AC mRNA in stress granules to increase mucus secretion | Human bronchial biopsies, bronchial epithelial cells, mice exposed to cold-air, rats exposed to cold-air or cigarette smoke | (L. Chen et al., 2016; Juan et al., 2016) |
| Elevated in serum and synovial fluid from patients with rheumatoid arthritis | Levels in synovial fluid strongly correlated with markers for rheumatoid arthritis disease activity; potential marker for rheumatoid arthritis | Human serum and synovial fluid | (Yoo et al., 2016) |
| Increases caspase-1, IL-1β and induced pyroptosis; CIRP injections into mice causes endothelial cell activation and significant lung damage | Activates assembly of the Nlrp3 inflammasome; possible mechanism for lung damage in sepsis and hemorrhagic shock | Mouse lung vascular endothelial cells (MLVEC), WT Mice | (Fein & Calalang-Colucci, 2000; W.-L. Yang et al., 2016) |
| Induces IL-1β, IL-8 and TNFα transcripts | Increases Iκκ phosphorylation, activating inflammation through NFκB pathway | Neonatal foreskin fibroblasts (NFhTrt cells) | (Brochu et al., 2013) |
| Activates NFκB in vivo and apoptosis in neurons | Positively regulates NFκB causing neuroinflammation | Mouse Fibroblasts (NIH3T3 cells), mouse models of neuroinflammation | (M. A. Lopez et al., 2016; M. Zhou et al., 2014) |
| Knockout mice have higher TNFα expression during initial wound healing phase | Decreases TNFα during the initial (inflammatory) phase of wound healing | Wild-type and CIRP knockout mice subjected to wounds | (Idrovo et al., 2016) |
Figure 3.
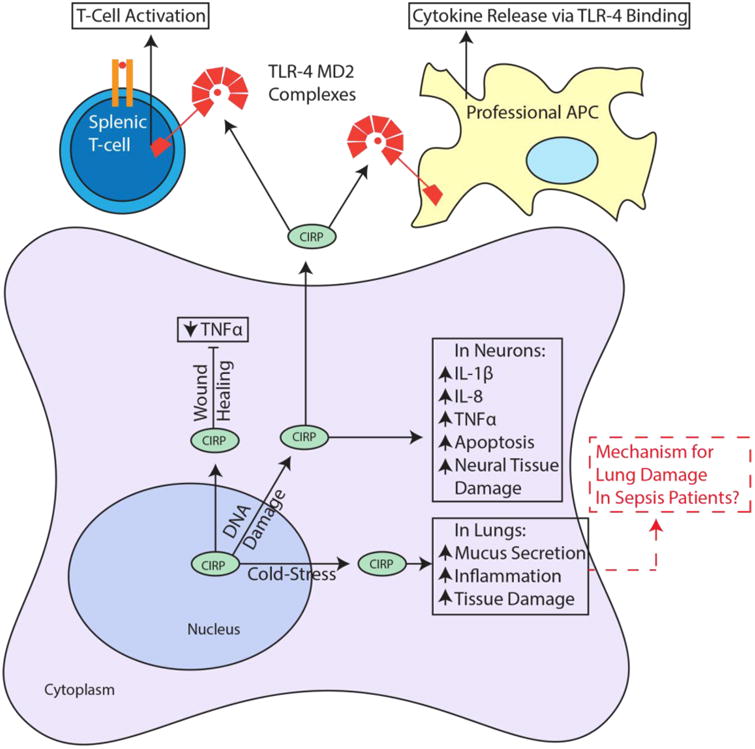
Roles of CIRP as a mediator of inflammation. In response to cold-stress CIRP stimulates mucus secretion, inflammation and tissue damage in the lung. In response to DNA damage, CIRP decreases TNFα to promote wound healing and increases IL1β, IL-8 and TNFα in neurons as well as inducing apoptosis and neural tissue damage. Also in response to DNA damage, CIRP is found extracellularly where it binds MD2 in TLR-4 complexes of professional antigen presenting cells (APC) and splenic T-cells. This results in cytokine release and T-cell activation. and Red dashed arrows indicate potential links to suggested CIRP functions. Arrows and boxes in black represent known roles from literature and red dashed arrows and boxes represent possible mechanisms or connections.
