Abstract
Pelvic venous insufficiency is now a well-characterized etiology of pelvic congestion syndrome (PCS). The prevalence of CPP is 15% in females aged 18 to 50 years in the United States and up to 43.4% worldwide. In addition to individual physical, emotional, and quality-of-life implications of CPP, there are profound healthcare and socioeconomic expenses with estimated annual direct and indirect costs in the United States in excess of 39 billion dollars. PCS consists of clinical symptoms with concomitant anatomic and physiologic abnormalities originating in venous insufficiency. The etiology of PCS is diverse involving both mechanical and hormonal factors contributing to venous dilatation (>5 mm) and insufficiency. Factors affecting the diagnosis of PCS include variance of causes and clinical presentations of pelvic pain and relatively low sensitivity of noninvasive diagnostic imaging and laparoscopy to identify insufficiency compared with catheter venogram. A systematic review of the literature evaluating patient outcomes following percutaneous treatment of PCS is presented.
Keywords: interventional radiology, pelvic congestion syndrome, pelvic pain, venous reflux, embolization
Objectives : Upon completion of this article, the reader will be able to describe the etiology of and risk factors contributing to the development of pelvic congestion syndrome. Readers will also be able to discuss the role of endovascular treatment for the disease process.
Accreditation : This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit : Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit ™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Pelvic venous insufficiency, initially described around 1850s 1 and correlated with pelvic pain in the 1940s to 1950s, 2 3 4 is now a well-characterized etiology of pelvic congestion syndrome (PCS). 1 5 6 7 Thirty percent to 40% of cases of chronic pelvic pain (CPP) 8 are associated with PCS. 2 9 Prevalence of CPP is 15% in females aged 18 to 50 years in the United States 10 and up to 43.4% worldwide. 11 12 13 CPP accounts for up to 40% of outpatient gynecologic visits 14 15 and up to 40% of gynecologic laparoscopies. 16 In addition to individual physical, emotional, and quality-of-life implications 13 of CPP, there are profound healthcare and socioeconomic expenses with estimated annual direct and indirect costs in the United States in excess of 39 billion dollars. 10 14
PCS consists of clinical symptoms with concomitant anatomic and physiologic abnormalities originating in venous insufficiency. 17 18 19 Clinical symptoms of PCS are consistently reported as chronic, noncyclic pelvic pain or heaviness which is commonly exacerbated by prolonged standing and often occurring in association with dysmenorrhea, dyspareunia, urinary urgency, and perineal or lower extremity varices. 1 5 7 11 13 17 19 20 21
The etiology of PCS is diverse involving both mechanical and hormonal factors contributing to venous dilatation (>5 mm) and insufficiency. 7 Absence or dysfunction of valves, variant anatomy, venous kinking from uterine malposition, and structural and hormonal changes of parity all correspond to primary PCS, 1 7 17 20 whereas extrinsic compression corresponds with secondary PCS. 17 Slow flow, inflammation, thrombosis, and insufficiency are thought to be responsible for symptom development as pelvic varices can be present in asymptomatic individuals. 7 22 23 24 25
Factors affecting the diagnosis of PCS include variance of causes and clinical presentations of pelvic pain 1 2 20 26 27 and relatively low sensitivity of noninvasive diagnostic imaging and laparoscopy 6 22 to identify insufficiency compared with catheter venogram. Despite diagnostic challenges, studies show promising results for percutaneous management of PCS delineating it as a treatable syndrome of significant prevalence, morbidity, and systemic costs. 10 14 15 28 A systematic review of literature evaluating patient outcomes following percutaneous treatment of PCS is presented.
Methods
Data Sources and Study Selection
A systematic review of the MEDLINE database was conducted using PubMed, Ovid SP, and Google Scholar search engines. Methodologic framework followed PRISMA guidelines. 29 Search parameters included full text articles published between 1974 and February 2015 using key words “pelvic congestion syndrome,” “pelvic congestion,” “pelvic varices,” and “ovarian vein embolotherapy.” Articles were manually reviewed for treatment of PCS, rendering 25 studies.
Inclusion Criteria
Inclusion criteria are as follows: (1) all studies with intervention for PCS with the method of intervention identified as percutaneous; (2) a minimum sample size of 10 study participants, excluding case reports or small case series; (3) studies that report assessment of patient symptoms pre- and posttreatment. Fourteen studies met inclusion criteria. 21 22 30 31 32 33 34 35 36 37 38 39 40 41
Exclusion Criteria
One study utilizing percutaneous ovarian vein embolization in patients with perineal and lower extremity varix without symptoms of PCS was excluded. Four studies utilizing surgical treatment were excluded. Six studies were excluded for having a sample population of less than 10 patients. Studies were excluded if the full text was not available. Review articles, letters, and editorials were also excluded.
