Abstract
Postpartum hemorrhage (PPH) is the leading cause of maternal perinatal morbidity and mortality worldwide. Defined as greater than 500 mL blood loss after vaginal delivery, and greater than 1,000 mL blood loss after cesarean delivery, PPH has many causes, including uterine atony, lower genital tract lacerations, coagulopathy, and placental anomalies. Correction of coagulopathy and identification of the cause of bleeding are mainstays of treatment. Medical therapies such as uterotonics, balloon tamponade, pelvic artery embolization, and uterine-sparing surgical options are available. Hysterectomy is performed when conservative therapies fail. Pelvic artery embolization is safe and effective, and is the first-line therapy for medically refractory PPH. A thorough knowledge of pelvic arterial anatomy is critical. Recognition of variant anatomy can prevent therapeutic failure. Pelvic embolization is minimally invasive, has a low complication rate, spares the uterus, and preserves fertility.
Keywords: postpartum hemorrhage, uterine artery, embolization, fertility, interventional radiology
Objectives : Upon completion of this article, the reader will be able to discuss the role of pelvic artery embolization in the treatment of postpartum hemorrhage.
Obstetrical hemorrhage remains the leading cause of maternal perinatal morbidity and mortality worldwide, accounting for 25 to 30% of all maternal deaths, with an estimated 127,000 deaths annually. 1 2 3 4 5 The risk of a woman dying from postpartum hemorrhage (PPH) is 1:6 in developing nations, 1:30,000 in Northern Europe, and is 1:3,700 in the United States. 5 6 7 Data from the United Kingdom estimate that for each woman who dies from PPH, there are 60 peripartum hemostatic hysterectomies. 8 Recent systematic reviews estimate the prevalence of PPH greater than 500 mL to be 6.3 to 13% and greater than 1,000 mL to be 1.9 to 2.8% in North America and Europe. 9 10 PPH is becoming more prevalent in the United States due to unknown factors. 11
PPH is commonly defined as greater than 500 mL blood loss during and following vaginal delivery, and greater than 1,000 mL blood loss for cesarean section. 3 12 However, since visual estimates of blood loss are largely inaccurate, and can err by greater than 50%, 13 14 15 16 additional definitions have been proposed, such as decrease in hematocrit greater than 6%. 17 18 Primary PPH occurs within the first 24 hours after delivery, whereas secondary PPH occurs after 24 hours. 19
PPH is a condition that produces significant morbidity and death; complications include hypovolemic shock, disseminated intravascular coagulation (DIC), renal failure, hepatic failure, and acute respiratory distress syndrome. 20 Multiple conservative treatments and minimally invasive techniques including pelvic artery embolization (PAE) and uterine balloon tamponade aim to prevent these complications and have successfully and progressively reduced the rate of peripartum hysterectomy from 1/1,000 to 1/2,000 in the last 20 years. 21 Brown et al performed the first PAE for PPH in 1979 for a primiparous patient with severe PPH who had failed conservative measures including vaginal packing and laceration suturing, as well as bilateral hypogastric artery ligation. 22 PAE has been suggested as the first-line therapy in patients who are refractory to conservative measures. 23
Pathophysiology of Postpartum Hemorrhage
Pregnancy increases circulating blood volume by 50% to perfuse the low resistance uteroplacental unit and to compensate for blood loss at delivery. Upon delivery, contraction of the myometrium compresses the spiral arteries, leading to hemostasis. 18 24
The most common cause of PPH is failure of mechanical compression of the spiral arteries caused by uterine atony. 24 Uterine atony accounts for 70% of cases of primary PPH and often results in hemorrhagic shock, which can progress to endothelial damage and DIC. 25 26
Additional causes of PPH include retained products of conception, abnormally invasive placenta (AIP; placenta accreta/increta/percreta spectrum), uterine rupture, lower genital tract laceration, and coagulopathy. 27
Risk Factors
Identification of patients at risk for PPH improves diagnosis and treatment of the condition. Several risk factors have been discovered, including obesity, induction and augmentation of labor, chorioamnionitis, magnesium sulfate use, and previous PPH. 28 29 30 Other factors identified include placental abnormalities such as accreta and previa, overdistension of the uterus in macrosomia and polyhydramnios, grand multiparity, uterine structural abnormalities, preexisting coagulopathic anemias, and Asian ethnic descent. 31 32
Cesarean deliveries are at increased risk of PPH in the presence of general anesthesia, amnionitis, preeclampsia, protracted second phase of labor, and increasing age. Prior cesarean delivery also increases risk. 26 33
While the above-listed causes are seemingly disparate and numerous, the general causes are nicely summarized by the useful mnemonic the “Four Ts” that outlines common causes of PPH:
Tone: uterine atony, multiple pregnancy, prolonged third stage of labor, general anesthesia.