CIRP in Inflammation and Cancer
Recent studies have begun to highlight the mechanistic link between CIRP's function as an inflammatory molecule and its oncogenic function. In a mouse model for colitis-associated cancer (CAC), CIRP promoted an increase of TNFα and IL-23 expression in inflammatory cells (Sakurai et al., 2014). CIRP knockout mice were less susceptible to CAC development and had reduced expression of chronic inflammation markers TNFα, IL-23 and anti-apoptotic proteins Bcl-2 and Bcl-XL in colonic lamina propria cells. Reduced expression of anti-apoptotic proteins Bcl-2 and Bcl-XL in these mice stimulated inflammatory cell apoptosis in the lamina propria. CIRP deficiency also decreased expression of stem cell marker Sox2 and the number of Dclk1+ cells, which is a gut associated cancer stem cell marker. Lastly, bone marrow transplants from CIRP knockout mice into wild-type mice reduced tumorigenesis, indicating that CIRP may function at the stem cell level in CAC. This study concluded that CIRP likely increased expression of IL-17/23 and TNFα via the NF-κB pathway, as CIRP knockout mice had lower levels of IκBα phosphorylation. Interestingly, CIRP levels are elevated in colonic mucosae of patients diagnosed with refractory ulcerative colitis (Sakurai et al., 2014). These patients are considered to be “at risk” for CAC development.
In a study of oral squamous cell carcinoma (OSCC), expression of TLR4 and CIRP were both elevated in OSCC tissues when compared to matched normal tissues. Both CIRP and TLR4 expression correlated with poor outcomes, implicating that both are markers of poor prognosis (Ren et al., 2014). Since CIRP is a known trigger of TLR4 through its function as a DAMP, it is possible that CIRP and TLR4 activity may lead to increased levels of pro-inflammatory cytokines in OSCC, although it has yet to be studied. Although CIRP is capable of triggering TLR4, upon TLR4 binding, CIRP was shown to promote a Th1 type of response, which is typically effective in eliminating cancer (Elinav et al., 2013).
Reinforcing CIRP's promotion of inflammation in cancer is a study of hepatocellular carcinoma (HCC) (Sakurai et al., 2015). In this study, CIRP was found to increase expression of IL-1β and IL-6 in Kupffer cells, liver specific macrophages. In addition, CIRP expression correlated with levels of reactive oxygen species (ROS) as well as with HCC recurrence. CIRP knockout mice exhibited attenuated tumorigenesis, reduced ROS accumulation and reduced expression of IL-1β and IL-6. CIRP deficiency also decreased expression of the cancer stem cell marker CD133, providing further evidence that CIRP may play a role in promoting stemness in HCC as well as in CAC and possibly in other forms of cancer. CIRP was shown to promote inflammation in the liver during ischemia/reperfusion injury, which promotes liver metastasis of colon cancer. This suggests a role for CIRP in metastasis (Doi et al., 2002; Godwin et al., 2015). These studies firmly establish CIRP's role as a molecular modulator of inflammation and cancer, providing several avenues for future studies (Fig. 4).
Figure 4.