Quality Assurance and Appraisal
Each eligible study was evaluated for methodology and potential bias.
Data Extraction
Included studies were reviewed to extract the following information from patient subsets undergoing percutaneous treatment for PCS: population size, study design, age, follow-up duration, type of intervention, embolic agent(s), and outcomes via change in pre- and posttreatment self-reported symptoms. Additional information including reported complications, symptom resolution, symptom type, parity, and postprocedure length of stay are reported according to the subset of studies providing these data points. Applicable PRISMA guidelines were followed in data collection, analysis, and reporting.
Cumulative Data Synthesis
Detailed description of extracted data was tabulated. Summary results are reported as proportions, medians (M) with corresponding interquartile range (IQR Q3–Q1 ), or weighted means (average w ), as applicable.
Limitations of Review
Studies meeting inclusion criteria for review are heterogeneous in method of treatment, endpoints, and in study populations and size precluding meta-analysis. Included studies consist of case series study design (lacking control groups). Use of subjective patient reporting to determine clinical success invites recall and interviewer bias. Nonetheless, there exist certain characteristics of patients, pitfalls, and percutaneous treatment, which are consistently described in the literature.
Results
Study Demographics
Fourteen studies of percutaneous treatment for PCS yield a total of 828 patients and 994 unique percutaneous interventions: 979 for initial sclerosis or embolization of ovarian or internal iliac veins (accounting for staged procedures), 21 22 30 31 32 33 34 35 36 37 38 39 40 41 14 repeat interventions for recurrent symptoms, 30 34 40 41 and 1 repeat intervention for technical failure at initial intervention. 41
Indication for intervention requires symptom(s) of PCS in combination with signs of pelvic venous incompetence on catheter-based venography. 21 22 30 31 32 33 34 35 36 37 38 39 40 41
Average w patient age is 40 years (range: 16–72 years). 21 22 30 31 32 33 34 35 36 37 38 39 40 41
Average w follow-up is 36.1 months (range: 1–288 months). 21 22 30 31 32 33 34 35 36 37 38 39 40 41
Postprocedure length of stay ranges from 4 to 24 hours with procedures occurring in the outpatient setting 30 32 33 35 40 41 or overnight observation. 21 22 37 40
Reported complications range from 0.85 to 10% (M: 4.95, IQR Q3–Q1 : 5.4) and were minor without sequelae: 6 cases of vessel perforation, 20 cases of nontarget embolization, 6 groin hematomas, 1 arrhythmias, 1 internal iliac venous thrombus, and 2 contrast reactions among 944 unique procedures. 21 22 31 32 35 36 37 38 39 40 41
Clinical Outcomes
Patients reporting improvement of clinical symptoms following percutaneous treatment ranges from 68.3 to 100% (M: 95.1, IQR Q3–Q1 : 17.4). 21 22 30 31 32 33 34 35 36 37 38 39 40 41 Of 828 patients, 762 (92%) patients complete respective study follow-up endpoints. 21 22 30 31 32 33 34 35 36 37 38 39 40 41 Following intervention, 697 (range: 68.3–100%, M: 95.1, IQR Q3–Q1 : 17.4) report some degree of symptomatic improvement, 57 (range: 0–31.7%, M: 4.6, IQR Q3–Q1 : 14.2) report no symptom change, and 6 (range: 0–4.1%, M: 0, IQR Q3–Q1 : 0) report worsening of symptoms. 21 22 30 31 32 33 34 35 36 37 38 39 40 41 Of studies reporting resolution, 191 of 488 (range: 7.5–87.5%, M: 58.2, IQR Q3–Q1 : 27.1) report complete symptom resolution at follow-up. 21 30 31 32 33 34 37 41
Of patients initially reporting symptom improvement, 18 (range: 0–18.2%, M: 2.1, IQR Q3–Q1 : 5.4) report symptom recurrence occurring over a range from 4 to 12 months. 21 22 30 31 32 34 35 36 38 39 40 41
Repeat Interventions of Percutaneous and Surgical Natures
Fourteen repeat percutaneous interventions were performed for recurrent symptoms: 1 case recurring 5 years after embolization following multiple pregnancies; 34 11 cases recurring in venous territories not originally embolized; 30 40 41 and 2 cases recurring in previously treated territories. 30 Follow-up of repeat interventions in these patients report improved symptoms for the patient experiencing recurrence following pregnancies 34 and in two of five patients retreated for new or persistent varices. 30
One study provides follow-up of nine patients undergoing hysterectomy due to lack of symptomatic improvement of pelvic pain following percutaneous treatment: hysterectomy yielded no additional symptom improvement. 31
Kim et al 22 reported a subset of 25 patients who failed to experience improvement of pelvic pain following hysterectomy, all 25 report symptomatic improvement following percutaneous treatment.
