Abstract
Lower extremity venous insufficiency and varicose veins are common conditions, affecting up to 25% of women. Herein, we review the pathophysiology of lower extremity venous insufficiency and varicose veins, the epidemiology of varicose veins, clinical diagnosis, and ultrasonographic diagnosis. We also discuss treatment rationale, algorithms, and techniques, with a focus on endovenous great saphenous vein ablation.
Keywords: varicose veins, venous insufficiency, laser ablation
Objectives : Upon completion of this article, the reader will be able to identify the clinical, physical, and ultrasonographic findings of insufficient lower extremity veins. Also, readers will be able to outline the basic indications, contraindications, expected results, techniques, and follow-up for treatment.
Definition and Pathophysiology
Lower extremity venous insufficiency, also known as reflux or incompetence, is a condition where the normal one-way return of venous blood back to the heart has been disrupted and blood flow is bidirectional. Thin, pliable valves normally present in all peripheral veins normally prevent retrograde flow of blood; failure or damage to the valves is thought to be responsible for venous insufficiency. Factors predisposing to insufficiency include lifestyle factors, central venous hypertension, thrombosis, or inherited variations in valve number or fragility. 1 When valves fail and veins are incompetent, this can lead to local venous hypertension, venous engorgement/enlargement, tissue edema, and changes in tissue perfusion. These changes may be localized or affect an entire extremity. Varicose veins, visibly enlarged tortuous superficial veins, are the externally visible manifestation of superficial lower extremity venous insufficiency. Any vein may be involved, including the great/small saphenous, perforators or small venules. Varicosities may be caused by incompetence in the vein itself or incompetent perforators that expose the superficial veins to high pressures from the deep system.
Important Anatomical Concepts
The veins of the lower extremity are split into three systems: the deep, superficial, and perforating venous systems. The muscular fascial layer, which is a dense fibrous membrane that surrounds the entirety of the lower extremity, separates the superficial and deep venous systems. The perforating veins connect the deep and superficial veins through the muscular fascia.
All the venous structures superficial to the muscular fascia are considered the superficial venous system. The main veins of the superficial system, the great saphenous vein (GSV) and small saphenous vein (SSV), lie within the saphenous compartment, a subcompartment of the superficial venous system that is bordered superficially by the saphenous fascia and deeply by the muscular fascia. External to the saphenous fascia are accessory saphenous, reticular, and saphenous tributaries. Accessory saphenous veins run parallel to the GSV and SSV. The reticular veins lie between the dermis and saphenous fascia, drain the skin and subcutaneous tissues, and communicate with the saphenous tributaries. The reticular veins can also communicate directly with the deep veins through the perforator veins.
Epidemiology
Varicose veins and venous insufficiency of the lower extremity are among the most common disease entities affecting the adult population with an estimated 25% of women and 15% of men older than 15 years affected. 2 The development of varicose veins has been associated with multiple predisposing factors such as age, gender, pregnancy, heredity, body habitus, and life style.
Nonmodifiable factors that increase the probability of developing varicose veins include age and gender. When divided into three age cohorts of 40-, 50-, and 60-year olds, the prevalence of varicose veins overall was 22, 35, and 41% respectively. 2 One of the major reasons for increased risk of varicosities in women is thought to be related to the hydrostatic and hormonal effects of pregnancy. The occurrence of new varicosities in pregnancy may be as high as 28%. 3 Heredity also plays a significant role in predisposition to varicose veins with the risk of varicosities reaching 90% in individuals where both parents are affected. 4
The major modifiable risk factors for varicosities are body habitus in women and life style. Women in the upper quartile of body mass index (BMI), that is, more than 30 kg/m 2 , have a higher probability of varicosities; however, in men there does not appear to be a correlation between BMI and varicosities. Multiple studies have demonstrated an independent link between working posture and risk for varicosities. In the Tampere study, 27% of individuals with sedentary working conditions had varicosities, whereas 36% of individuals with standing jobs had varicosities. 5
Varicosities associated with venous disease (leg pain, swelling, night cramps, skin changes, etc.) have been a topic of interest due to its link to decreased quality of life (QOL). The Venous Insufficiency Epidemiological and Economical Study (VEINS) showed that 65.2% of subjects with varicose veins had concomitant venous disease and that physical and mental QOL scores decreased as the severity of venous disease increased. 6 7 For the most severe cases of venous disease, QOL scores were worse than individuals suffering from chronic lung disease, back pain, and arthritis. 6 7
Clinical Diagnosis
The diagnosis of varicosities and chronic venous insufficiency is predominately clinical, initially consisting of history and physical examination. Duplex ultrasound imaging is reserved for clinical confirmation. The Clinical-Etiology-Anatomy-Pathophysiology (CEAP; Table 1 ) scoring system provides a useful framework for classifying the clinical severity of venous disease and describing contributing pathology.
