Abstract
Magnetic-resonance–guided focused ultrasound (MRgFUS), also called high-intensity focused ultrasound (HIFU) is an effective, noninvasive uterine-preserving treatment for symptomatic uterine fibroids. As the use of this therapeutic modality is not yet widespread, it may remain unfamiliar to many interventional radiologists. The purpose of this review is to discuss MRgFUS, including technology, patient selection, technique, outcomes, complications, and recent data on fertility and comparative effectiveness.
Keywords: High-intensity focused ultrasound, fibroids, menorrhagia, interventional radiology
Objectives : Upon completion of this article, the reader will be able to describe the role of MRgFUS in the treatment of fibroids, including patient selection, technical factors, and clinical outcomes associated with the technique.
Uterine fibroids are the most common pelvic tumor among women of reproductive age and approximately half of women with fibroids are symptomatic. 1 2 Many women with symptomatic uterine fibroids seek minimally invasive, uterine-preserving treatment options including medical therapy such as oral contraceptives or gonadotropin-releasing hormone (GnRH) agonists, myomectomy, radiofrequency ablation, uterine artery embolization (UAE), and magnetic-resonance–guided focused ultrasound (MRgFUS).
MRgFUS, also referred to as high-intensity focused ultrasound (HIFU), is a noninvasive method of tissue ablation that uses tightly focused, high-energy ultrasound waves to instantaneously destroy tissue, under MR guidance. 3 The ExAblate 2000 device (Insightec, Haifa, Israel) received approval from the Food and Drug Administration (FDA) in 2004 for the treatment of symptomatic uterine fibroids. Performed as an outpatient procedure using moderate sedation without the need for ionizing radiation, MRgFUS has a fast recovery time of 1 to 3 days and is a viable alternative to myomectomy and UAE.
Basic Principles and Updates in Technology
The core principle of focused ultrasound is targeted delivery of pulses of high-intensity ultrasound waves focused onto a small volume of tissue, a process called sonication. The high-energy focused ultrasound heats tissue at the focal zone to a temperature of 55 to 85°C, causing coagulative necrosis and cell death. Due to the small size of the tissue ablated (∼6 mm × 25 mm), multiple sonications are required to treat each fibroid. Focused ultrasound can be performed with ultrasound or MRI guidance (1.5 or 3 T). The benefits of coupling the therapy with MR guidance include optimal tissue targeting resulting from the contrast, spatial resolution, and multidimensional capabilities of MRI in addition to MR thermometry which offers real-time thermal imaging of the ablated area. 4
Per Insightec's ExAblate labeling, the most recent updated version of the technology is the UF-3 system which allows for transducer elevation (up and down movement of the ultrasound transducer) resulting in improved fibroid treatment. The system also has the ability to turn off specific ultrasound crystals generating ultrasound energy for a specific area to avoid nontarget sonication. Additionally, the software updates have allowed for patient motion detection and optimization of the treatment planning. Overall, in the authors' opinions, all updates have resulted in a more efficient and user-friendly system.