Percutaneous Techniques
Clinical improvement following coil embolization ranges from 82.1 to 100% (M: 95.8, IQR Q3–Q1 : 6.1) in 473patients. 21 31 34 35 37 38 39 Clinical improvement following embolization with glue and lipiodized oil ranges from 68.3 to 73.7% (M: 71, IQR Q3–Q1 : 5.4) in 60 patients. 30 32 A single study of isolated ovarian vein sclerosis reports 100% clinical success in 33 patients. 33 Clinical success of studies involving mixed percutaneous methods including sclerosant with coil and/or Gelfoam embolization ranges from 83 to 100% (M: 94.9, IQR Q3–Q1 : 17.1) in 196 patients 22 36 40 41 ( Figs. 1 2 3 ).
Fig. 1.
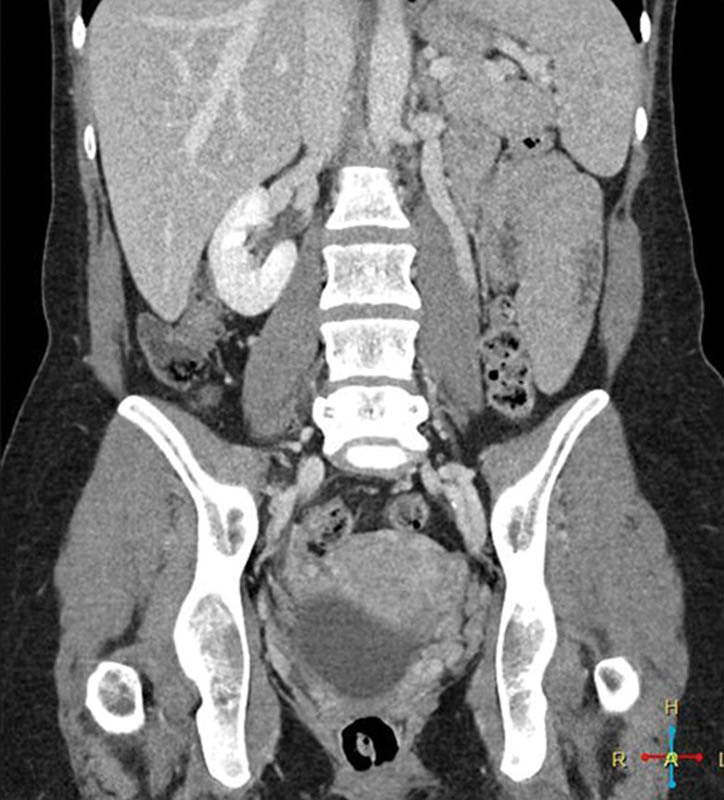
Coronal CT venogram with dilated left gonadal vein.
Fig. 2.
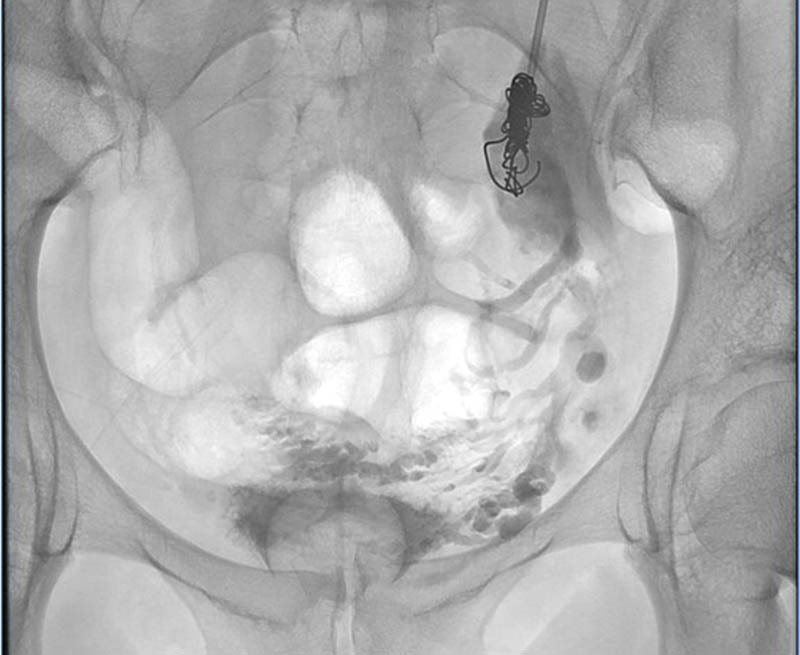
Fluoroscopic view of the abdomen and pelvis demonstrates sodium tetradecyl sulfate and contrast in the deep pelvic varices with a coil in the left gonadal vein.
Fig. 3.
