Abstract
Breast cancer is the most common women's malignancy in the United States and is the second leading cause of cancer death. More than half of patients with breast cancer will develop hepatic metastases; this portends a poorer prognosis. In the appropriately selected patient, there does appear to be a role for curative (surgery, ablation) or palliative (intra-arterial treatments) locoregional therapy. Gynecologic malignancies are less common and metastases to the liver are most often seen in the setting of disseminated disease. The role of locoregional therapies in these patients is not well reported. The purpose of this article is to review the outcomes data of locoregional therapies in the treatment of hepatic metastases from breast and gynecologic malignancies.
Keywords: breast cancer, gynecologic malignancies, liver metastases, chemoembolization, radioembolization, interventional radiology
Objectives : Upon completion of this article, the reader will be able to identify the available ablative and intra-arterial therapies and outcomes for liver metastases from breast cancer and gynecologic malignancies.
Accreditation : This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit : Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit ™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Breast cancer is the most common women's malignancy in the United States and is the second leading cause of cancer death. The American Cancer Society (ACS) estimates that in 2017, a total of 255,180 Americans will be diagnosed with breast cancer and with 41,070 deaths. 1 In fact, one in eight women in the United States will be diagnosed with breast cancer within their lifetime. 2 With regard to gynecologic malignancies, the ACS estimates 107,470 diagnoses in 2017, with 31,600 deaths. 1 Of these malignancies, ovarian cancer is the leading cause of death from gynecologic cancer in the United States. 1 The management of hepatic metastatic disease in women with breast and gynecologic malignancies continues to pose a difficult clinical management scenario. In metastatic breast cancer (MBC), more than 50% of patients develop liver metastases over the course of their disease. 3 4 5 Gynecologic cancers that metastasize to the liver are most often seen in the setting of disseminated disease. 6 7 8 The majority of patients in these groups are not candidates for surgical resection secondary to extent of disease spread. For these patients, locoregional therapy may play a role in stabilizing hepatic tumors and/or palliating cancer-related symptoms.
Breast Cancer
Metastatic breast cancer to the liver portends a poor prognosis, with hepatic failure causing death in 20% of patients. 3 Furthermore, compromised liver function necessitates chemotherapy dosing modification, which can limit its antitumor effectiveness. 9 10 11 The National Comprehensive Cancer Network (NCCN) Breast Cancer Panel updated its clinical practice guidelines in May 2016 to reflect several emerging trends in the treatment of MBC. 12 These offer a glimpse into a shifting paradigm for the treatment of MBC patients toward newer, less invasive, and more targeted therapies specific to the location and biology of disease. Importantly, minimizing toxicity and preserving quality of life is prioritized. These concepts, meant to promote the application of systemic therapies in patients with MBC, also highlight the potential impact of locoregional treatments provided by interventional radiologists.
Liver metastases are considered a manifestation of systemic disease, requiring palliative/noncurative management. Surgical resection may play a role in the management of MBC patients, but few present with isolated liver disease, limiting candidacy to less than 1% of patients. Surgical resection is best performed following rigorous selection criteria. The ideal candidates for surgical resection are patients with solitary lesions (<4–5 cm), with stable disease following neoadjuvant systemic therapy, and a delay between primary lesions and metastases of longer than 2 years (a marker for favorable biology). 13 Ideally, a radical resection can be performed. In these patients, results are mixed, with median time to recurrence of 10 to 36 months, 14 and 5-year overall survival (OS) rates ranging from 12 to 75%. Tumor recurrence is seen in 60% of patients. 13 14
Given the limitations of surgical resection, other locoregional therapies have been explored. Several studies have investigated the role of thermal ablation in the treatment of liver metastases from breast cancer. Ablation may be considered in patients with limited tumor burden not amenable to surgery due to comorbidities, including patients with small solitary lesions, metachronous lesions, and unresectable lesions that demonstrate partial or complete response following neoadjuvant chemotherapy. 15 The local tumor response rates from thermal ablation range from 63 to 97%, 16 17 18 19 20 21 with median survival ranging from 10.9 to 60 months. 19 20 21 22 Sofocleous et al 19 and Meloni et al 21 have independently reported 5-year OS rates of approximately 30% following radiofrequency (RF) ablation. Limited data with microwave ablation exist in the setting of MBC; 23 24 these case series have comparable results to RF ablation. 15 Unfortunately, most patients do not present with a tumor burden amendable to ablation.