Patient Selection
Indications for the treatment of symptomatic uterine fibroids with MRgFUS are similar to that for UAE and myomectomy. And similar to UAE, appropriate patient selection is essential for successful MRgFUS outcomes. A preprocedure clinic visit should be performed and include assessment of symptoms as well as evaluation for the standard contraindications to MRI including presence of ferromagnetic implants, pacemakers, and metallic foreign bodies. If a patient has an intrauterine device (IUD), it is removed prior to MRgFUS to prevent local heating. 5 A physical exam is necessary to evaluate the skin of the lower abdomen and pelvis for scars or marks such as tattoos which may be in the path of the ultrasound beam and be at risk for skin burns. In addition, if the patient has large amounts of subcutaneous fat, higher ultrasound energies will be required to reach the fibroid and can increase the risk of skin burns. 6
A contrast-enhanced MRI of the pelvis should be obtained prior to the treatment to evaluate the MR characteristics, size, number, and location of the fibroids which will be used to determine if a patient is an appropriate candidate for MRgFUS. The imaging must also be reviewed for skin scars, surgical clips and IUD, and nontarget organs in the path of the ultrasound beam to avoid skin burns, local tissue heating, and organ injury, respectively. 7
Fibroids with homogenously hypointense signal on T2-weighted imaging are considered to have better response to MRgFUS compared with fibroids with hyperintense or heterogeneous T2 signal. 8 9 This is likely due to the increased cellularity and proliferative activity of the fibroid cells that are T2 hyperintense or heterogeneous, resulting in inadequate heating with increased likelihood of persistent growth in areas that were poorly ablated. 9 However, some studies have also shown no difference in outcomes based on the T2 signal characteristics of the uterine fibroids. 10 11 Higher levels of acoustic energy are required to ablate fibroids with hyperintense or heterogeneous T2 signal due to the increased cellularity of the fibroid cells, which may increase rates of skin burns and procedural pain. 8 Peripheral calcifications of fibroids may be a relative contraindication, due to reflection of the ultrasound beam. 5
Although there are no absolute limits regarding fibroid size or number, better results are expected after MRgFUS in patients with fewer than four fibroids or fibroid volumes less than 500 mL. 5 7 One study demonstrated best results in fibroid volumes less than 50 mL. 10 In addition, the use of GnRH agonists for 3 months before MRgFUS to reduce fibroid and uterine size has also been reported. 12
Fibroid location is important for MRgFUS planning. Submucosal fibroids can be safely treated with MRgFUS, with size reduction up to 90.1% at 24 months; in one study, 58% of patients underwent vaginal passage of necrotic tissue for up to 3 months after treatment. 13 MRgFUS has also been shown to be safe and effective in treating pedunculated fibroids with stalk-sparing ablation, with decrease in fibroid size and symptoms, and no reported stalk separation or other adverse outcome. 14 Lastly, fibroids must be located within 12 cm of the anterior abdominal wall to ensure adequate ultrasound penetration to achieve full treatment.
Technique
To provide moderate sedation during the MRgFUS, the patient should fast for 6 hours prior to the procedure. The lower abdominal and pelvic skin should be shaved from the umbilicus to the pubis, and then cleaned with alcohol to remove any lotions or powders, to prevent skin burns. A Foley catheter should be inserted into the bladder due to the prolonged duration of the procedure and the need to keep the bladder collapsed or to instill it with saline to displace surrounding structures out of the path of the beam. Compression stockings to prevent deep venous thrombosis are recommended. The patient is positioned prone on the ExAblate table within the MRI scanner, with pelvis over the transducer.
Multiplanar gradient-echo localizer sequences are obtained to confirm proper patient positioning. If intestine is present anterior to the uterus ( Fig. 1 ), manipulation of the transducer to oblique the path of the beam or filling the bladder to elevate the uterus and filling the rectum to displace the bowel can help avoid injury to the bowel ( Fig. 2 ). In cases where the fibroid is deep within the pelvis and exceeds the recommend 12 cm range from the skin to the posterior aspect of the fibroid ( Fig. 3 ), filling of the rectum with ultrasound gel can displace the uterus anteriorly, within the treatment range ( Fig. 4 ).
Fig. 1.