Trauma: lacerations of the uterus, cervix, or vagina, episiotomy.
Tissue: retained placenta, placenta accreta.
Thrombin: preexisting or acquired (DIC) coagulopathy. 31
Treatment Options
Several therapies exist for the treatment of PPH. The least invasive therapies are attempted initially, followed by more invasive maneuvers and procedures if hemorrhage persists. Treatment centers on resuscitation of the patient and stopping the source of bleeding. 34
Active management of the third stage of labor is standard practice in obstetrics, and consists of uterotonic administration such as oxytocin upon delivery of the baby, early cord clamping and cutting, and gentle cord traction with uterine counter traction. 5 This has been shown to reduce the incidence of PPH by 60 to 70%. 35 The antifibrinolytic tranexamic acid has shown mortality benefit when given within the first 3 hours after hemorrhage. 36 Recombinant factor VIIa may be beneficial, though thrombotic complications must be weighed and routine use is not recommended. 31 37 Additional conservative therapies include uterine massage, vaginal packing, uterine balloon tamponade (Bakri balloon), and repair of lower genital tract lacerations. 34 Balloon tamponade has shown clinical success rates ranging from 75 to 86%. 38 39 40 After integration of balloon tamponade into the PPH treatment algorithm, Laas et al noted decrease in embolization rates from 8.2 to 2.3%. 38
When conservative measures fail, uterine-sparing surgical intervention and PAE are the next line of therapy. Surgical options include stepwise uterine devascularization and uterine compression sutures; however, arterial ligation has a historically high rate of failure due to the rich collateral vasculature of the female pelvis. 41 42 More recent data cite clinical success rates of uterine-sparing surgery ranging from 71 to 75%. 39 43 44 A meta-analysis by Doumouchtsis showed surgical options were not more effective than PAE. 45 Surgical options also carry the additional risks of bleeding, infection, and ureteral damage. 46
Pelvic Artery Embolization
Patient Selection
PAE is a safe and effective treatment for PPH and is now the first-line therapy for medically refractory PPH. 47 48 49 50 51 PAE is indicated for women with PPH who have failed conservative management for any cause of primary or secondary PPH ( Fig. 1 ). A recent review found no difference in PAE effectiveness in vaginal deliveries or cesarean deliveries. 48 Notably, PAE is also an effective therapy in patients with persistent PPH even after arterial ligation or hysterectomy. Procedural success is not related to the cause of PPH, transfusion requirements, or time from delivery to embolization. 19 47 50 51 52 53 It is our observation from years of practice at a variety of hospitals that PAE remains an underutilized method of control of PPH.
Fig. 1.