Table 1. CEAP comprehensive classification system for chronic venous disorders.
| Clinical | Etiologic | Anatomic | Pathophysiologic |
|---|---|---|---|
| C0—No visible or palpable signs of venous disease | C—Congenital | S—Superficial veins | R—reflux (insufficiency) |
| C1—Telangiectasias or reticular veins | P—Primary | D—Deep veins | O—Obstruction |
| C2—Varicose veins | S—Secondary (postthrombotic | P—Perforator veins | |
| C3—Edema | |||
| C4a—Pigmentation or eczema | |||
| C4b—Lipodermatosclerosis or atrophie blanche | |||
| C5—Healed venous ulcer | |||
| C6—Active venous ulcer |
Although many individuals seek treatment for varicose veins related to their undesirable cosmetic appearance, varicose veins can be symptomatic with the most frequent symptoms being leg pain, night cramps, fatigue, heaviness, or restlessness. Often, venous pain is worse with prolonged standing and relieved by elevation. More severe cases can lead to chronic venous insufficiency with physical exam findings such as lower extremity swelling, eczema, pigmentation, hemorrhage, and ulceration. The main purpose of the history and physical exam is to make sure the patient symptoms are related to venous disease rather than orthopedic, neurologic, or arterial vascular disease. Arterial vascular disease can usually be excluded in the office-based setting with an ankle–brachial index (ABI).
Confirmatory Diagnosis with Ultrasound
Rationale for Duplex
If a patient is clinically suspected of having venous disease, the diagnosis can be confirmed by the presence of reflux identified on duplex ultrasound. The benefit of duplex ultrasound is twofold in that the exam is excellent in documenting the nature and extent of disease and serves as a reliable means for tracking disease progress over time. The main goal of duplex ultrasonography in a patient at the time of first diagnosis is to identify normal versus abnormal venous pathways, determine locations of incompetence and obstruction, and facilitate the diagnosis of atypical causes of reflux. 8
Duplex Indications
Duplex ultrasonography is indicated for evaluating individuals with symptomatic varicose veins or in those with asymptomatic visible varicose veins who are considering treatment. Duplex ultrasound can also be helpful in the evaluation of patients who have symptoms of venous hypertension without visible varicose veins and in those who have recurrence after treatment.
Duplex Technique and Findings
Examination of varicosities should be performed in the standing position with most of the patient's weight centered on the contralateral leg, as this position most accurately simulates the physiology experienced in venous insufficiency while also allowing the patient to relax the muscles of the leg being examined allowing for maximum venous distention. The entire length of the GSV should be examined beginning at the saphenofemoral junction. Next, the SSV should be evaluated with the patient turned away from the operator with knee in slight flexion. Evaluation of the SSV should start at the calf with the probe moved proximal until its termination is established. Complete venous duplex evaluation typically includes examination of the deep veins as well, as this has implications for treatment.