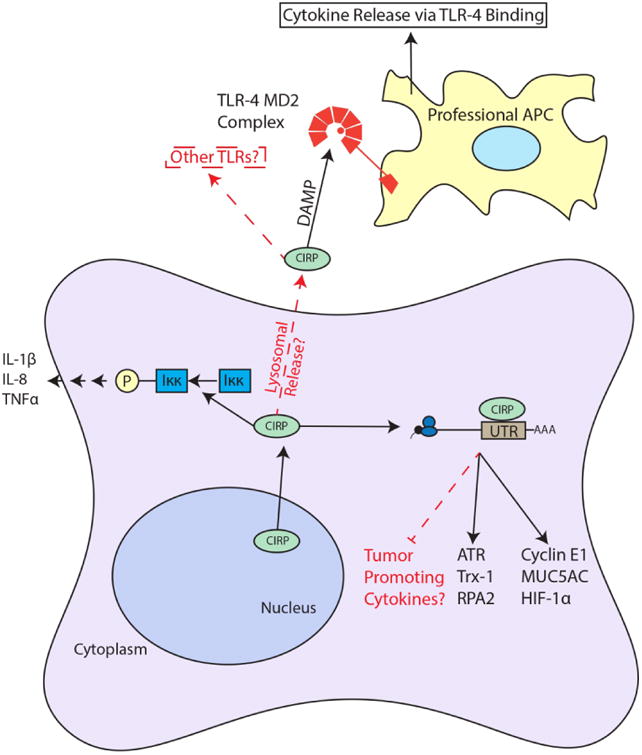
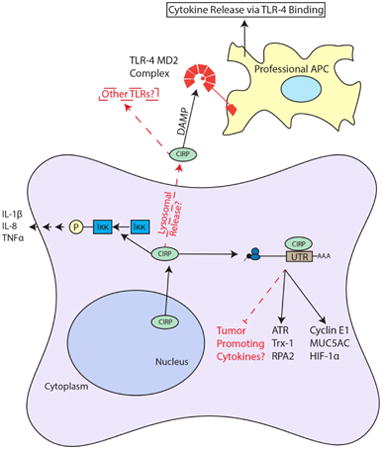
Summary of CIRP Roles in Inflammation and Cancer. CIRP shuttles from the nucleus to the cytoplasm to bind the 3′ UTRs of target mRNA to increase their translation. It is possible that CIRP can bind tumor promoting cytokines in certain contexts. CIRP in found extracellularly in patients with sepsis (likely through lysosomal secretion) where it binds the TLR-4 MD2 complex, functioning as a DAMP and stimulating cytokine release from APCs. Also, CIRP increases Iκκ phosphorylation through an unknown mechanism. Arrows and boxes in black represent known roles from literature and red dashed arrows and boxes represent possible mechanisms or connections.
Conclusion
This review summarizes historical and recent studies into the roles of CIRP in cancer, inflammation, and cancer-associated inflammation (see Graphical Abstract). One of the most notable recent developments is CIRP's influence on cancer through its effects on inflammation. The function of CIRP in cancer appeared to be mainly driven though its functions as an RBP, promoting the stability and translation of specific mRNAs encoding cancer-associated proteins (Kim, Hur, & Jeong, 2009). It is becoming clear that CIRP also functions as a modulator of inflammation in several forms of cancer as well as in other diseases. Playing a role in inflammation is not a new role for RBP's in general (Katsanou et al., 2005; Kim et al., 2009; Zhu et al., 2016). A recent study integrated diverse public domain datasets to catalogue 1344 RBPs and perform a meta-analysis of the RBPome (Neelamraju, Hashemikhabir, & Janga, 2015). An important finding was that RBPs are significantly associated with inflammatory diseases and immune responses, as well as all major types of cancer. CIRP was noted in this report. Recent findings expand the scope of the CIRP's capabilities and provoke several new questions. For instance, it is now known that CIRP is capable of promoting higher levels of inflammatory cytokines in certain cancers, but also appears to be capable of decreasing inflammation to accelerate wound healing. This dichotomy suggests that the influence of CIRP on inflammation is context dependent and highlights the importance of detailing the mechanisms by which CIRP modulates inflammation in various contexts. Since it is now clear that CIRP is capable of influencing cancer as both an RBP and as an inflammatory modulator, it is possible that CIRP plays more than one role.
The most intriguing unknowns include the mechanism for CIRP's extracellular localization (Qiang et al., 2013; Ren et al., 2014; Sakurai et al., 2014) and CIRP's binding of the TLR4-MD2 complex when found extracellularly, which provokes the question of whether it has the ability to bind other toll-like receptors, and how this binding alters function. Similarly, determining how CIRP increases Iκκ and IκBα phosphorylation, is both intriguing and necessary for understanding CIRP's role in the NFκB pathway. CIRP is pro-inflammatory via its function as a stabilizing RBP, but also decreases inflammation by unknown mechanisms. Future studies should be aimed at elucidating the mechanistic roles CIRP plays in these various contexts.