Intra-arterial therapies are most often employed in the treatment of MBC to the liver in the salvage setting (i.e., patients with none or limited systemic options) or during systemic chemotherapy holidays/breaks. These therapies are considered for patients who have no extrahepatic disease or stable limited extrahepatic disease and progression of hepatic tumors. The goal of these therapies is to target the hepatic disease burden while preserving liver function; tumor debulking offers palliation of abdominal pain from large hepatic masses. 25 26 These treatments deliver therapeutic agents directly to the tumor vasculature, achieving high locoregional tumor doses without the toxicity profile seen with similar doses administered systemically. There are several intra-arterial therapies that can be provided to treat primary/secondary hepatic malignancies. The patient selection criteria, rationale for intra-arterial therapies, and technical considerations for these therapies have been previously described. 27 28 29 30
Hepatic arterial infusion (HAI) chemotherapy involves the placement of an indwelling infusion catheter into the hepatic artery following surgical implantation of a chemotherapy pump to deliver sustained intra-arterial chemotherapy. This technique is more commonly employed for the treatment of patients with metastatic colon cancer to the liver; there is limited published evidence to support its application for patients with MBC to the liver. A small series by Ang et al reported HAI chemotherapy with combination systemic chemotherapy in nine patients with MBC to the liver. 31 These patients were heavily pre-treated, receiving a median of six lines of systemic therapy. The overall response rate was 78%, similar to prior HAI studies. 32 The median time to progression of liver disease was 6 months, with a median OS of 17 months. Another small series by Nielsen et al reported HAI in 16 patients (9 of who had pump implantation) using capecitabine and oxaliplatin. The majority of patients (11/16) had liver and bone metastatic disease. Patients had previously undergone a median of two prior lines of chemotherapy. The authors reported a response rate of 50% by Response Evaluation Criteria In Solid Tumors (RECIST), with median progression-free survival of 7.9 months, and median OS of 19.2 months. 33
Conventional transarterial chemoembolization (cTACE) delivers cytotoxic chemotherapeutic drug(s) mixed with iodinated oil (lipiodol). The lipiodol emulsifies the chemotherapy and aids visualization during administration. An embolic agent (particles or Gelfoam) completes the therapy; this prevents drug washout (increasing drug dwell time and reducing systemic bioavailability) and promotes tumor ischemia. In a large series, Vogl et al reported outcomes of cTACE with mitomycin C ± gemcitabine in 208 patients with MBC. By RECIST criteria, partial response was seen in 13% (27/208), stable disease in 50.5% (105/208), and progressive disease in 36.5% (76/208). The 1- and 3-year survival rates following cTACE were 69 and 33%, respectively. The median OS was reported to be 18.5 months. 34 A follow-up series by Vogl et al used the same cTACE regimen in 161 patients with MBC being down-staged to laser-induced thermotherapy. 35 The authors reported a mean OS of 32.5 months. Eichler et al reported results of cTACE in 43 patients with gemcitabine three times, over a 4-week interval. 36 By RECIST criteria, partial response was seen in 3 patients (7%), stable disease in 16 (37%), and progressive disease occurred in 22 (51%). The authors reported a median OS of 10.2 months similar to prior smaller series. 37
Drug-eluting embolic chemoembolization involves the administration of microspheres loaded with a chemotherapeutic (often doxorubicin) to the tumor vasculature. There is limited published data: Martin et al reported doxorubicin-based DEE-TACE in 40 patients. 38 Within the cohort, the majority of patients (62%) had less than 25% liver disease burden, while 57% had extrahepatic disease. All patients in the cohort had previously undergone at least two lines of prior systemic chemotherapy. At 3 months of follow-up, 58% of patients ( n = 23) had an imaging response by mRECIST criteria, with a reported median OS of 47 months. 38 A recent study by Lin et al examined the use of doxorubin loaded 75 to 150 µm diameter DEEs in the treatment of 23 patients with MBC refractory to two or more lines of systemic chemotherapy. 39 At 3-month follow-up, 13 (57%) patients had a stable disease, 6 (26%) patients had partial response, and 4 (17%) patients demonstrated progressive disease. The authors reported a median OS of 17 months.