The patient can be instructed to Valsalva to identify the first competent valve. 9 Color Doppler can rapidly identify abnormal vein segments by running the probe along the vein while manually compressing and releasing upstream vein segments. Reflux is best identified, quantified, and documented when using pulsed wave Doppler immediately after an abrupt compression and release of a more peripheral venous segment. 9 Images of varicosities at their junction with the GSV/SSV and the length of the refluxing segments should be documented. It is also necessary to image and document incompetent perforator veins, as these too lead to the symptoms and physical exam findings of superficial venous insufficiency. The typical diameter of the GSV in the upright position is 4 mm or less with the SSV typically measuring 3 mm or less. When incompetent, the GSV is usually dilated sometimes up to 20 mm in diameter. The diameter of the GSV and SSV usually increases just below an area of incompetence and will decrease after the takeoff of a refluxing tributary or competent perforator. 8
When determining the presence of reflux, evaluation of both the overall reflux time and volume (magnitude of reflux) is important. In normal valves, brief reflux prior to valve closure is normal. The most commonly used criteria for reflux is reversed flow lasting longer than 0.5 seconds. 10 Generally, the magnitude of reflux is obvious by identifying abnormal flow by spectral Doppler after releasing a superficial venous segment downstream from the area of interrogation ( Fig. 1 ). It is important to note that a small, brief blip of color after releasing a downstream venous segment is usually normal. The same criteria of reversed venous flow for greater than 0.5 seconds can be used for perforator veins. Additionally, 90% of perforator veins that have a diameter of greater than 3.5 mm are incompetent. 11
Fig. 1.
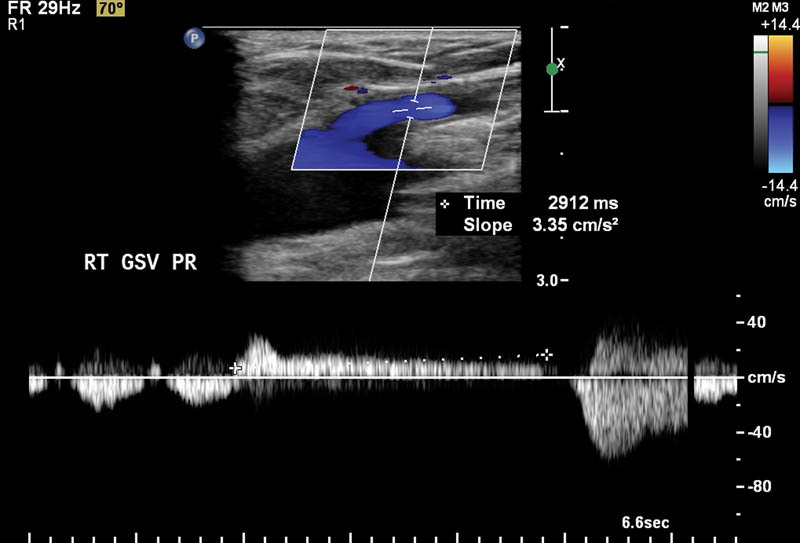
Great saphenous reflux of 2.9 seconds identified on Doppler.
Management of Saphenous Reflux
Treatment Goals
The goal of treatment in patients with chronic superficial venous insufficiency is to improve symptoms, reduce edema, treat lipodermatosclerosis, and promote venous ulcer healing. Initially, most patients are treated conservatively with a combination of leg elevation, compression therapy, dermatologic agents, and ulcer wound management. Generally, 3 months of conservative therapy are required by insurance companies before authorizing symptomatic patients to receive ablative therapy.
Open versus Minimally Invasive Therapies for Nonmedical Treatment
Prior to the advent of endovascular therapy, the mainstay of treatment for chronic superficial venous insufficiency was open surgical vein ligation or stripping. Given significant pain, morbidity, and longer recovery times associated with open surgical treatment, percutaneous endovascular techniques were developed that significantly decrease pain and recovery times. Treatment typically centers around the principal of occluding incompetent veins and redirecting blood flow into competent or less visible segments of the venous system.