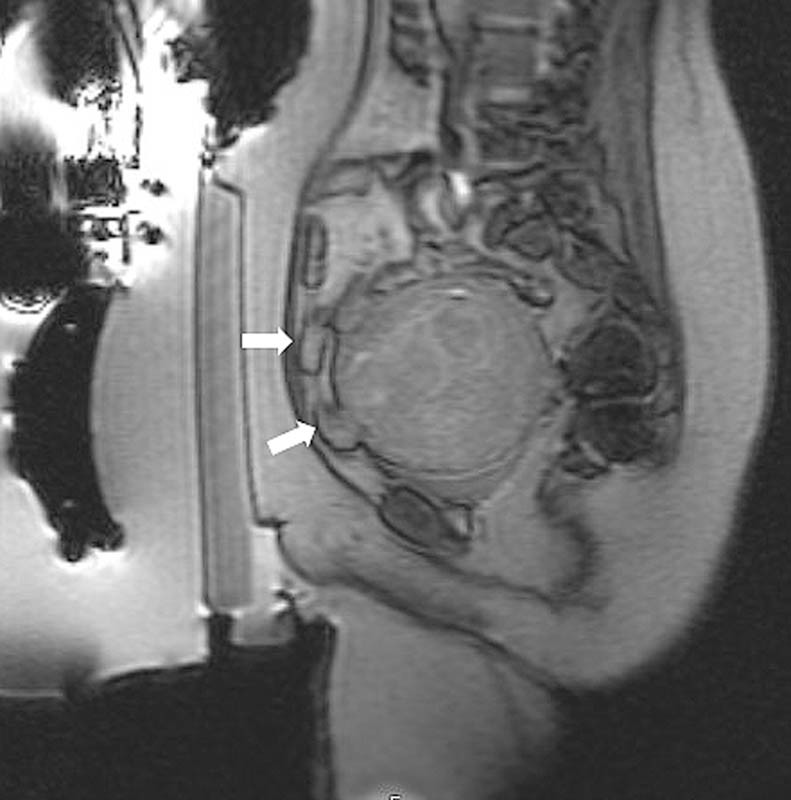
Sagittal GRE image demonstrates loops of intestine (white arrows) anterior to the fibroid, in the intended treatment path.
Fig. 2.
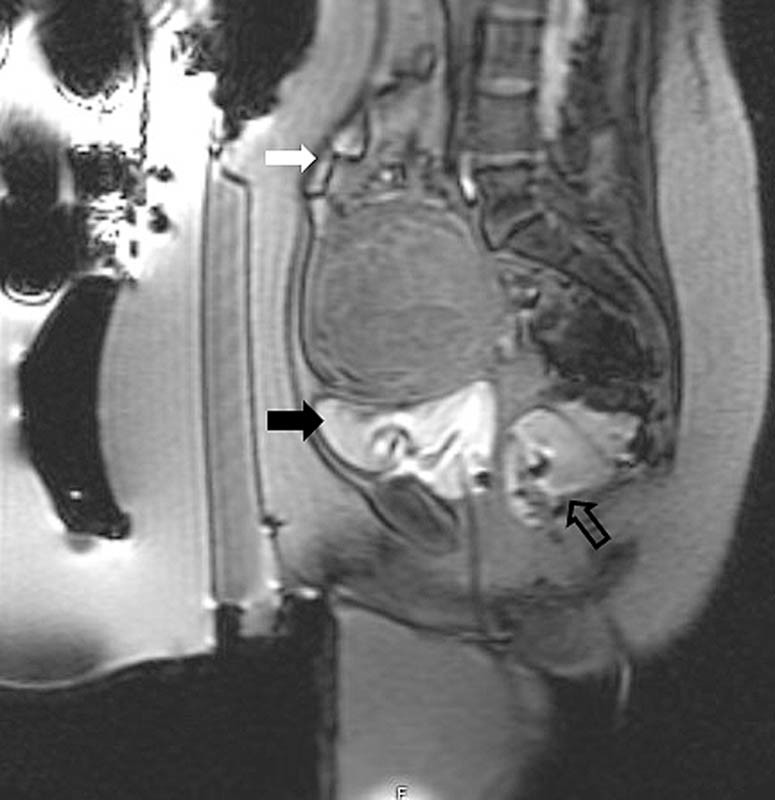
Following filling of the bladder (black arrow) and rectum (open arrow), the intestine (white arrow) has been displaced superiorly and out of the treatment path.
Fig. 3.