Radioembolization employs small microembolic microspheres (20–40 µm) to deliver a β-emitting isotope, yttrium-90 (Y90), to hepatic tumors. Haug et al studied radioembolization with resin microspheres in 58 patients with MBC. 40 The majority of patients (66%) had less than 25% hepatic disease burden, with 38 patients (66%) presenting with extrahepatic disease. This cohort had been heavily pretreated, with a mean of 3.1 prior lines of chemotherapy. Partial response was observed in 25.6 and 11.6% had progressive disease by RECIST. Based on 18 F-FDG PET/CT, the response rate was 51% (22/43) defined as a 30% decreased in SUV max . The authors found a median OS of 11 months following radioembolization. Cianni et al treated 52 patients with radioembolization. 41 Within this cohort, patients had previously undergone a median of four prior lines of systemic chemotherapy. The authors reported a partial response rate of 56%, and stable disease in 35% by RECIST criteria. PET/CT imaging response defined as any reduction in metabolic activity was 81%. Overall, the authors found median OS of 11.5 months in this cohort, with a significant difference in median OS between patients with and without extrahepatic disease (8.2 vs. 14.3 months, respectively, p < 0.0001). These results were similar to an earlier study by Jakobs et al in 30 patients, with a reported partial response of 61%, and stable disease in 35% by RECIST criteria, with a median OS of 11.7 months. 42 Saxena et al reported on a series on 40 patients with an overall response rate of 31.6% with partial response in 20%, stable disease in 39%, and progressive disease in 28.9% of patients by RECIST criteria The median OS was 13.6 months in this series. 43 Gordon et al described radioembolization with glass microspheres in 75 patients with breast cancer liver metastases and stable extrahepatic disease. 44 Partial response was seen in 35.3%, stable disease in 63.2%, and progressive disease in 1.5% of patients by RECIST criteria. On multivariate analysis, negative prognosticators for survival included hepatic tumor burden greater than 25% and serum bilirubin greater than 1.1 mg/dL. For the entire cohort, the median OS was 6.6 months, with a median OS of 9.3 months for patients with less than 25% tumor burden. Fendler et al treated 81 patients with radioembolization using resin microspheres. 45 The authors noted a 52% response rate with follow-up 18 F-FDG PET/CT defined as a 30% decrease in SUV max . On multivariate analysis, the authors found tumor-to-liver ratio greater than 50% was a negative prognosticator for OS. In this cohort, the median OS was 8.2 months. Pieper et al published on a cohort of 44 patients treated with radioembolization with resin microspheres. 46 This was a heavily pretreated cohort, with 73% ( n = 32) having previously undergone more than five lines of systemic chemotherapy. The majority of patients ( n = 25, 57%) had greater than 25% tumor burden. By RECIST criteria, partial response was seen in 39.5%, stable disease in 42.1%, and progressive disease in 18.4% of patients. The authors reported a median OS of 6.1 months.
The cumulative published evidence, while not prospective or randomized, suggests that radioembolization provides lower rates of targeted disease progression than cTACE in the setting of MBC ( Table 1 ). A final concept is the potential for combining systemic and locoregional therapies for patients with MBC; a clear limitation of locoregional therapy is that it only treats existing and targeted disease in the organ of interest (often the liver). Combining these therapies is especially appealing with radioembolization and radiosensitizing systemic agents active against MBC. A recent prospective study demonstrated the safety of radioembolization with glass microspheres and concomitant oral capecitabine. 25 Four (5%) of the patients in this study had MBC to the liver ( Fig. 1 ). Future studies should evaluate the synergistic effect of these therapies and the impact on disease control and OS.