Indications and Contraindications for Saphenous Ablation
Ablative therapy is indicated to treat both great and small saphenous vein reflux in patients who have continued symptoms after 3 months of conservative therapy and documented reflux by duplex ultrasound. 1 Patients with visible varicose veins or telangiectasias should undergo saphenous vein ablation prior to focal management of varicosities, as this may reduce the need for treatment. Saphenous vein ablation should also be performed first in those patients who are found to have a combination of saphenous and perforator reflux, as perforator reflux often resolves following saphenous ablation. 12
Contraindications to treatment with ablative therapy are listed in Table 2 .
Table 2. General contraindications to venous ablation.
| Pregnancy or breast feeding |
| Inability to ambulate |
| Deep vein thrombosis or obstruction |
| Klippel–Trénaunay syndrome or other congenital venous anomalies (especially of the deep system) |
| Arterial occlusive disease |
| General poor health or limited life span |
Patients who have a combination of superficial venous reflux as well as deep venous reflux typically are not good candidates for saphenous ablation given the usual presence of coexisting medical issues. In this patient population, varicose vein recurrence rates are significantly higher than in those with isolated superficial venous reflux, given the global venous hypertension. Given these findings, medical management (to include compression therapy) is the mainstay of therapy in those with coexisting deep venous reflux. 13
Endovascular Treatment of Saphenous Reflux
Overarching Treatment Principles
When significant venous reflux is identified, the overarching goal is to eliminate the site of reflux from its deep venous origin by vein obliteration and thrombosis. With all forms of saphenous vein ablation (great or small), image guidance is used to place the catheter and ablative device at the proximal extent of the planned ablation, for example, at the saphenofemoral junction or the saphenopopliteal junction. Subsequently, a chemical sclerosant or energy source is used to cause endothelial and vein wall damage that leads to vein closure.
Modality Overview, Special Considerations, and Discharge Recommendations
Venous ablation is performed as an outpatient procedure in the office, ambulatory surgical center, or hospital setting. Pertinent history is reviewed and medication allergies are noted. In patients without risk factors, preprocedural laboratories are not typically necessary. Informed consent is obtained; typical risks from this procedure include failure to close vein or improve symptoms, deep vein thrombosis, dermal pigmentation, ecchymosis, thermal nerve injury (typically cutaneous), and other risks common to most endovascular procedures (allergic reaction, pain, infection, etc.).
The procedure may be performed under local anesthetic only or light to moderate sedation, depending on patient preference. Typically, only ultrasound guidance is needed as visualization of superficial venous structures and devices are excellent with a high-frequency linear transducer ( Fig. 2 ). Percutaneous access is obtained with ultrasound guidance and micropuncture technique. As the veins targeted can be small and prone to spasm, we attempt to puncture the targeted vein as peripheral as possible, moving central if unsuccessful punctures cause spasm or occlusion. Increasing the ambient temperature or warm blankets may also facilitate puncture. Additionally, some operators find puncturing the vein in the longitudinal ultrasound orientation helpful to visualize the entire course of the needle. Wire placement and progress is followed with ultrasound until access is obtained into the central vein. The device/catheter is then placed over the wire according to manufacturer's instructions.
Fig. 2.