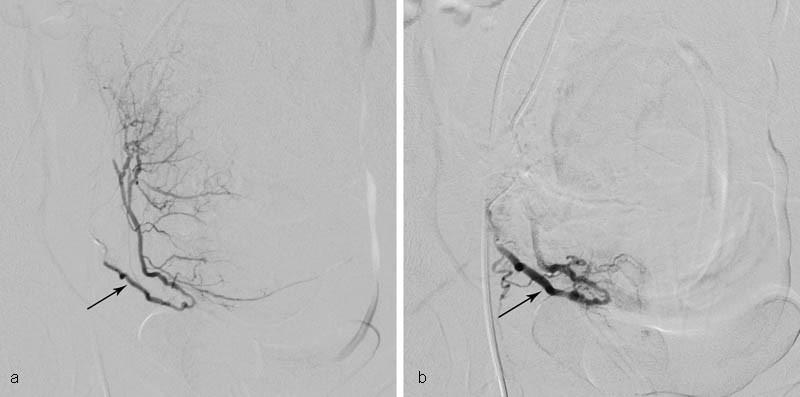
Medically refractory postpartum hemorrhage from uterine atony in a 16-year-old hemodynamically unstable female who presented by ambulance with estimated 2,350 mL blood loss after dilation and evacuation abortion at 22.5 weeks. The patient was refractory to multiple medical therapies as well as Bakri balloon tamponade and vaginal packing. She was brought emergently to IR, where angiography and selective bilateral uterine artery embolization were performed. Clinical success was attained, and the patient had an uneventful postprocedural course. ( a ) Selective right uterine artery (arrow) angiography before embolization. Contrast extravasation was not appreciated; such lack of extravasation is commonly the case. ( b ) Right uterine artery angiography after embolization with gelatin sponge pledgets to stasis (arrow).
Normal Anatomy
The internal iliac artery typically bifurcates and gives rise to anterior and posterior trunks. The anterior trunk gives rise to visceral branches, including the uterine artery. The uterine artery usually arises from the medial aspect of the anterior trunk. Also arising from the anterior trunk are the superior vesical, middle rectal, inferior rectal, and vaginal arteries. The uterine artery gives rise to arcuate arteries, which extend into the myometrium. The arcuate arteries supply radial arteries that project toward the uterine cavity. The spiral arteries arise from the radial arteries, and project into the endometrium. 24 Anatomic variants of the uterine artery origin are common. A rich collateral supply exists in the female pelvis. Anastomoses are routinely noted between the right and left internal iliac arteries, and between the internal iliac and inferior mesenteric arteries, lumbar, iliolumbar, and sacral arteries. 24
The venous plexus runs parallel to the arterial tree, and opacifies late. This should not be misidentified as contrast extravasation. 24
Variant Anatomy
Anatomic variations are often discovered in cases of PAE failure. 54 The uterine artery may originate from the anterior or lateral aspects of the internal iliac artery, from the main internal iliac artery, or directly from the aorta. 55 A common trunk may exist, supplying the uterine and vesical arteries; recognition of this variant is essential to prevent nontarget embolization of the bladder. 56
The ovarian artery may also supply the uterus. While not routinely embolized, interrogation of the ovarian artery is crucial in cases of refractory or recurrent PPH. 57 58 The uterus may also be supplied by the round ligament artery. 59 60 The round ligament artery commonly arises from the inferior epigastric artery or directly from the external iliac artery, and represents a collateral pathway between the external iliac and internal iliac circulation. 56 59 60 61
Procedural Technique
There are many equally effective ways to perform uterine artery embolization; our preferred method follows. Right common femoral artery access is obtained and a vascular sheath is placed. A SOS Omni catheter (AngioDynamics, Latham, NY) is used to cross the aortic bifurcation. A Glidecath catheter (Terumo, Tokyo, Japan) is advanced over the aortic bifurcation and a Waltman loop 62 is formed. The Glidecath is directed into the anterior division of the internal iliac artery. Angiography is performed to confirm catheter position, and identify the origin of the uterine artery. A microcatheter (Maestro; Merit Medical, South Jordan, UT; or Renegade Hi Flo; Boston Scientific, Marlborough, MA) and microwire (Headliner 90; Terumo) are used to select the uterine artery. Embolization is performed with gelatin sponge pledgets (Gelfoam; Pharmacia & Upjohn, Kalamazoo, MI) and/or Embospheres (500–700 or 700–900 µm; Merit Medical). The microcatheter is removed, and the base catheter is redirected into the contralateral internal iliac artery. Embolization of the contralateral uterine and internal iliac is performed. Postembolization pelvic angiography is performed to confirm adequacy of embolization and to identify collateral or accessory vasculature.