Table 1. Results of intra-arterial therapy for metastatic breast cancer.
| Chemoembolization | |||
|---|---|---|---|
| Author | Year | N | Disease control (RECIST) |
| Lin et al 39 | 2017 | 23 | 83% |
| Eichler et al 36 | 2013 | 43 | 44% |
| Martin et al 38 | 2012 | 40 | 58% |
| Vogl et al 35 | 2011 | 161 | 56% |
| Cho et al 37 | 2010 | 10 | 40% |
| Vogl et al 34 | 2010 | 208 | 63% |
| Overall | 485 | 59% | |
| Radioembolization | |||
| Pieper et al 46 | 2016 | 44 | 71.1% |
| Gordon et al 44 | 2014 | 75 | 98.5% |
| Cianni et al 41 | 2013 | 52 | 91.4% |
| Saxena et al 43 | 2013 | 40 | 71.1% |
| Haug et al 40 | 2012 | 58 | 88% |
| Fendler et al 45 | 2016 | 81 | 52% |
| Overall | 335 | 78% | |
Abbreviation: RECIST, Response Evaluation Criteria in Solid Tumors.
Note: Disease control: complete response + partial response + stable disease by RECIST criteria.
Fig. 1.
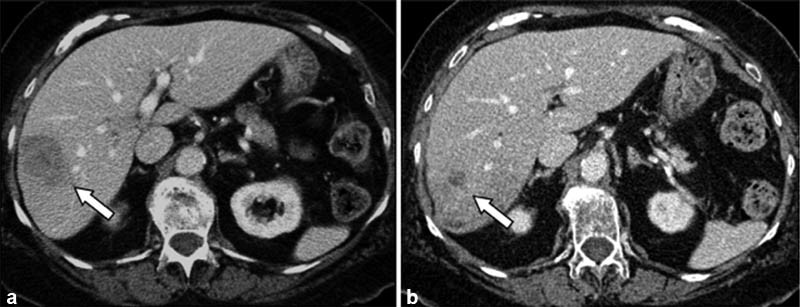
( a ) An 82-year-old woman with a solitary tumor in the right lobe measuring 4.5 cm × 3.3 cm (arrow). This patient was treated on a clinical trial with capecitabine and radioembolization. ( b ) CT 5 months later demonstrated a reduction in bidimensional size to 1.3 cm × 1.3 cm (arrow). This patient had isolated liver metastases and remained free of extrahepatic disease 666 days after the first Y90 treatment.
Gynecologic Malignancies
Of gynecologic malignancies, ovarian cancer has the highest mortality, with the majority of patients presenting with advanced disease 47 48 49 and a 5-year OS ranging from 5 to 20%. Tumors often spread through direct seeding of the peritoneal cavity, with lymphatic dissemination seen in the minority of patients. 50 Isolated hepatic parenchymal metastases (rather than peritoneal seeding) are rare (<10% of cases), with liver metastases seen in the setting of disseminated disease, with resultant poor survival. 49 Endometrial and cervical cancer metastases to the liver are also rare, ranging from 7 to 10% of metastatic disease. 51 With ovarian cancer specifically, patients with unresectable parenchymal metastases have poorer OS and rapid progression compared with patients with peritoneal hepatic seeding amenable to resection. 52 In few patients who are candidates for surgical resection, median survival times vary widely ranging from 7 to 27 months in small case series. 53 54 55 Palliative therapeutic options are thus the mainstay for treatment for these patients. Experience with liver-directed therapy in these patients is limited to small case series. 56 57 In the largest published series, Vogl et al reported outcomes following cTACE in 65 women with unresectable ovarian hepatic parenchymal metastases following progression on third line chemotherapy. According to RECIST criteria, partial response was seen in 16.9%, stable disease in 58.5%, and progressive disease in 24.6%. The median OS was 14 months, with 1- and 3-year OS rates of 58 and 13%, respectively. 56
Conclusion
Locoregional therapies have an acceptable safety profile and prove efficacious in the setting of chemotherapy refractory hepatic metastases from breast cancer. Limited experience in metastatic gynecologic malignancies also demonstrates technical feasibility and safety. However, the available data are from heterogeneous cohorts studied retrospectively, with varying technique, patient selection, and response criteria. Given these limitations, the current data should be used to guide future prospective trial design and multicenter data registries. These efforts will further clarify the role of locoregional therapy in the treatment of patients with hepatic metastatic disease from breast and gynecologic cancers.