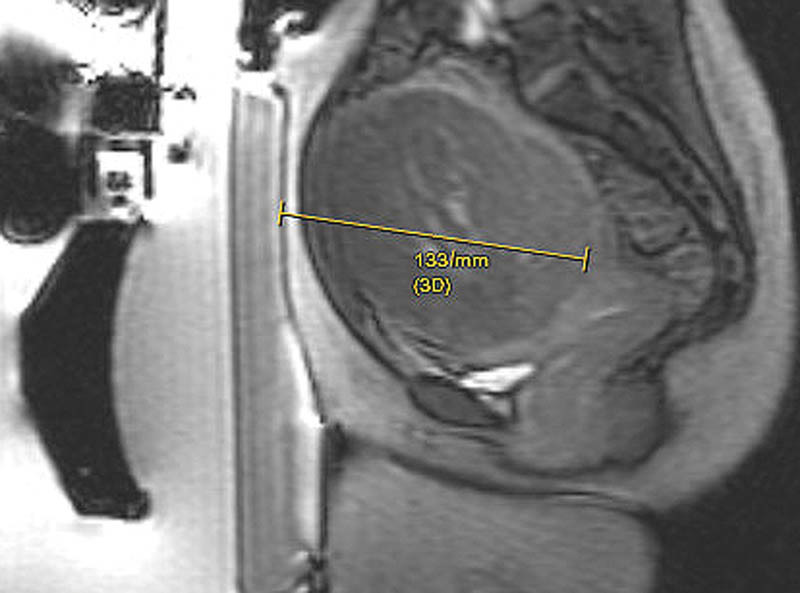
Sagittal GRE image demonstrates the distance from the skin to the posterior aspect of the fibroid to be 13.3 cm, which is outside the 12 cm range recommended for optimal therapy.
Fig. 4.
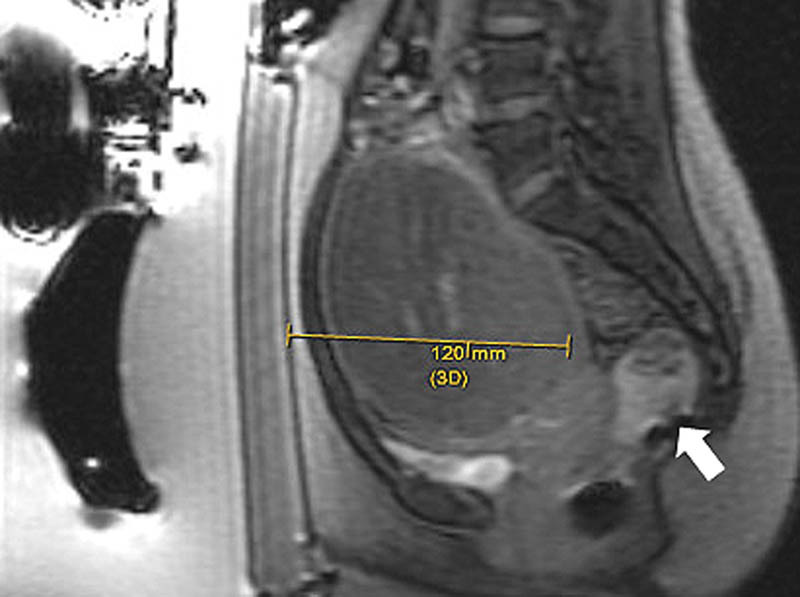
Following rectal fill (arrow), the uterus and the fibroid are displaced anteriorly, within the recommended 12 cm range.