Acknowledgments
This work was funded by the University of New Mexico Research Allocation Committee, the American Association of Anatomists Fellows Grant Award Program and by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award (1 F31 CA213933-01A1)
Contributor Information
Daniel A. Lujan, Department of Cell Biology and Physiology, University of New Mexico School of Medicine and University of New Mexico Comprehensive Cancer Center, Albuquerque, NM
Joey L. Ochoa, Department of Cell Biology and Physiology, University of New Mexico School of Medicine and University of New Mexico Comprehensive Cancer Center, Albuquerque, NM
Rebecca S. Hartley, Department of Cell Biology and Physiology, University of New Mexico School of Medicine and University of New Mexico Comprehensive Cancer Center, Albuquerque, NM
References
- Akincilar SC, Unal B, Tergaonkar V. Reactivation of telomerase in cancer. Cellular and Molecular Life Sciences. 2016;73(8):1659–1670. doi: 10.1007/s00018-016-2146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artero-Castro A, Callejas FB, Castellvi J, Kondoh H, Carnero A, Fernández-Marcos PJ, et al. Lleonart ME. Cold-inducible RNA-binding protein bypasses replicative senescence in primary cells through extracellular signal-regulated kinase 1 and 2 activation. Molecular and cellular biology. 2009;29(7):1855–1868. doi: 10.1128/MCB.01386-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauséjour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. The EMBO journal. 2003;22(16):4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biade S, Marinucci M, Schick J, Roberts D, Workman G, Sage E, et al. Johnson S. Gene expression profiling of human ovarian tumours. British journal of cancer. 2006;95(8):1092–1100. doi: 10.1038/sj.bjc.6603346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognese AC, Sharma A, Yang WL, Nicastro J, Coppa GF, Wang P. Cold-inducible RNA-binding protein activates splenic T cells during sepsis in a TLR4-dependent manner. Cellular & Molecular Immunology. 2016 doi: 10.1038/cmi.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochu C, Cabrita MA, Melanson BD, Hamill JD, Lau R, Pratt MC, McKay BC. NF-κB-dependent role for cold-inducible RNA binding protein in regulating interleukin 1β. PloS one. 2013;8(2):e57426. doi: 10.1371/journal.pone.0057426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang ET, Parekh PR, Yang Q, Nguyen DM, Carrier F. Heterogenous ribonucleoprotein A18 (hnRNP A18) promotes tumor growth by increasing protein translation of selected transcripts in cancer cells. Oncotarget. 2016;7(9):10578. doi: 10.18632/oncotarget.7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Ran D, Xie W, Xu Q, Zhou X. Cold-inducible RNA-binding protein mediates cold air inducible airway mucin production through TLR4/NF-κB signaling pathway. International Immunopharmacology. 2016;39:48–56. doi: 10.1016/j.intimp.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, et al. Gerald W. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436(7051):725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, et al. DePinho RA. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. cell. 1999;97(4):527–538. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- De Leeuw F, Zhang T, Wauquier C, Huez G, Kruys V, Gueydan C. The cold-inducible RNA-binding protein migrates from the nucleus to cytoplasmic stress granules by a methylation-dependent mechanism and acts as a translational repressor. Experimental cell research. 2007;313(20):4130–4144. doi: 10.1016/j.yexcr.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Doi K, Horiuchi T, Uchinami M, Tabo T, Kimura N, Yokomachi J, et al. Tanaka K. Hepatic ischemia–reperfusion promotes liver metastasis of colon cancer. Journal of Surgical Research. 2002;105(2):243–247. doi: 10.1006/jsre.2002.6356. [DOI] [PubMed] [Google Scholar]
- Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nature Reviews Cancer. 2013;13(11):759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- Fein AM, Calalang-Colucci MG. Acute lung injury and acute respiratory distress syndrome in sepsis and septic shock. Critical care clinics. 2000;16(2):289–317. doi: 10.1016/s0749-0704(05)70111-1. [DOI] [PubMed] [Google Scholar]
- Fornace AJ, Alamo I, Hollander MC. DNA damage-inducible transcripts in mammalian cells. Proceedings of the National Academy of Sciences. 1988;85(23):8800–8804. doi: 10.1073/pnas.85.23.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Ma JJ, Lu C. Prognostic value of cyclin E expression in breast cancer: a meta-analysis. Tumor Biology. 2013;34(6):3423–3430. doi: 10.1007/s13277-013-0915-8. [DOI] [PubMed] [Google Scholar]
- Gerstberger S, Hafner M, Tuschl T. A census of human RNA-binding proteins. Nature Reviews Genetics. 2014;15(12):829–845. doi: 10.1038/nrg3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin A, Yang WL, Sharma A, Khader A, Wang Z, Zhang F, et al. Wang P. Blocking Cold-Inducible RNA-Binding Protein (CIRP) Protects Liver from Ischemia/Reperfusion Injur. Shock (Augusta, Ga) 2015;43(1):24. doi: 10.1097/SHK.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Wu Y, Hartley RS. Cold-inducible RNA-binding protein contributes to human antigen R and cyclin E1 deregulation in breast cancer. Molecular carcinogenesis. 2010;49(2):130–140. doi: 10.1002/mc.20582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid AA, Mandai M, Fujita J, Nanbu K, Kariya M, Kusakari T, et al. Fujii S. Expression of cold-inducible RNA-binding protein in the normal endometrium, endometrial hyperplasia, and endometrial carcinoma. International journal of gynecological pathology. 2003;22(3):240–247. doi: 10.1097/01.PGP.0000070851.25718.EC. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Idrovo JP, Jacob A, Yang WL, Wang Z, Yen HT, Nicastro J, et al. Wang P. A deficiency in cold-inducible RNA-binding protein accelerates the inflammation phase and improves wound healing. International journal of molecular medicine. 2016;37(2):423–428. doi: 10.3892/ijmm.2016.2451. [DOI] [PubMed] [Google Scholar]
- Jian F, Chen Y, Ning G, Fu W, Tang H, Chen X, et al. Wang W. Cold inducible RNA binding protein upregulation in pituitary corticotroph adenoma induces corticotroph cell proliferation via Erk signaling pathway. Oncotarget. 2016;7(8):9175–9187. doi: 10.18632/oncotarget.7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan Y, Haiqiao W, Xie W, Huaping H, Zhong H, Xiangdong Z, et al. Perelman JM. Cold-inducible RNA-binding protein mediates airway inflammation and mucus hypersecretion through a post-transcriptional regulatory mechanism under cold stress. The International Journal of Biochemistry & Cell Biology. 2016;78:335–348. doi: 10.1016/j.biocel.2016.07.029. [DOI] [PubMed] [Google Scholar]
- Katsanou V, Papadaki O, Milatos S, Blackshear PJ, Anderson P, Kollias G, Kontoyiannis DL. HuR as a negative posttranscriptional modulator in inflammation. Molecular cell. 2005;19(6):777–789. doi: 10.1016/j.molcel.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Keyomarsi K, Tucker SL, Buchholz TA, Callister M, Ding Y, Hortobagyi GN, et al. Lowe M. Cyclin E and survival in patients with breast cancer. New England Journal of Medicine. 2002;347(20):1566–1575. doi: 10.1056/NEJMoa021153. [DOI] [PubMed] [Google Scholar]
- Kim MY, Hur J, Jeong SJ. Emerging roles of RNA and RNA-binding protein network in cancer cells. BMB reports. 2009;42(3):125–130. doi: 10.5483/bmbrep.2009.42.3.125. [DOI] [PubMed] [Google Scholar]
- Lee HN, Ahn SM, Jang HH. Cold-inducible RNA-binding protein, CIRP, inhibits DNA damage-induced apoptosis by regulating p53. Biochemical and Biophysical Research Communications. 2015;464(3):916–921. doi: 10.1016/j.bbrc.2015.07.066. [DOI] [PubMed] [Google Scholar]