Acknowledgments
A.C.G. is a student of the Medical Scientist Training Program and is funded by the National Institutes of Health, grant no. T32GM008152.
References
- 1.Siegel R L, Miller K D, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(01):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center M M, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(02):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Zinser J W, Hortobagyi G N, Buzdar A U, Smith T L, Fraschini G. Clinical course of breast cancer patients with liver metastases. J Clin Oncol. 1987;5(05):773–782. doi: 10.1200/JCO.1987.5.5.773. [DOI] [PubMed] [Google Scholar]
- 4.Atalay G, Biganzoli L, Renard F et al. Clinical outcome of breast cancer patients with liver metastases alone in the anthracycline-taxane era: a retrospective analysis of two prospective, randomised metastatic breast cancer trials. Eur J Cancer. 2003;39(17):2439–2449. doi: 10.1016/s0959-8049(03)00601-4. [DOI] [PubMed] [Google Scholar]
- 5.Cummings M C, Simpson P T, Reid L E et al. Metastatic progression of breast cancer: insights from 50 years of autopsies. J Pathol. 2014;232(01):23–31. doi: 10.1002/path.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chi D S, Fong Y, Venkatraman E S, Barakat R R. Hepatic resection for metastatic gynecologic carcinomas. Gynecol Oncol. 1997;66(01):45–51. doi: 10.1006/gyno.1997.4727. [DOI] [PubMed] [Google Scholar]
- 7.Chi D S, Welshinger M, Venkatraman E S, Barakat R R. The role of surgical cytoreduction in Stage IV endometrial carcinoma. Gynecol Oncol. 1997;67(01):56–60. doi: 10.1006/gyno.1997.4838. [DOI] [PubMed] [Google Scholar]
- 8.Kim G E, Lee S W, Suh C O et al. Hepatic metastases from carcinoma of the uterine cervix. Gynecol Oncol. 1998;70(01):56–60. doi: 10.1006/gyno.1998.5037. [DOI] [PubMed] [Google Scholar]
- 9.Eklund J W, Trifilio S, Mulcahy M F.Chemotherapy dosing in the setting of liver dysfunction Oncology (Williston Park) 200519081057–1063., discussion 1063–1064, 1069 [PubMed] [Google Scholar]
- 10.Field K M, Dow C, Michael M. Part I: Liver function in oncology: biochemistry and beyond. Lancet Oncol. 2008;9(11):1092–1101. doi: 10.1016/S1470-2045(08)70279-1. [DOI] [PubMed] [Google Scholar]
- 11.Field K M, Michael M. Part II: Liver function in oncology: towards safer chemotherapy use. Lancet Oncol. 2008;9(12):1181–1190. doi: 10.1016/S1470-2045(08)70307-3. [DOI] [PubMed] [Google Scholar]
- 12.Gradishar W J, Anderson B O, Balassanian R et al. NCCN guidelines insights: breast cancer, version 1.2017. J Natl Compr Canc Netw. 2017;15(04):433–451. doi: 10.6004/jnccn.2017.0044. [DOI] [PubMed] [Google Scholar]
- 13.Golse N, Adam R. Liver metastases from breast cancer: what role for surgery? Indications and results. Clin Breast Cancer. 2017;17(04):256–265. doi: 10.1016/j.clbc.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Elsberger B, Roxburgh C S, Horgan P G. Is there a role for surgical resections of hepatic breast cancer metastases? Hepatogastroenterology. 2014;61(129):181–186. [PubMed] [Google Scholar]
- 15.Vogl T J, Farshid P, Naguib N N, Zangos S. Thermal ablation therapies in patients with breast cancer liver metastases: a review. Eur Radiol. 2013;23(03):797–804. doi: 10.1007/s00330-012-2662-4. [DOI] [PubMed] [Google Scholar]
- 16.Livraghi T, Goldberg S N, Solbiati L, Meloni F, Ierace T, Gazelle G S. Percutaneous radio-frequency ablation of liver metastases from breast cancer: initial experience in 24 patients. Radiology. 2001;220(01):145–149. doi: 10.1148/radiology.220.1.r01jl01145. [DOI] [PubMed] [Google Scholar]