Once there is proper positioning of the uterus in line with the transducer without intervening bowel or nontarget organs, multiplanar T2-weighted images are obtained. These images are then used on the ExAblate workstation for treatment planning. The treating physician manually outlines the skin line, the fibroids to be treated, and places barriers to prevent the high-intensity sound waves from traversing structures such as bowel, nerve roots, pubic bone, and skin scars. Fiducial markers are also placed around the uterus as reference to monitor movement during the treatment session. Following treatment planning, therapy is initiated with a few low-energy test sonications for final calibration and testing of power output. Multiple sonications are then performed until a sufficient fibroid volume has been treated. During the treatment, the physician can continuously change the parameters of the treatment, by increasing the energy or the direction of the beam to enhance the therapy. Each sonication lasts approximately 20 to 30 seconds followed by a cooling period of 80 to 100 seconds. A typical therapy is approximately 3 hours long and consists of close to 90 to 100 sonications. During the procedure, the patient is given a “panic button” and is instructed to use it if she experience skin burning, intense pain, internal heating, or pain radiation down the leg. 7
During the procedure, real-time thermometry is plotted with temperature measurements over the course of the sonication. If suboptimal temperatures are obtained, the parameters can be altered and sonication of the same spot may be repeated. A blue overlay represents treated areas, corresponding to the ablated, nonenhancing fibroid tissue ( Fig. 5a, b ). Following the procedure, multiplanar fat-saturated T1-weighted unenhanced and gadolinium-enhanced MR images are obtained to assess and calculate the nonperfused volume (NPV; Fig. 6a, b ). As the FDA cautions against MRgFUS following gadolinium administration for fear of the release of toxic-free gadolinium, a second session of treatment may be necessary if initial treatment is found to be inadequate.
Fig. 5.
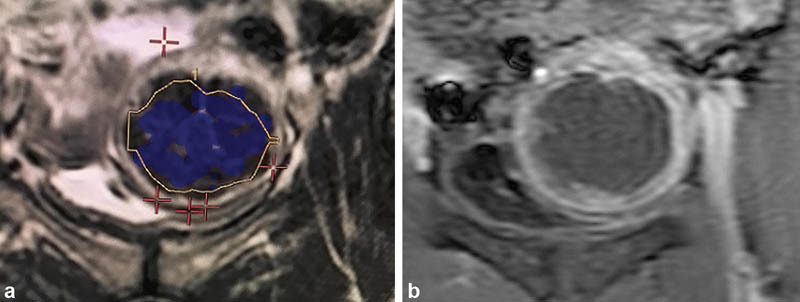
The blue overlay ( a ) corresponds to the nonenhancing portion of the fibroid ( b ).
Fig. 6.
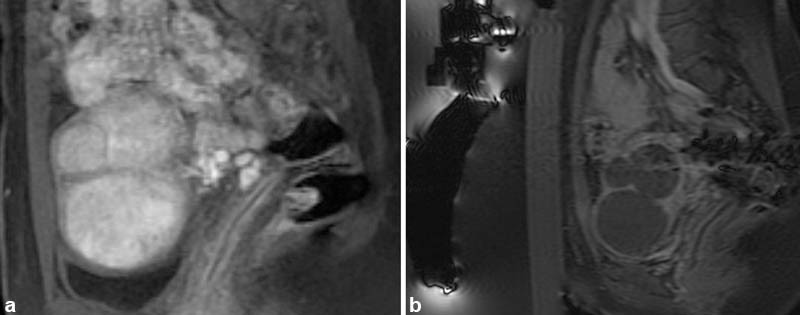
Sagittal T1-weighted postcontrast fast-saturated images. ( a ) Multiple enhancing fibroids are present in the uterus. ( b ) Following magnetic-resonance–guided focused ultrasound, none of the fibroids are enhancing resulting in a high nonperfused volume.
Following the procedure, the patient is monitored until she returns to her presedation state. In most cases, patients are discharged with instructions to take only nonsteroidal anti-inflammatory analgesics 15 and most patients return to work 1 to 3 days following the procedure.