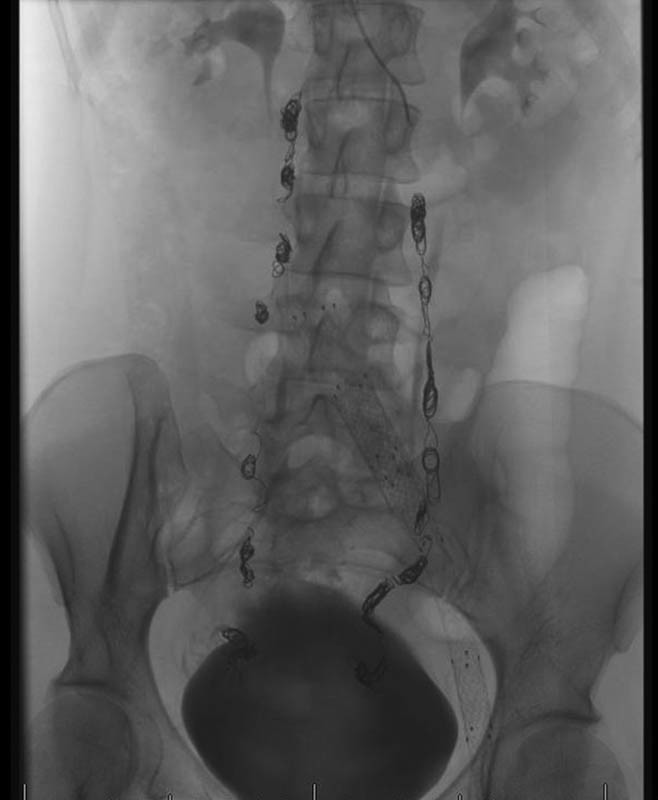
Fluoroscopic view of the abdomen and pelvis demonstrates coils in the bilateral gonadal veins.
Territory of Intervention
Six studies report treatment of ovarian veins with clinical improvement in 198 of 228 patients ranging from 68.3 to 100% (M: 91.1, IQR Q3–Q1 : 29). 30 31 32 33 34 35 Five studies report treatment of ovarian and internal iliac veins with clinical improvement in 334 of 348 patients ranging from 93.9 to 100% (M: 95.8, IQR Q3–Q1 : 5.9). 21 36 37 38 39 Three studies report treatment of ovarian, internal iliac, and additional pelvic varices with clinical improvement in 165 of 186 patients ranging from 83 to 96% (M: 93.9, IQR Q3–Q1 : 13.4). 22 40 41
Studies specify 483 bilateral and 290 unilateral ovarian vein interventions, almost invariably left sided. 21 22 30 31 32 33 34 35 37 38 39 40
Variceal Diameter
The diameter of ovarian veins based on catheter venogram ranges from 6.2 to 14.6 mm; no correlation is identified comparing diameter to symptom improvement. 21 31 32 33 34 35
Patient Assessment
Studies utilize various endpoints to assess clinical success including patient report of symptom improvement, 30 31 pre- and posttreatment questionnaires, 32 33 34 decrease in variceal size, 30 33 and a VAS. 21 22 35 36 37 38 39 40 41
Parity
Of 655 patients in studies reporting parity, 86.6% were parous 21 22 30 31 32 35 36 37 38 having an average w parity of 2.6 (range: 0–8). 21 30 31 32 35 36 37 38 39 41 Kwon et al 36 reported no statistically significant difference between parity (P1 through P5) and outcomes. Maleux et al 32 reported no statistically significant difference in outcomes between multiparous and uniparous patients. Five studies include nulliparous patients, 22 30 35 39 41 of which two studies having 21% 30 and 63% 22 nulliparous patients report no significant differences in outcomes between parous and nulliparous subpopulations.
Symptom Breakdown
From a total of 266 patients, 75.2% of patients report improvement of dysmenorrhea. 21 37 From a total of 210 patients, 85.2% of patients report improvement of dyspareunia. 21 30 32 33 34 37 41 From a total of 149 patients, 98.7% of patients report improvement of urinary urgency. 21 32 33 37
Discussion
Efficacy of Percutaneous Treatment in PCS
Percutaneous embolization for PCS is an effective method of treatment having a high percentage of symptom improvement (reported in 697 of 762 patients). 21 22 30 31 32 33 34 35 36 37 38 39 40 41 Studies utilizing a VAS as a quantitative measure of symptom improvement 42 report statistically significant overall symptom improvement comparing posttreatment and pretreatment values with an average w decrease of 5.7 within 0 to 10 scale. 21 22 35 36 37 38 39 40 41
Safety
Procedural complications in percutaneous treatment of PCS are minor and uncommon, reported in 36 of 944 procedures. 21 22 31 32 35 36 37 38 39 40 41 Reports of worsening symptoms after percutaneous treatment for PCS are rare, reported in 6 of 710 patients. 22 31 No additional treatment-related sequelae were identified.