- 17.Lawes D, Chopada A, Gillams A, Lees W, Taylor I. Radiofrequency ablation (RFA) as a cytoreductive strategy for hepatic metastasis from breast cancer. Ann R Coll Surg Engl. 2006;88(07):639–642. doi: 10.1308/003588406X149129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunabushanam G, Sharma S, Thulkar Set al. Radiofrequency ablation of liver metastases from breast cancer: results in 14 patients J Vasc Interv Radiol 200718(1, Pt 1):67–72. [DOI] [PubMed] [Google Scholar]
- 19.Sofocleous C T, Nascimento R G, Gonen M et al. Radiofrequency ablation in the management of liver metastases from breast cancer. AJR Am J Roentgenol. 2007;189(04):883–889. doi: 10.2214/AJR.07.2198. [DOI] [PubMed] [Google Scholar]
- 20.Jakobs T F, Hoffmann R T, Schrader A et al. CT-guided radiofrequency ablation in patients with hepatic metastases from breast cancer. Cardiovasc Intervent Radiol. 2009;32(01):38–46. doi: 10.1007/s00270-008-9384-7. [DOI] [PubMed] [Google Scholar]
- 21.Meloni M F, Andreano A, Laeseke P F, Livraghi T, Sironi S, Lee F T., Jr Breast cancer liver metastases: US-guided percutaneous radiofrequency ablation–intermediate and long-term survival rates. Radiology. 2009;253(03):861–869. doi: 10.1148/radiol.2533081968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrafiello G, Fontana F, Cotta E et al. Ultrasound-guided thermal radiofrequency ablation (RFA) as an adjunct to systemic chemotherapy for breast cancer liver metastases. Radiol Med (Torino) 2011;116(07):1059–1066. doi: 10.1007/s11547-011-0697-2. [DOI] [PubMed] [Google Scholar]
- 23.Lorentzen T, Skjoldbye B O, Nolsoe C P. Microwave ablation of liver metastases guided by contrast-enhanced ultrasound: experience with 125 metastases in 39 patients. Ultraschall Med. 2011;32(05):492–496. doi: 10.1055/s-0029-1246002. [DOI] [PubMed] [Google Scholar]
- 24.Liang P, Dong B, Yu X et al. Prognostic factors for percutaneous microwave coagulation therapy of hepatic metastases. AJR Am J Roentgenol. 2003;181(05):1319–1325. doi: 10.2214/ajr.181.5.1811319. [DOI] [PubMed] [Google Scholar]
- 25.Hickey R, Mulcahy M F, Lewandowski R J et al. Chemoradiation of hepatic malignancies: prospective, phase 1 study of full-dose capecitabine with escalating doses of yttrium-90 radioembolization. Int J Radiat Oncol Biol Phys. 2014;88(05):1025–1031. doi: 10.1016/j.ijrobp.2013.12.040. [DOI] [PubMed] [Google Scholar]
- 26.Gordon A C, Salem R, Lewandowski R J. Yttrium-90 radioembolization for breast cancer liver metastases. J Vasc Interv Radiol. 2016;27(09):1316–1319. doi: 10.1016/j.jvir.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Lewandowski R J, Geschwind J F, Liapi E, Salem R. Transcatheter intraarterial therapies: rationale and overview. Radiology. 2011;259(03):641–657. doi: 10.1148/radiol.11081489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salem R, Thurston K G. Radioembolization with 90yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 2: special topics. J Vasc Interv Radiol. 2006;17(09):1425–1439. doi: 10.1097/01.RVI.0000235779.88652.53. [DOI] [PubMed] [Google Scholar]
- 29.Salem R, Thurston K G. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol. 2006;17(08):1251–1278. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- 30.Lewandowski R J, Sato K T, Atassi B et al. Radioembolization with 90Y microspheres: angiographic and technical considerations. Cardiovasc Intervent Radiol. 2007;30(04):571–592. doi: 10.1007/s00270-007-9064-z. [DOI] [PubMed] [Google Scholar]