Outcomes
Technical success can be gauged on immediate postprocedure imaging based on the extent of the NPV of the treated fibroids. There is a correlation between the NPV and symptom resolution, with longer lasting symptom relief noted in patients with NPV greater than 50 to 60%. 8 Additionally, in cases of NPV greater than 80%, there is more reduction in fibroid volume compared with cases where the NPV is less than 80%. 16 Typically, pelvic pain and pressure improve soon after the procedure and there is decrease in uterine volume and improvement in menorrhagia approximately after three menstrual cycles. 6
Gorny et al 17 demonstrated that the reintervention rate after an average follow-up of 2.8 years was 4% at 12 months, 13% at 24 months, 19% at 36 months, and 23% at 48 months. These numbers are comparable to the reintervention rates of UAE and myomectomy. Other trials have demonstrated higher reintervention rates with 30% at 24 months 18 19 and 31% at 30 months. 20 This discrepancy may be due to older age of the patients in the trial by Gorny et al, in addition to the number of secondary MRgFUS procedures performed in that patient population.
Gorny et al also demonstrated that patient's age is a significant predictor of treatment success such that older age at treatment is associated with lower risk of reintervention. In addition, patients with homogenously hypointense T2-weighted fibroids had improvement treatment success compared with patients with heterogeneous or hyperintense T2 signal. 17 This finding was in keeping with prior reports demonstrating the increased cellularity and proliferative activity of fibroid cells which are hyperintense or heterogeneous on T2-weighted imaging. 9
Fertility
Fertility after MRgFUS has not been clinically evaluated in randomized controlled trials. However, successful pregnancies and deliveries following the procedure have been reported. The largest series describes 54 pregnancies in 51 women with 41% deliveries, 20% ongoing pregnancies, and 28% spontaneous abortions. 21 These results are similar to the delivery rate (48%) and abortion rate (23%) following myomectomy compared with the deliver rate (19%) and abortion rate (64%) following UAE. 22 In 2015, the FDA approved the next-generation ExAblate system to treat symptomatic uterine fibroids and changed the labeling to allow consideration for women who desire to maintain fertility. Prior to that, the FDA had approved MRgFUS for fibroid therapy only in women who were family-complete.
Complications
Complications following MRgFUS are uncommon but include skin burns, sciatic nerve injury, vaginal discharge, focal abdominal wall edema, deep vein thrombosis, and bowel injury. 6 Skin burns can be avoided by shaving and cleaning the skin so air does not get trapped in the hair. Scars can also be a source of skin burns. Nerves and bones in the far field can be heated and injured during the treatment. To avoid this, the minimal distance between the posterior treatment area and bone is 4 cm. 7 In the reported cases, nerve injury resolves after varying degree of time. Injury to the intestine is the most severe complication of MRgFUS and can result from undetected internal movement of the bowel. Vigilance to the fiducial markers placed during treatment planning is important to monitor internal structure movement and avoid nontarget sonication during the procedure.
Comparative Effectiveness
In a prospective comparative cohort study, Froeling et al 19 demonstrated the long-term outcomes of 77 women treated with UAE and MRgFUS. The authors illustrated that following both MRgFUS and UAE, women had significant improvement in symptom severity score (SSS) and total health-related quality of life (HRQOL) score. However, at long-term follow-up of 60.7 to 61.9 months, the reintervention rate was significantly lower after UAE (12.2%) than MRgFUS (66.7%, p < 0.001). It should be noted that the majority of women in the MRgFUS group (47/50; 94%) were treated with the modified FDA treatment guidelines which limited ablation to 50% or 150 mL of the fibroid volume. Additionally, the two groups were different, which may have complicated treatment comparisons. The women in the UAE group had larger uteri, larger fibroid volume and more fibroids, higher SSS, and lower HRQOL scores and were older.
In a prospective, randomized controlled trial comparing MRgFUS to UAE, Barnard et al 23 demonstrated that after UAE, women had longer recovery times and used more prescription medication. The long-term follow-up results of this trial are pending.
Challenges
Several challenges have inhibited mainstream adoption of MRgFUS. One major factor is the prolonged duration of most procedures, which can be prohibitive in many practice settings given the limited availability and high cost of MR scanner time. Another barrier is patient eligibility with numerous exclusion criteria and restrictive selection criteria. Finally, more comparative trials are needed to assess MRgFUS against other more established uterine-preserving treatments. The relative paucity of comparative data may be an impediment to widespread use of MRgFUS in itself. In addition, it may impact the availability of insurance coverage for this potentially costly procedure. Table 1 illustrates the pros and cons of MRgFUS.