- Lee HN, Ahn SM, Jang HH. Cold-inducible RNA-binding protein promotes epithelial-mesenchymal transition by activating ERK and p38 pathways. Biochemical and Biophysical Research Communications. 2016;477(4):1038–1044. doi: 10.1016/j.bbrc.2016.07.028. [DOI] [PubMed] [Google Scholar]
- Lleonart M. A new generation of proto-oncogenes: cold-inducible RNA binding proteins. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2010;1805(1):43–52. doi: 10.1016/j.bbcan.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Lopez M, Meier D, Müller A, Franken P, Fujita J, Fontana A. Tumor necrosis factor and transforming growth factor β regulate clock genes by controlling the expression of the cold inducible RNA-binding protein (CIRBP) Journal of Biological Chemistry. 2014;289(5):2736–2744. doi: 10.1074/jbc.M113.508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MA, Meier D, Wong WWL, Fontana A. TNF induced inhibition of Cirbp expression depends on RelB NF-κB signalling pathway. Biochemistry and Biophysics Reports. 2016;5:22–26. doi: 10.1016/j.bbrep.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan DA, Garcia S, Vanderhoof J, Sifuentes J, Brandt Y, Wu Y, et al. Hathaway HJ. Cold-inducible RNA binding protein in mouse mammary gland development. Tissue and Cell. 2016;48(6):577–587. doi: 10.1016/j.tice.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Lukong KE, Chang Kw, Khandjian EW, Richard S. RNA-binding proteins in human genetic disease. Trends in Genetics. 2008;24(8):416–425. doi: 10.1016/j.tig.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Mangé A, Lacombe J, Bascoul-Mollevi C, Jarlier M, Lamy PJ, Rouanet P, et al. Solassol J. Serum autoantibody signature of ductal carcinoma in situ progression to invasive breast cancer. Clinical Cancer Research. 2012;18(7):1992–2000. doi: 10.1158/1078-0432.CCR-11-2527. [DOI] [PubMed] [Google Scholar]
- Neelamraju Y, Hashemikhabir S, Janga SC. The human RBPome: from genes and proteins to human disease. Journal of proteomics. 2015;127:61–70. doi: 10.1016/j.jprot.2015.04.031. [DOI] [PubMed] [Google Scholar]
- Nishiyama H, Itoh K, Kaneko Y, Kishishita M, Yoshida O, Fujita J. A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth. The Journal of cell biology. 1997;137(4):899–908. doi: 10.1083/jcb.137.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri S, de Cicco RL, Santucci-Pereira J, Slifker M, Ross EA, Russo IH, et al. Zeleniuch-Jacquotte A. Defining the genomic signature of the parous breast. BMC medical genomics. 2012;5(1):46. doi: 10.1186/1755-8794-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri S, de Cicco RL, Santucci-Pereira J, Slifker M, Ross EA, Russo IH, et al. Russo J. Defining the genomic signature of the parous breast. BMC Med Genomics. 2012;5:46. doi: 10.1186/1755-8794-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang X, Yang WL, Wu R, Zhou M, Jacob A, Dong W, et al. Wang H. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nature medicine. 2013;19(11):1489–1495. doi: 10.1038/nm.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren WH, Zhang LM, Liu HQ, Gao L, Chen C, Qiang C, et al. Huang C. Protein overexpression of CIRP and TLR4 in oral squamous cell carcinoma: an immunohistochemical and clinical correlation analysis. Medical Oncology. 2014;31(8):1–9. doi: 10.1007/s12032-014-0120-7. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Itoh K, Higashitsuji H, Nonoguchi K, Liu Y, Watanabe H, et al. Fujita J. Cirp protects against tumor necrosis factor-α-induced apoptosis via activation of extracellular signal-regulated kinase. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2006;1763(3):290–295. doi: 10.1016/j.bbamcr.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Kashida H, Watanabe T, Hagiwara S, Mizushima T, Iijima H, et al. Kudo M. Stress response protein cirp links inflammation and tumorigenesis in colitis-associated cancer. Cancer research. 