Selective uterine artery embolization is strongly preferred as efficacy of hemostasis is markedly improved, but in cases of severe vasoconstriction, embolization of the anterior division of the internal iliac artery may be considered. 48 Cervical and vaginal branches should be interrogated in the case of genital tract laceration. 48 56 63 Iatrogenic pseudoaneurysm should be suspected in patients with secondary PPH after cesarean delivery ( Fig. 2 ). 19 Persistent bleeding after embolization of the uterine arteries warrants investigation of the ovarian and round ligament arteries. 61
Fig. 2.

Infected uterine artery pseudoaneurysm causing secondary postpartum hemorrhage in a 32-year-old woman who underwent cesarean delivery. Primary postpartum hemorrhage attributed to uterine atony was treated conservatively with medications and transfusion. The patient presented to the emergency department 10 days after delivery with fevers, leukocytosis, and hematuria. ( a ) Transvaginal ultrasound demonstrated a uterine artery pseudoaneurysm (arrow). ( b ) CTA confirms left uterine artery pseudoaneurysm (arrow). ( c ) Selective left uterine artery angiography immediately prior to embolization shows large pseudoaneurysm (arrow) arising from the mid to distal left uterine artery. ( d ) Left internal iliac arteriography after embolization with gelatin sponge pledgets to stasis. There is no residual filling of the pseudoaneurysm. The procedure was clinically successful, and the patient recovered after a course of antibiotics.
Procedural Advantages and Disadvantages
PAE is a minimally invasive procedure that can be performed under moderate sedation. Advantages of PAE include shorter hospital stay, preservation of fertility, and avoidance of surgical risks. 48 50 Menstruation is not affected by PAE. 64 The overall complication rate of PAE is low, ranging from 6 to 9% in the hands of an experienced interventional radiologist. 19 65 66
Risks and disadvantages of PAE exist. Patients are usually transported to the interventional radiology suite, limiting treatment of unstable patients who are unable to travel. Embolization ostensibly carries risks such as necrosis of the bladder, uterus, and rectum; transient neurologic sequelae such as paresthesia have been described. 49 50 56 67 Virtually all are related to operator inexperience or nonselective internal iliac artery embolization rather than superselective uterine artery treatment. The risk of uterine necrosis is greater with small particles, as was described when the uterus was embolized with gelatin sponge and 150 to 250 µm polyvinyl alcohol (PVA) particles. 68
Embolic Materials
Gelatin sponge is the most common embolic material used in the treatment of PPH, and is commonly used to embolize both uterine arteries or both internal iliac arteries. 48 49 50 69 Gelatin sponge can be cut into pledgets or torpedoes. 48 69 However, it should be noted that gelatin sponge relies on the clotting cascade, and is functionally impaired in the setting of DIC. 70 Gelatin sponge temporarily occludes the target vessel, which recanalizes in approximately 3 to 6 weeks. 23
Calibrated microspheres and PVA are commonly used in conjunction with gelatin sponge. Microspheres leave the capillary bed intact, and there is limited potential for recanalization. 66 To avoid uterine necrosis, 500 to 900 µm particles are preferred; necrosis has been described with both absorbable and nonabsorbable embolics. 68 71 72
N -butyl cyanoacrylate has been rarely used for PAE and is said to be useful in cases where total vessel occlusion is necessary, such as recurrent PPH after PAE or pseudoaneurysm. 70 73 Pregnancy and birth has been demonstrated after NBCA embolization of the uterine arteries. 74 Onyx (ev3, Irvine, CA) has also been successfully used for PPH refractory to gelatin sponge embolization. 75 Metallic coils are useful in treating pseudoaneurysms. 19
Outcomes
Clinical success is defined as control of bleeding without the need for additional procedures. 19 A recent meta-analysis by Sathe et al demonstrated clinical success rates of PAE ranging from 58 to 98% with median success rate of 89%. 76 Reembolization after failed PAE has also been described with similar success. 54 77
Predictors of Failure
Several factors have been identified in patients who have failed PAE. DIC has been singled out as the most important predictor of failure. 49 50 54 77 Patients with variant anatomy and prominent collateral supply such as ovarian and round ligament arteries are more likely to experience persistent hemorrhage after bilateral uterine artery embolization, as are those patients with retained placenta and advanced maternal age. 58 61 78
Future Fertility
A major advantage of PAE is that it spares the uterus, allowing for future pregnancy if desired. Pregnancy rates following uterine artery embolization are comparable to age-adjusted rates in the general population. 79 Pregnancy and childbirth remains possible even in the face of multiple procedures. Sentilhes et al described a case of a woman who had successful pregnancy and childbirth after four different uterine-sparing procedures including PAE. 80 Recent studies suggest a small but increased incidence of placental disorders and recurrent PPH after PAE. 80 81 82 83 Spontaneous abortion rate was also found to be higher in patients treated with PAE for PPH in one study. 84 None of these retrospective studies have been validated by prospective data; it is our opinion that PAE for PPH produces little or no effect on future fertility.