Table 1. Pros and cons of magnetic resonance-guided focused ultrasound.
| Pros | Cons |
|---|---|
| Nonionizing radiation | Prolonged procedural duration |
| Noninvasive | Limited eligibility |
| Outpatient | Difficulty with insurance coverage |
| No impact on ovarian function | Lack of comparative trials |
The future of MRgFUS for fibroid therapy remains optimistic. In order for this technology to become mainstream, it should be offered to patients who have the highest chance of successful outcomes. Patient selection remains the most important factor for clinical success. Based on the currently available evidence, the “ideal” patient to maximally benefit from MRgFUS is older patient, with a small number of small-volume fibroids which are T2 hypointense and homogenously enhancing, with a clear treatment path from skin to fibroid.
Conclusion
MRgFUS is an effective noninvasive outpatient therapy for symptomatic fibroids in appropriately selected patients. Although some challenges have impeded widespread use of MRgFUS, additional comparative effectiveness data and more consistent insurance coverage could potentially overcome some of these barriers to make it a more mainstream uterine-sparing treatment modality for symptomatic fibroids.
References
- 1.Wallach E E, Vlahos N F. Uterine myomas: an overview of development, clinical features, and management. Obstet Gynecol. 2004;104(02):393–406. doi: 10.1097/01.AOG.0000136079.62513.39. [DOI] [PubMed] [Google Scholar]
- 2.Divakar H. Asymptomatic uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22(04):643–654. doi: 10.1016/j.bpobgyn.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Jacoby V L, Kohi M P, Poder L et al. PROMISe trial: a pilot, randomized, placebo-controlled trial of magnetic resonance guided focused ultrasound for uterine fibroids. Fertil Steril. 2016;105(03):773–780. doi: 10.1016/j.fertnstert.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Coakley F V, Foster B R, Farsad K et al. Pelvic applications of MR-guided high intensity focused ultrasound. Abdom Imaging. 2013;38(05):1120–1129. doi: 10.1007/s00261-013-9999-2. [DOI] [PubMed] [Google Scholar]
- 5.Roberts A. Magnetic resonance-guided focused ultrasound for uterine fibroids. Semin Intervent Radiol. 2008;25(04):394–405. doi: 10.1055/s-0028-1102999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silberzweig J E, Powell D K, Matsumoto A H, Spies J B. Management of uterine fibroids: a focus on uterine-sparing interventional techniques. Radiology. 2016;280(03):675–692. doi: 10.1148/radiol.2016141693. [DOI] [PubMed] [Google Scholar]
- 7.Hesley G K, Gorny K R, Woodrum D A. MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovasc Intervent Radiol. 2013;36(01):5–13. doi: 10.1007/s00270-012-0367-3. [DOI] [PubMed] [Google Scholar]
- 8.Mikami K, Murakami T, Okada A, Osuga K, Tomoda K, Nakamura H. Magnetic resonance imaging-guided focused ultrasound ablation of uterine fibroids: early clinical experience. Radiat Med. 2008;26(04):198–205. doi: 10.1007/s11604-007-0215-6. [DOI] [PubMed] [Google Scholar]
- 9.Funaki K, Fukunishi H, Funaki T, Sawada K, Kaji Y, Maruo T. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. Am J Obstet Gynecol. 2007;196(02):1840–1.84E8. doi: 10.1016/j.ajog.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Trumm C G, Stahl R, Clevert D A et al. Magnetic resonance imaging-guided focused ultrasound treatment of symptomatic uterine fibroids: impact of technology advancement on ablation volumes in 115 patients. Invest Radiol. 2013;48(06):359–365. doi: 10.1097/RLI.0b013e3182806904. [DOI] [PubMed] [Google Scholar]