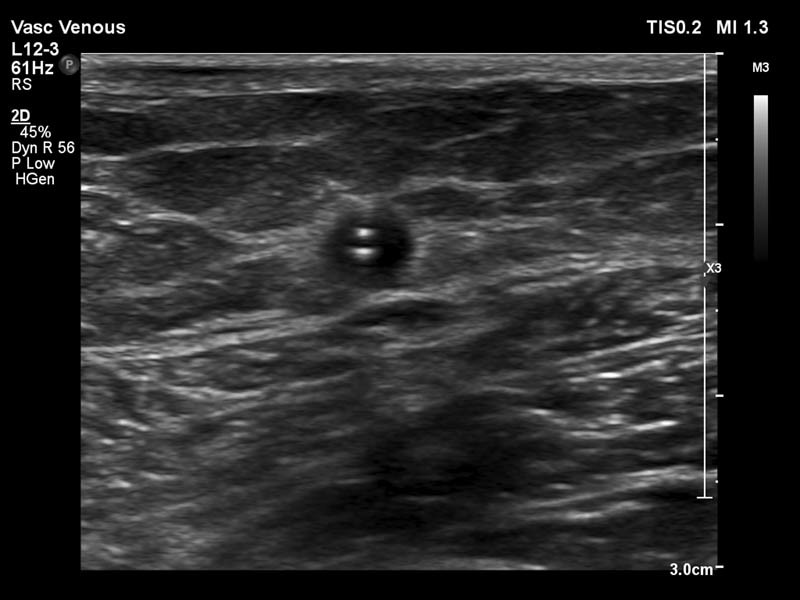
Laser sheath present within great saphenous vein.
To provide anesthesia for the procedure and help prevent thermal injury to the surrounding tissues, tumescent anesthesia is applied around the vein for the entire length of treatment. In this technique, large volumes (∼10 mL/1 cm of vein) of dilute anesthetic (typically 0.1% lidocaine) are applied circumferentially around the length of vein to be treated. Application is with a 21-G needle (the micropuncture needle works well) and a pedal operated peristaltic pump, given the large injection volumes. Contact between the device and the vein wall may be improved by tumescent infiltration or by compression of the vein by ultrasound probe during treatment.
Radiofrequency ablation (RFA) and endovenous laser ablation (EVLA) : Both RFA and EVLA work in similar fashion, by applying thermal energy to the wall of a vein through percutaneous placed device, which serves to damage the endothelium/vein wall and promote thrombosis. Several different devices are available in the United States; typical examples are the VNUS RFA system (Angiodynamics, Queensbury, NY) and the VenaCure EVLA system (Angiodynamics). The laser devices are available with a multitude of different wavelengths (some available are 810, 940, 980, 1,320, and 1,470 nm) and tip construction. Devices should be used according to manufacturer's instructions, with appropriate laser eye protection for the staff and patients. The tip of the endovenous device is placed to 2 to 3 cm peripheral to the junction of the treated vein ( Fig. 3 ) with the deep venous system and withdrawn in a slow continuous fashion. Although most experience with RFA/EVLA is with GSV ablation, other veins/perforators may be treated so long as their course is straight enough to accept the probe catheter. 14
Fig. 3.
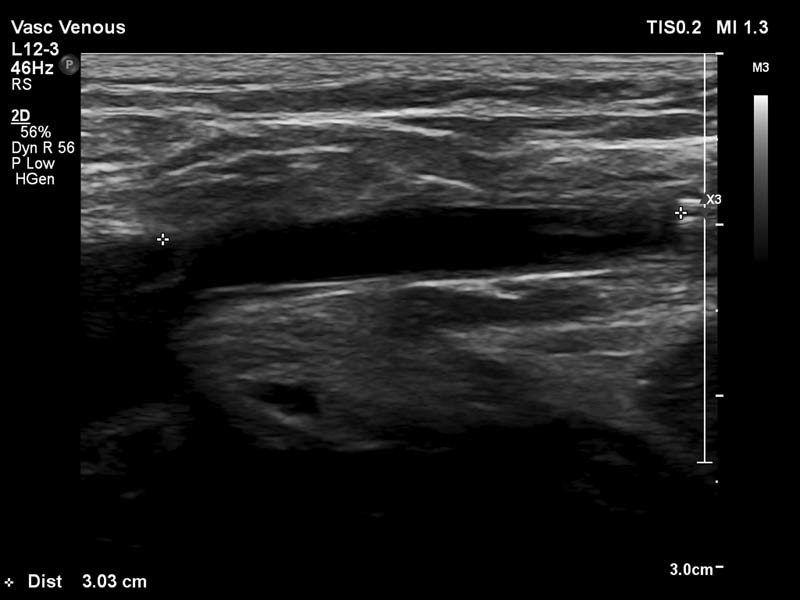
Laser device in great saphenous vein ∼3 cm from the great saphenous vein/common femoral vein junction.