To date, no studies specifically address attempted conception following percutaneous treatment for PCS. Galkin et al reported a series of ovarian varix embolization to treat infertility, with improvement of clinical symptoms, laboratory tests, and 14 of 19 patients conceiving. 43 Capasso et al 31 reported no significant change in menstrual cycle posttreatment. Kim et al 22 reported no change in pre- and postembolization levels of follicle-stimulating hormone, luteinizing hormone, or estradiol. Notably, percutaneous gonadal vein variceal embolization is recommended as treatment for both pain and infertility in the male population. 44 45
Interval of Clinical Improvement
Studies reporting intervals of symptom change demonstrate improvement in the early postprocedural period ranging from 1 day to 3 months. 30 31 32 35 38 40 A large-scale study with 5-year follow-up 21 reports greatest decreases in VAS scores occurring within the first 6 months. Moreover, time frames may be falsely elevated reflecting documentation at the time of follow-up rather than time to symptom improvement.
Similarly, studies report little to no increase of symptom improvement beyond the early postprocedural period. Pieri et al 34 reported changes in symptom levels and characteristics at 1-month follow-up, but no further changes at subsequent follow-up intervals. Kwon et al 36 reported that for patients without symptom improvement during the initial 3 months, no improvement was experienced within the follow-up period. Chung and Huh 35 and Nasser et al 41 reported average VAS scores for follow-up intervals, showing marked improvement at 1-month follow-up with mild gradual improvement at 3, 6, and 12-month intervals.
Recurrence, Reintervention, and Predictors
Percutaneous intervention for PCS incurs minimal reported symptom recurrence, 21 22 30 31 32 33 34 35 36 37 38 39 40 41 which may be artificially low compared with clinical practice given time frame of and reporting within studies. The majority of reported symptom recurrence occurs in territories not previously embolized. 30 40 41
Repeat percutaneous intervention is sparsely reported with mixed results. 30 34 40 41
Percutaneous treatment has been reported effective when prior interventions including hysterectomy 22 and medication 29 have failed.
No consistent predictors of outcome following percutaneous intervention for PCS are identified within the studies. Specifically, parity, varix size, symptom severity, or territory embolized were not found to be independent predictors of outcome.
Percutaneous Technique
Symptom improvement is similar between coil embolization, sclerosant, and combined use of agents, whereas that of glue and lipiodized oil is relatively lower.
Treatment Territory
Study techniques vary in territory and laterality of embolization, without accounting for different combinations of territories in reported results. There are divergent opinions as to whether limited 30 31 39 41 or complete embolization 21 40 should be performed. Combination of ovarian, internal iliac, and additional variceal intervention 22 40 41 has a range of clinical success lower than that of ovarian and iliac interventions, 21 36 37 38 39 though median values are similar between these groups and isolated ovarian intervention. 30 31 32 33 34 35
Studies reporting outcomes analysis comparing unilateral and bilateral embolization 30 32 report no statistically significant difference. Capasso et al 31 noted that 76.9% of patients treated with left ovarian vein embolization did not develop right ovarian varices at follow-up.
Pitfalls of Diagnosis
Patients with PCS are generally described as multiparous and premenopausal with reports of symptom resolution occurring at menopause. 19 31 32 Five studies include nulliparous patients 22 30 35 39 41 and no statistical difference in outcomes is identified based on number of pregnancies or between parous and nulliparous patients. 22 30 31 32 Eight studies include patients aged 56 years and older 21 30 31 32 33 39 40 41 exceeding an average age of postmenopausal patients as determined by a large-scale population study. 46 Nulliparous and postmenopausal patients present with PCS and these populations should not be overlooked.
Imaging findings of PCS are well described by Knuttinen et al including dilated ovarian, pelvic, arcuate, perineal, and lower extremity veins demonstrating slow flow, stasis, or reflux. 18 However, studies report insensitivity of noninvasive imaging compared with catheter venogram. Cross-sectional imaging has a wide variance of reported sensitivities for detection of pelvic varices including, 12.5% on CT 22 and 58.6 to 100% on MRI, 18 22 47 48 related to venous drainage with supine positioning and lack of dynamic imaging sequences. 1 19 20 40 Ultrasound offers advantages in dynamic and positional image acquisition allowing demonstration of venous reflux with upright positioning and Valsalva maneuver, 49 yet studies report insensitivity 50 compared with catheter venography with ultrasound identification of varices in as little as 53% 51 and 20% 22 of cases. Diagnostic laparoscopy underestimates the presence and number of varices as both positioning and insufflation pressure facilitate drainage or effacement of varices 40 50 with reports of pelvic variceal identification ranging from less than 20% 6 to 40%. 22
Adding to diagnostic confusion, ovarian vein dilatation, though associated 52 and predictive, 18 is not synonymous with venous incompetence or symptoms. 22 23 24 25
Conclusion
PCS is a prevalent and treatable condition for which percutaneous treatment is safe and effective. Thorough clinical and imaging evaluation by a provider who is familiar with the associated diagnostic pitfalls is imperative, as the indication for treatment of PCS requires both clinical symptom(s) and associated venous incompetence. Catheter-directed venography demonstrates improved sensitivity in detecting venous insufficiency compared with noninvasive imaging, as well as the benefit of simultaneous diagnosis and treatment, and a high rate of success in improving clinical symptoms.