2014;74(21):6119–6128. doi: 10.1158/0008-5472.CAN-14-0471. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Yada N, Watanabe T, Arizumi T, Hagiwara S, Ueshima K, et al. Kudo M. Cold-inducible RNA-binding protein promotes the development of liver cancer. Cancer science. 2015;106(4):352–358. doi: 10.1111/cas.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh MS, Carrier F, Papathanasiou MA, Hollander MC, Zhan Q, Yu K, Fornace AJ. Identification of several human homologs of hamster DNA damage-inducible transcripts cloning and characterization of a novel uv-inducible cDNA that codes for a putative RNA-binding protein. Journal of Biological Chemistry. 1997;272(42):26720–26726. doi: 10.1074/jbc.272.42.26720. [DOI] [PubMed] [Google Scholar]
- Wilusz J, Feig DI, Shenk T. The C proteins of heterogeneous nuclear ribonucleoprotein complexes interact with RNA sequences downstream of polyadenylation cleavage sites. Molecular and cellular biology. 1988;8(10):4477–4483. doi: 10.1128/mcb.8.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, et al. Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445(7128):656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Carrier F. The UV-inducible RNA-binding protein A18 (A18 hnRNP) plays a protective role in the genotoxic stress response. Journal of Biological Chemistry. 2001;276(50):47277–47284. doi: 10.1074/jbc.M105396200. [DOI] [PubMed] [Google Scholar]
- Yang R, Weber DJ, Carrier F. Post-transcriptional regulation of thioredoxin by the stress inducible heterogenous ribonucleoprotein A18. Nucleic acids research. 2006;34(4):1224–1236. doi: 10.1093/nar/gkj519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Zhan M, Nalabothula NR, Yang Q, Indig FE, Carrier F. Functional significance for a heterogenous ribonucleoprotein A18 signature RNA motif in the 3′-untranslated region of ataxia telangiectasia mutated and Rad3-related (ATR) transcript. Journal of Biological Chemistry. 2010;285(12):8887–8893. doi: 10.1074/jbc.M109.013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WL, Sharma A, Wang Z, Li Z, Fan J, Wang P. Cold-inducible RNA-binding protein causes endothelial dysfunction via activation of Nlrp3 inflammasome. Scientific reports. 2016;6 doi: 10.1038/srep26571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo IS, Lee SY, Park CK, Lee JC, Kim Y, Yoo SJ, et al. Kang SW. Serum and synovial fluid concentrations of cold-inducible RNA-binding protein in patients with rheumatoid arthritis. International Journal of Rheumatic Diseases. 2016 doi: 10.1111/1756-185X.12892. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Kulkarni P, Inoue T, Getzenberg RH. Down-regulating cold shock protein genes impairs cancer cell survival and enhances chemosensitivity. Journal of cellular biochemistry. 2009;107(1):179–188. doi: 10.1002/jcb.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wu Y, Mao P, Li F, Han X, Zhang Y, et al. Liu D. Cold-inducible RNA-binding protein CIRP/hnRNP A18 regulates telomerase activity in a temperature-dependent manner. Nucleic acids research. 2015:gkv1465. doi: 10.1093/nar/gkv1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Yang WL, Ji Y, Qiang X, Wang P. Cold-inducible RNA-binding protein mediates neuroinflammation in cerebral ischemia. Biochimica et Biophysica Acta (BBA)-General Subjects. 2014;1840(7):2253–2261. doi: 10.1016/j.bbagen.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Dong H, Zhong Y, Huang J, Lv J, Li J. The Cold-Inducible RNA-Binding Protein (CIRP) Level in peripheral blood predicts sepsis outcome. PloS one. 2015;10(9):e0137721. doi: 10.1371/journal.pone.0137721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Bührer C, Wellmann S. Cold-inducible proteins CIRP and RBM3, a unique couple with activities far beyond the cold. Cellular and Molecular Life Sciences. 2016:1–21. doi: 10.1007/s00018-016-2253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