Follow-up care after vein ablation can be broken down into three categories: pain management, patient instructions, and postoperative imaging. Some patients notice a feeling of tightness or a palpable cord at the site of vein treatment. Pain control is typically easily managed with nonsteroidal anti-inflammatory drug (NSAID). If there is a contraindication to NSAIDs, then an opioid analgesic can be used.
At the time of discharge, the patient should be instructed to ambulate normally and walk every hour before bedtime for 2 days then daily for 15 to 30 minutes. When seated, patients should be instructed to elevate the treated leg. Additionally, patients should be instructed to wear high grade (30–40 mm Hg) compression stockings continuously for the first 48 hours postprocedure and then during the day for the next 2 weeks. These measures assist in vein closure and decrease the risk of thrombus propagation to the deep system.
A repeat duplex ultrasound is usually performed 2 days following saphenous vein ablation to assess for deep venous thrombosis (DVT). If thrombus extends up to but not into the saphenofemoral or saphenopopliteal junction, then repeat ultrasound should be performed within a week to evaluate for propagation of thrombus ( Fig. 4 ). If thrombus extends into the deep venous system, then appropriate anticoagulation should be initiated. Strenuous exercise is generally avoided for 2 weeks and the areas of treatment should not be exposed to the sun because of the risk of hyperpigmentation.
Fig. 4.
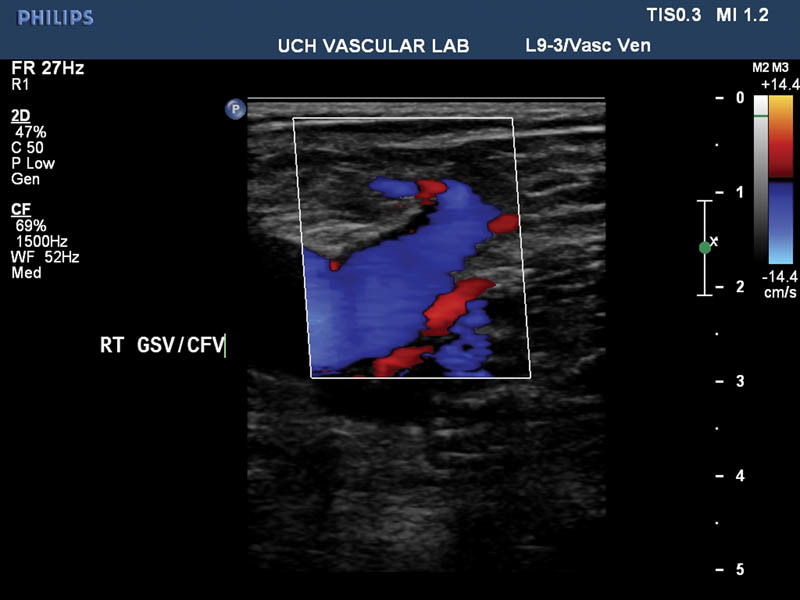
Mostly thrombosed great saphenous vein (not pictured) with a patent stump and common femoral vein 48 hours posttreatment.