References
- 1.Ganeshan A, Upponi S, Hon L Q, Uthappa M C, Warakaulle D R, Uberoi R. Chronic pelvic pain due to pelvic congestion syndrome: the role of diagnostic and interventional radiology. Cardiovasc Intervent Radiol. 2007;30(06):1105–1111. doi: 10.1007/s00270-007-9160-0. [DOI] [PubMed] [Google Scholar]
- 2.Duncan C H, Taylor H C., Jr A psychosomatic study of pelvic congestion. Am J Obstet Gynecol. 1952;64(01):1–12. doi: 10.1016/s0002-9378(16)38730-0. [DOI] [PubMed] [Google Scholar]
- 3.Taylor H C., Jr Vascular congestion and hyperemia; their effect on function and structure in the female reproductive organs; the clinical aspects of the congestion-fibrosis syndrome. Am J Obstet Gynecol. 1949;57(04):637–653. doi: 10.1016/0002-9378(49)90704-8. [DOI] [PubMed] [Google Scholar]
- 4.Taylor H C. Pelvic pain based on a vascular and autonomic nervous system disorder. Am J Obstet Gynecol. 1954;67(06):1177–1196. doi: 10.1016/s0002-9378(16)38487-3. [DOI] [PubMed] [Google Scholar]
- 5.Beard R W, Reginald P W, Wadsworth J. Clinical features of women with chronic lower abdominal pain and pelvic congestion. Br J Obstet Gynaecol. 1988;95(02):153–161. doi: 10.1111/j.1471-0528.1988.tb06845.x. [DOI] [PubMed] [Google Scholar]
- 6.Beard R W, Highman J H, Pearce S, Reginald P W.Diagnosis of pelvic varicosities in women with chronic pelvic pain Lancet 19842(8409):946–949. [DOI] [PubMed] [Google Scholar]
- 7.Phillips D, Deipolyi A R, Hesketh R L, Midia M, Oklu R. Pelvic congestion syndrome: etiology of pain, diagnosis, and clinical management. J Vasc Interv Radiol. 2014;25(05):725–733. doi: 10.1016/j.jvir.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 8.Beard R W, Kennedy R G, Gangar K F et al. Bilateral oophorectomy and hysterectomy in the treatment of intractable pelvic pain associated with pelvic congestion. Br J Obstet Gynaecol. 1991;98(10):988–992. doi: 10.1111/j.1471-0528.1991.tb15336.x. [DOI] [PubMed] [Google Scholar]
- 9.Soysal M E, Soysal S, Vicdan K, Ozer S. A randomized controlled trial of goserelin and medroxyprogesterone acetate in the treatment of pelvic congestion. Hum Reprod. 2001;16(05):931–939. doi: 10.1093/humrep/16.5.931. [DOI] [PubMed] [Google Scholar]
- 10.Mathias S D, Kuppermann M, Liberman R F, Lipschutz R C, Steege J F. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87(03):321–327. doi: 10.1016/0029-7844(95)00458-0. [DOI] [PubMed] [Google Scholar]
- 11.Latthe P, Latthe M, Say L, Gülmezoglu M, Khan K S. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health. 2006;6:177–183. doi: 10.1186/1471-2458-6-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahangari A. Prevalence of chronic pelvic pain among women: an updated review. Pain Physician. 2014;17(02):E141–E147. [PubMed] [Google Scholar]
- 13.Zondervan K T, Yudkin P L, Vessey M P et al. The community prevalence of chronic pelvic pain in women and associated illness behaviour. Br J Gen Pract. 2001;51(468):541–547. [PMC free article] [PubMed] [Google Scholar]
- 14.Kuligowska E, Deeds L, III, Lu K., III Pelvic pain: overlooked and underdiagnosed gynecologic conditions. Radiographics. 2005;25(01):3–20. doi: 10.1148/rg.251045511. [DOI] [PubMed] [Google Scholar]
- 15.Klock S. St. Louis: Mosby; 1995. Psychosomatic issues in obstetrics and gynecology; pp. 399–402. [Google Scholar]
- 16.Howard F M. The role of laparoscopy in chronic pelvic pain: promise and pitfalls. Obstet Gynecol Surv. 1993;48(06):357–387. doi: 10.1097/00006254-199306000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Durham J D, Machan L. Pelvic congestion syndrome. Semin Intervent Radiol. 2013;30(04):372–380. doi: 10.1055/s-0033-1359731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knuttinen M G, Xie K, Jani A, Palumbo A, Carrillo T, Mar W. Pelvic venous insufficiency: imaging diagnosis, treatment approaches, and therapeutic issues. AJR Am J Roentgenol. 2015;204(02):448–458. doi: 10.2214/AJR.14.12709. [DOI] [PubMed] [Google Scholar]