- 31.Ang C, Jhaveri K, Patel D, Gewirtz A, Seidman A, Kemeny N. Hepatic arterial infusion and systemic chemotherapy for breast cancer liver metastases. Breast J. 2013;19(01):96–99. doi: 10.1111/tbj.12050. [DOI] [PubMed] [Google Scholar]
- 32.Arai Y, Sone Y, Inaba Y, Ariyoshi Y, Kido C.Hepatic arterial infusion chemotherapy for liver metastases from breast cancer Cancer Chemother Pharmacol 199433(Suppl):S142–S144. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen D L, Nørgaard H, Vestermark L W et al. Intrahepatic and systemic therapy with oxaliplatin combined with capecitabine in patients with hepatic metastases from breast cancer. Breast. 2012;21(04):556–561. doi: 10.1016/j.breast.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Vogl T J, Naguib N NN, Nour-Eldin N-EA, Eichler K, Zangos S, Gruber-Rouh T. Transarterial chemoembolization (TACE) with mitomycin C and gemcitabine for liver metastases in breast cancer. Eur Radiol. 2010;20(01):173–180. doi: 10.1007/s00330-009-1525-0. [DOI] [PubMed] [Google Scholar]
- 35.Vogl T J, Naguib N N, Nour-Eldin N E et al. Repeated chemoembolization followed by laser-induced thermotherapy for liver metastasis of breast cancer. AJR Am J Roentgenol. 2011;196(01):W66–72. doi: 10.2214/AJR.09.3836. [DOI] [PubMed] [Google Scholar]
- 36.Eichler K, Jakobi S, Gruber-Rouh T, Hammerstingl R, Vogl T J, Zangos S. Transarterial chemoembolisation (TACE) with gemcitabine: phase II study in patients with liver metastases of breast cancer. Eur J Radiol. 2013;82(12):e816–e822. doi: 10.1016/j.ejrad.2013.08.046. [DOI] [PubMed] [Google Scholar]
- 37.Cho S W, Kitisin K, Buck D et al. Transcatheter arterial chemoembolization is a feasible palliative locoregional therapy for breast cancer liver metastases. Int J Surg Oncol. 2010;2010:251621. doi: 10.1155/2010/251621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin R CG, Robbins K, Fagés J F et al. Optimal outcomes for liver-dominant metastatic breast cancer with transarterial chemoembolization with drug-eluting beads loaded with doxorubicin. Breast Cancer Res Treat. 2012;132(02):753–763. doi: 10.1007/s10549-011-1926-z. [DOI] [PubMed] [Google Scholar]
- 39.Lin Y-T, Médioni J, Amouyal G, Déan C, Sapoval M, Pellerin O. Doxorubicin-loaded 70-150 μm microspheres for liver-dominant metastatic breast cancer: results and outcomes of a pilot study. Cardiovasc Intervent Radiol. 2017;40(01):81–89. doi: 10.1007/s00270-016-1465-4. [DOI] [PubMed] [Google Scholar]
- 40.Haug A R, Tiega Donfack B P, Trumm C et al. 18F-FDG PET/CT predicts survival after radioembolization of hepatic metastases from breast cancer. J Nucl Med. 2012;53(03):371–377. doi: 10.2967/jnumed.111.096230. [DOI] [PubMed] [Google Scholar]
- 41.Cianni R, Pelle G, Notarianni E et al. Radioembolisation with (90)Y-labelled resin microspheres in the treatment of liver metastasis from breast cancer. Eur Radiol. 2013;23(01):182–189. doi: 10.1007/s00330-012-2556-5. [DOI] [PubMed] [Google Scholar]
- 42.Jakobs T F, Hoffmann R-T, Fischer T et al. Radioembolization in patients with hepatic metastases from breast cancer. J Vasc Interv Radiol. 2008;19(05):683–690. doi: 10.1016/j.jvir.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Saxena A, Kapoor J, Meteling B, Morris D L, Bester L. Yttrium-90 radioembolization for unresectable, chemoresistant breast cancer liver metastases: a large single-center experience of 40 patients. Ann Surg Oncol. 2014;21(04):1296–1303. doi: 10.1245/s10434-013-3436-1. [DOI] [PubMed] [Google Scholar]