Endovenous sclerotherapy : With endovenous sclerotherapy, a sclerosant is injected into the venous system of interest to damage the endothelium and cause thrombosis. There are numerous different sclerosants in use, the most commonly used sclerosant in the United States is Sotradecol (STS). STS is available in 3 and 1% concentrations and may be diluted as necessary. Lower concentrations (as low as 0.125%) are used in smaller veins. 15 Usually, a foam is created by pumping Sotradecol contained within a syringe that is connected via a three-way stopcock to a second syringe containing air. This enhances ultrasound visibility ( Fig. 5a, b ) and creates greater wall contact/blood displacement for a given volume of sclerosant. Through the usage of occlusion balloons, sclerosant can be held in place for a longer period thus enhancing treatment response. Additionally, occlusion balloons decrease the likelihood of injuring the deep venous structure due to sclerosant migration. Sclerotherapy is usually reserved for smaller veins where the anatomy is not appropriate for EVLA/RFA and control of sclerosant is easier. Postprocedural care is similar to EVLA/RFA, although follow-up ultrasound is not routine. Exposure to the sun should be avoided for 2 weeks because of the risk of hyperpigmentation.
Fig. 5.
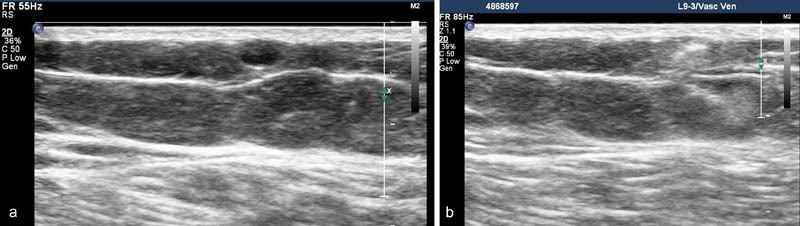
Superficial varicosity before ( a ) and after ( b ) foam sclerosant injection.
Other newer devices : The ClariVein device (Quincy, MA), uses a combination of a mechanically rotating tip and controlled sclerosant injection to induce vein closure. VenaSeal (Medtronic) delivers n-butyl-cyanoacrylate to the inside of the vein wall physically closing the vein and inciting inflammation. Both devices have the possible advantage of being nonthermal and obviating the need for tumescent anesthesia.
Results
The rates of successful EVLA closure of the GSV are high with most published rates ranging between 90 and 98% at 2 years or more 16 with some suggestion of improved long-term closure rates with the 1,470-nm fiber versus a 910-nm fiber. 17 Published closure rates with perforator veins are in excess of 94%. 18 Endovenous procedures (RFA, EVLA, and ultrasound-guided foam injection) have all demonstrated improvement in venous symptom scores in controlled trials. Additionally, endovenous procedures may be associated with shorter recovery times and more rapid return to work compared with surgical stripping. 19
Summary
Lower extremity venous insufficiency and varicose veins are an exceedingly common condition in women. In addition to their appearance, varicose veins are often associated with symptoms and a lower QOL scored by patients. With modern imaging and ablation techniques, insufficient veins can often be safely and rapidly treated in the outpatient setting with good response rates and high patient satisfaction scores.
References
- 1.Min R J. Lower extremity superficial venous insufficiency: percutaneous techniques of management. Endovasc Manag Venous Dis. 2000;3:54–59. [Google Scholar]
- 2.Fan C-M. Epidemiology and pathophysiology of varicose veins. Tech Vasc Interv Radiol. 2003;6(03):108–110. doi: 10.1053/s1089-2516(03)00060-x. [DOI] [PubMed] [Google Scholar]
- 3.Stansby G.Women, pregnancy, and varicose veins Lancet 2000355(9210):1117–1118. [DOI] [PubMed] [Google Scholar]
- 4.Cornu-Thenard A, Boivin P, Baud J M, De Vincenzi I, Carpentier P H. Importance of the familial factor in varicose disease. Clinical study of 134 families. J Dermatol Surg Oncol. 1994;20(05):318–326. doi: 10.1111/j.1524-4725.1994.tb01631.x. [DOI] [PubMed] [Google Scholar]