- 19.Koo S, Fan C M. Pelvic congestion syndrome and pelvic varicosities. Tech Vasc Interv Radiol. 2014;17(02):90–95. doi: 10.1053/j.tvir.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Ignacio E A, Dua R, Sarin S et al. Pelvic congestion syndrome: diagnosis and treatment. Semin Intervent Radiol. 2008;25(04):361–368. doi: 10.1055/s-0028-1102998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laborda A, Medrano J, de Blas I, Urtiaga I, Carnevale F C, de Gregorio M A. Endovascular treatment of pelvic congestion syndrome: visual analog scale (VAS) long-term follow-up clinical evaluation in 202 patients. Cardiovasc Intervent Radiol. 2013;36(04):1006–1014. doi: 10.1007/s00270-013-0586-2. [DOI] [PubMed] [Google Scholar]
- 22.Kim H S, Malhotra A D, Rowe P C, Lee J M, Venbrux A C.Embolotherapy for pelvic congestion syndrome: long-term results J Vasc Interv Radiol 200617(2, Pt 1):289–297. [DOI] [PubMed] [Google Scholar]
- 23.Dos Santos S J, Holdstock J M, Harrison C C, Lopez A J, Whiteley M S. Ovarian vein diameter cannot be used as an indicator of ovarian venous reflux. Eur J Vasc Endovasc Surg. 2015;49(01):90–94. doi: 10.1016/j.ejvs.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Nascimento A B, Mitchell D G, Holland G. Ovarian veins: magnetic resonance imaging findings in an asymptomatic population. J Magn Reson Imaging. 2002;15(05):551–556. doi: 10.1002/jmri.10098. [DOI] [PubMed] [Google Scholar]
- 25.Rozenblit A M, Ricci Z J, Tuvia J, Amis E S., Jr Incompetent and dilated ovarian veins: a common CT finding in asymptomatic parous women. AJR Am J Roentgenol. 2001;176(01):119–122. doi: 10.2214/ajr.176.1.1760119. [DOI] [PubMed] [Google Scholar]
- 26.Latthe P, Mignini L, Gray R, Hills R, Khan K.Factors predisposing women to chronic pelvic pain: systematic review BMJ 2006332(7544):749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stones R W, Price C. Health services for women with chronic pelvic pain. J R Soc Med. 2002;95(11):531–535. doi: 10.1258/jrsm.95.11.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheong Y C, Smotra G, Williams A C. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;3(03):CD008797. doi: 10.1002/14651858.CD008797.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman D G; PRISMA Group.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement PLoS Med 2009607e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meneses L, Fava M, Diaz P et al. Embolization of incompetent pelvic veins for the treatment of recurrent varicose veins in lower limbs and pelvic congestion syndrome. Cardiovasc Intervent Radiol. 2013;36(01):128–132. doi: 10.1007/s00270-012-0389-x. [DOI] [PubMed] [Google Scholar]
- 31.Capasso P, Simons C, Trotteur G, Dondelinger R F, Henroteaux D, Gaspard U. Treatment of symptomatic pelvic varices by ovarian vein embolization. Cardiovasc Intervent Radiol. 1997;20(02):107–111. doi: 10.1007/s002709900116. [DOI] [PubMed] [Google Scholar]
- 32.Maleux G, Stockx L, Wilms G, Marchal G. Ovarian vein embolization for the treatment of pelvic congestion syndrome: long-term technical and clinical results. J Vasc Interv Radiol. 2000;11(07):859–864. doi: 10.1016/s1051-0443(07)61801-6. [DOI] [PubMed] [Google Scholar]
- 33.Venbrux A C, Chang A H, Kim H Set al. Pelvic congestion syndrome (pelvic venous incompetence): impact of ovarian and internal iliac vein embolotherapy on menstrual cycle and chronic pelvic pain J Vasc Interv Radiol 200213(2, Pt 1):171–178. [DOI] [PubMed] [Google Scholar]
- 34.Pieri S, Agresti P, Morucci M, de' Medici L.Percutaneous treatment of pelvic congestion syndrome Radiol Med (Torino) 2003105(1-2):76–82. [PubMed] [Google Scholar]
- 35.Chung M H, Huh C Y. Comparison of treatments for pelvic congestion syndrome. Tohoku J Exp Med. 2003;201(03):131–138. doi: 10.1620/tjem.201.131. [DOI] [PubMed] [Google Scholar]
- 36.Kwon S H, Oh J H, Ko K R, Park H C, Huh J Y. Transcatheter ovarian vein embolization using coils for the treatment of pelvic congestion syndrome. Cardiovasc Intervent Radiol. 2007;30(04):655–661. doi: 10.1007/s00270-007-9040-7. [DOI] [PubMed] [Google Scholar]