- 11.Zhao W P, Chen J Y, Zhang L et al. Feasibility of ultrasound-guided high intensity focused ultrasound ablating uterine fibroids with hyperintense on T2-weighted MR imaging. Eur J Radiol. 2013;82(01):e43–e49. doi: 10.1016/j.ejrad.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 12.Smart O C, Hindley J T, Regan L, Gedroyc W M. Magnetic resonance guided focused ultrasound surgery of uterine fibroids--the tissue effects of GnRH agonist pre-treatment. Eur J Radiol. 2006;59(02):163–167. doi: 10.1016/j.ejrad.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Wang Y, Wang T, Wang J, Wang L, Tang J. Safety and efficacy of US-guided high-intensity focused ultrasound for treatment of submucosal fibroids. Eur Radiol. 2012;22(11):2553–2558. doi: 10.1007/s00330-012-2517-z. [DOI] [PubMed] [Google Scholar]
- 14.Park H, Yoon S W, Kim K A, Jung Kim D, Jung S G. Magnetic resonance imaging-guided focused ultrasound treatment of pedunculated subserosal uterine fibroids: a preliminary report. J Vasc Interv Radiol. 2012;23(12):1589–1593. doi: 10.1016/j.jvir.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Hudson S B, Stewart E A. Magnetic resonance-guided focused ultrasound surgery. Clin Obstet Gynecol. 2008;51(01):159–166. doi: 10.1097/GRF.0b013e318161e91f. [DOI] [PubMed] [Google Scholar]
- 16.Park M J, Kim Y S, Rhim H, Lim H K. Safety and therapeutic efficacy of complete or near-complete ablation of symptomatic uterine fibroid tumors by MR imaging-guided high-intensity focused US therapy. J Vasc Interv Radiol. 2014;25(02):231–239. doi: 10.1016/j.jvir.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Gorny K R, Borah B J, Brown D L, Woodrum D A, Stewart E A, Hesley G K. Incidence of additional treatments in women treated with MR-guided focused US for symptomatic uterine fibroids: review of 138 patients with an average follow-up of 2.8 years. J Vasc Interv Radiol. 2014;25(10):1506–1512. doi: 10.1016/j.jvir.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Froeling V, Meckelburg K, Scheurig-Muenkler C et al. Midterm results after uterine artery embolization versus MR-guided high-intensity focused ultrasound treatment for symptomatic uterine fibroids. Cardiovasc Intervent Radiol. 2013;36(06):1508–1513. doi: 10.1007/s00270-013-0582-6. [DOI] [PubMed] [Google Scholar]
- 19.Froeling V, Meckelburg K, Schreiter N F et al. Outcome of uterine artery embolization versus MR-guided high-intensity focused ultrasound treatment for uterine fibroids: long-term results. Eur J Radiol. 2013;82(12):2265–2269. doi: 10.1016/j.ejrad.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 20.Kim H S, Baik J H, Pham L D, Jacobs M A. MR-guided high-intensity focused ultrasound treatment for symptomatic uterine leiomyomata: long-term outcomes. Acad Radiol. 2011;18(08):970–976. doi: 10.1016/j.acra.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabinovici J, David M, Fukunishi H, Morita Y, Gostout B S, Stewart E A; MRgFUS Study Group.Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids Fertil Steril 20109301199–209. [DOI] [PubMed] [Google Scholar]
- 22.Mara M, Maskova J, Fucikova Z, Kuzel D, Belsan T, Sosna O. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31(01):73–85. doi: 10.1007/s00270-007-9195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnard E P, AbdElmagied A M, Vaughan L E et al. Periprocedural outcomes comparing fibroid embolization and focused ultrasound: a randomized controlled trial and comprehensive cohort analysis. Am J Obstet Gynecol. 2017;216(05):5000–5.0E13. doi: 10.1016/j.ajog.2016.12.177. [DOI] [PMC free article] [PubMed] [Google Scholar]