- 44.Gordon A C, Gradishar W J, Kaklamani V Get al. Yttrium-90 radioembolization stops progression of targeted breast cancer liver metastases after failed chemotherapy J Vasc Interv Radiol 201425101523–1532., 1532.e1–1532.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fendler W P, Lechner H, Todica A et al. Safety, efficacy, and prognostic factors after radioembolization of hepatic metastases from breast cancer: a large single-center experience in 81 patients. J Nucl Med. 2016;57(04):517–523. doi: 10.2967/jnumed.115.165050. [DOI] [PubMed] [Google Scholar]
- 46.Pieper C C, Meyer C, Wilhelm K E et al. Yttrium-90 radioembolization of advanced, unresectable breast cancer liver metastases—a single-center experience. J Vasc Interv Radiol. 2016;27(09):1305–1315. doi: 10.1016/j.jvir.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 47.Forstner R. Radiological staging of ovarian cancer: imaging findings and contribution of CT and MRI. Eur Radiol. 2007;17(12):3223–3235. doi: 10.1007/s00330-007-0736-5. [DOI] [PubMed] [Google Scholar]
- 48.Aletti G D, Podratz K C, Cliby W A, Gostout B S. Stage IV ovarian cancer: disease site-specific rationale for postoperative treatment. Gynecol Oncol. 2009;112(01):22–27. doi: 10.1016/j.ygyno.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 49.Cormio G, Rossi C, Cazzolla A et al. Distant metastases in ovarian carcinoma. Int J Gynecol Cancer. 2003;13(02):125–129. doi: 10.1046/j.1525-1438.2003.13054.x. [DOI] [PubMed] [Google Scholar]
- 50.Tangjitgamol S, Levenback C F, Beller U, Kavanagh J J. Role of surgical resection for lung, liver, and central nervous system metastases in patients with gynecological cancer: a literature review. Int J Gynecol Cancer. 2004;14(03):399–422. doi: 10.1111/j.1048-891x.2004.14326.x. [DOI] [PubMed] [Google Scholar]
- 51.Kurra V, Krajewski K M, Jagannathan J, Giardino A, Berlin S, Ramaiya N. Typical and atypical metastatic sites of recurrent endometrial carcinoma. Cancer Imaging. 2013;13(01):113–122. doi: 10.1102/1470-7330.2013.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lim M C, Kang S, Lee K S et al. The clinical significance of hepatic parenchymal metastasis in patients with primary epithelial ovarian cancer. Gynecol Oncol. 2009;112(01):28–34. doi: 10.1016/j.ygyno.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 53.Bosquet J G, Merideth M A, Podratz K C, Nagorney D M. Hepatic resection for metachronous metastases from ovarian carcinoma. HPB. 2006;8(02):93–96. doi: 10.1080/13651820500472119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Merideth M A, Cliby W A, Keeney G L, Lesnick T G, Nagorney D M, Podratz K C. Hepatic resection for metachronous metastases from ovarian carcinoma. Gynecol Oncol. 2003;89(01):16–21. doi: 10.1016/s0090-8258(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 55.Liu P C, Benjamin I, Morgan M A, King S A, Mikuta J J, Rubin S C. Effect of surgical debulking on survival in stage IV ovarian cancer. Gynecol Oncol. 1997;64(01):4–8. doi: 10.1006/gyno.1996.4396. [DOI] [PubMed] [Google Scholar]
- 56.Vogl T J, Naguib N N, Lehnert T et al. Initial experience with repetitive transarterial chemoembolization (TACE) as a third line treatment of ovarian cancer metastasis to the liver: indications, outcomes and role in patient's management. Gynecol Oncol. 2012;124(02):225–229. doi: 10.1016/j.ygyno.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Sato K T, Lewandowski R J, Mulcahy M F et al. Unresectable chemorefractory liver metastases: radioembolization with 90Y microspheres–safety, efficacy, and survival. Radiology. 2008;247(02):507–515. doi: 10.1148/radiol.2472062029. [DOI] [PubMed] [Google Scholar]