- 37.Creton D, Hennequin L, Kohler F, Allaert F A. Embolisation of symptomatic pelvic veins in women presenting with non-saphenous varicose veins of pelvic origin - three-year follow-up. Eur J Vasc Endovasc Surg. 2007;34(01):112–117. doi: 10.1016/j.ejvs.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Asciutto G, Asciutto K C, Mumme A, Geier B. Pelvic venous incompetence: reflux patterns and treatment results. Eur J Vasc Endovasc Surg. 2009;38(03):381–386. doi: 10.1016/j.ejvs.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 39.Hocquelet A, Le Bras Y, Balian E et al. Evaluation of the efficacy of endovascular treatment of pelvic congestion syndrome. Diagn Interv Imaging. 2014;95(03):301–306. doi: 10.1016/j.diii.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Neĭmark A I, Shelkovnikova N V. Endovascular treatment of persistent dysuria and chronic pelvic pain in women with pelvic varicose veins [in Russian] Urologiia. 2012;4(04):20–24. [PubMed] [Google Scholar]
- 41.Nasser F, Cavalcante R N, Affonso B B, Messina M L, Carnevale F C, de Gregorio M A. Safety, efficacy, and prognostic factors in endovascular treatment of pelvic congestion syndrome. Int J Gynaecol Obstet. 2014;125(01):65–68. doi: 10.1016/j.ijgo.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Grossman S A, Sheidler V R, McGuire D B, Geer C, Santor D, Piantadosi S. A comparison of the Hopkins Pain Rating Instrument with standard visual analogue and verbal descriptor scales in patients with cancer pain. J Pain Symptom Manage. 1992;7(04):196–203. doi: 10.1016/0885-3924(92)90075-s. [DOI] [PubMed] [Google Scholar]
- 43.Galkin E V, Grakova L S, Naumova E B. Roentgeno-endovascular surgery of hypofunctional ovaries in varicosities of the ovarian veins [in Russian] Vestn Rentgenol Radiol. 1991;5(05):51–59. [PubMed] [Google Scholar]
- 44.Bittles M A, Hoffer E K. Gonadal vein embolization: treatment of varicocele and pelvic congestion syndrome. Semin Intervent Radiol. 2008;25(03):261–270. doi: 10.1055/s-0028-1085927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Practice Committee of the American Society for Reproductive Medicine.Report on varicocele and infertility Fertil Steril 2006860501S93–S95. [DOI] [PubMed] [Google Scholar]
- 46.Triebner K, Johannessen A, Puggini L et al. Menopause as a predictor of new-onset asthma: a longitudinal Northern European population study. J Allergy Clin Immunol. 2015;6749(15):1243–1249. doi: 10.1016/j.jaci.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 47.Asciutto G, Mumme A, Marpe B, Köster O, Asciutto K C, Geier B. MR venography in the detection of pelvic venous congestion. Eur J Vasc Endovasc Surg. 2008;36(04):491–496. doi: 10.1016/j.ejvs.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 48.Yang D M, Kim H C, Nam D H, Jahng G H, Huh C Y, Lim J W.Time-resolved MR angiography for detecting and grading ovarian venous reflux: comparison with conventional venography Br J Radiol 201285(1014):e117–e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giacchetto C, Cotroneo G B, Marincolo F, Cammisuli F, Caruso G, Catizone F. Ovarian varicocele: ultrasonic and phlebographic evaluation. J Clin Ultrasound. 1990;18(07):551–555. doi: 10.1002/jcu.1870180705. [DOI] [PubMed] [Google Scholar]
- 50.Liddle A D, Davies A H. Pelvic congestion syndrome: chronic pelvic pain caused by ovarian and internal iliac varices. Phlebology. 2007;22(03):100–104. doi: 10.1258/026835507780807248. [DOI] [PubMed] [Google Scholar]
- 51.Park S J, Lim J W, Ko Y T et al. Diagnosis of pelvic congestion syndrome using transabdominal and transvaginal sonography. AJR Am J Roentgenol. 2004;182(03):683–688. doi: 10.2214/ajr.182.3.1820683. [DOI] [PubMed] [Google Scholar]
- 52.Belenky A, Bartal G, Atar E, Cohen M, Bachar G N. Ovarian varices in healthy female kidney donors: incidence, morbidity, and clinical outcome. AJR Am J Roentgenol. 2002;179(03):625–627. doi: 10.2214/ajr.179.3.1790625. [DOI] [PubMed] [Google Scholar]