- 5.Laurikka J O, Sisto T, Tarkka M R, Auvinen O, Hakama M. Risk indicators for varicose veins in forty- to sixty-year-olds in the Tampere varicose vein study. World J Surg. 2002;26(06):648–651. doi: 10.1007/s00268-001-0283-1. [DOI] [PubMed] [Google Scholar]
- 6.Kurz X, Lamping D L, Kahn S R et al. Do varicose veins affect quality of life? Results of an international population-based study. J Vasc Surg. 2001;34(04):641–648. doi: 10.1067/mva.2001.117333. [DOI] [PubMed] [Google Scholar]
- 7.Abenhaim L, Kurz X; VEINES Group.The VEINES study (VEnous Insufficiency Epidemiologic and Economic Study): an international cohort study on chronic venous disorders of the leg Angiology 1997480159–66. [DOI] [PubMed] [Google Scholar]
- 8.Khilnani N M, Min R J. Duplex ultrasound for superficial venous insufficiency. Tech Vasc Interv Radiol. 2003;6(03):111–115. doi: 10.1053/s1089-2516(03)00057-x. [DOI] [PubMed] [Google Scholar]
- 9.Masuda E M, Kistner R L, Eklof B. Prospective study of duplex scanning for venous reflux: comparison of Valsalva and pneumatic cuff techniques in the reverse Trendelenburg and standing positions. J Vasc Surg. 1994;20(05):711–720. doi: 10.1016/s0741-5214(94)70158-x. [DOI] [PubMed] [Google Scholar]
- 10.van Bemmelen P S, Bedford G, Beach K, Strandness D E. Quantitative segmental evaluation of venous valvular reflux with duplex ultrasound scanning. J Vasc Surg. 1989;10(04):425–431. doi: 10.1067/mva.1989.14123. [DOI] [PubMed] [Google Scholar]
- 11.Sandri J L, Barros F S, Pontes S, Jacques C, Salles-Cunha S X. Diameter-reflux relationship in perforating veins of patients with varicose veins. J Vasc Surg. 1999;30(05):867–874. doi: 10.1016/s0741-5214(99)70011-x. [DOI] [PubMed] [Google Scholar]
- 12.O'Donnell T F., Jr The present status of surgery of the superficial venous system in the management of venous ulcer and the evidence for the role of perforator interruption. J Vasc Surg. 2008;48(04):1044–1052. doi: 10.1016/j.jvs.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Puggioni A, Lurie F, Kistner R L, Eklof B. How often is deep venous reflux eliminated after saphenous vein ablation? J Vasc Surg. 2003;38(03):517–521. doi: 10.1016/s0741-5214(03)00413-0. [DOI] [PubMed] [Google Scholar]
- 14.Rosenblatt M. Percutaneous management of varicose veins. Sci Sess Sci Poster Abstr. 2002;13:258–262. [Google Scholar]
- 15.Gloviczki P, Comerota A J, Dalsing M Cet al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guideline of the Society for Vascular Surgery and the American Venous Forum J Vasc Surg 201153(5 Suppl):2S–48S. [DOI] [PubMed] [Google Scholar]
- 16.Kabnick L S, Cayne N, Jacobowitz G et al. Endovenous procedures in varicose veins: What is the best choice today? Phlebology. 2008;8:229–235. [Google Scholar]
- 17.Ahn S H, Gill G, Prince E A et al. Endovenous laser ablation (EVLA) performed with 1470 nm laser: long term outcomes and comparison with 980 nm laser. J Vasc Interv Radiol. 2013;24:S144. [Google Scholar]
- 18.Chehab M, Dixit P, Antypas E, Juncaj M, Wong O, Bischoff M. Endovenous laser ablation of perforating veins: feasibility, safety, and occlusion rate using a 1,470-nm laser and bare-tip fiber. J Vasc Interv Radiol. 2015;26(06):871–877. doi: 10.1016/j.jvir.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Darwood R J, Theivacumar N, Dellagrammaticas D, Mavor A I, Gough M J. Randomized clinical trial comparing endovenous laser ablation with surgery for the treatment of primary great saphenous varicose veins. Br J Surg. 2008;95(03):294–301. doi: 10.1002/bjs.6101. [DOI] [PubMed] [Google Scholar]