Prophylactic PAE for Abnormally Invasive Placenta
Abnormally invasive placenta is a spectrum including placenta accreta, increta, and percreta, and is a key risk factor for the development of PPH. 85 Improvements in prenatal ultrasound diagnosis of AIP has made it possible to perform PAE immediately after scheduled cesarean section, thereby avoiding PPH-related complications. 86 Several different approaches have been suggested for prophylactic intervention to prevent AIP-related PPH. These include balloon occlusion of the internal iliac arteries, combined balloon occlusion and embolization, prophylactic catheter placement for later embolization if necessary, embolization followed by hysterectomy, and embolization immediately prior to scheduled cesarean delivery. 23 85 86 87 88 89 90 No single prophylactic method has been shown to be clearly superior to date.
Conclusion
PPH is prevalent and is a frequent cause of morbidity and mortality in new mothers. PAE is a safe and effective minimally invasive treatment for PPH when conservative measures fail and should be an integral part of the modern care of the obstetric patient.
References
- 1.Say L, Chou D, Gemmill A et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(06):e323–e333. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 2.Devine P C. Obstetric hemorrhage. Semin Perinatol. 2009;33(02):76–81. doi: 10.1053/j.semperi.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 3.WHO.WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage Geneva: World Health Organisation; 2012 [PubMed] [Google Scholar]
- 4.WHO, UNFPA, The World Bank. Trends in Maternal Mortality: 1990 to 2010 - WHO, UNICEF, UNFPA and the World Bank Estimates;2012
- 5.Kumar N. Postpartum hemorrhage; a major killer of woman: review of current scenario. Obstet Gynecol Int J. 2016;4(04):116. [Google Scholar]
- 6.Ronsmans C, Graham W J; Lancet Maternal Survival Series steering group.Maternal mortality: who, when, where, and why Lancet 2006368(9542):1189–1200. [DOI] [PubMed] [Google Scholar]
- 7.Karoshi M, Keith L. Challenges in managing postpartum hemorrhage in resource-poor countries. Clin Obstet Gynecol. 2009;52(02):285–298. doi: 10.1097/GRF.0b013e3181a4f737. [DOI] [PubMed] [Google Scholar]
- 8.Knight M, Kurinczuk J J, Spark P, Brocklehurst P; United Kingdom Obstetric Surveillance System Steering Committee.Cesarean delivery and peripartum hysterectomy Obstet Gynecol 20081110197–105. [DOI] [PubMed] [Google Scholar]
- 9.Carroli G, Cuesta C, Abalos E, Gulmezoglu A M. Epidemiology of postpartum haemorrhage: a systematic review. Best Pract Res Clin Obstet Gynaecol. 2008;22(06):999–1012. doi: 10.1016/j.bpobgyn.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Calvert C, Thomas S L, Ronsmans C, Wagner K S, Adler A J, Filippi V. Identifying regional variation in the prevalence of postpartum haemorrhage: a systematic review and meta-analysis. PLoS One. 2012;7(07):e41114. doi: 10.1371/journal.pone.0041114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callaghan W M, Kuklina E V, Berg C J. Trends in postpartum hemorrhage: United States, 1994-2006. Am J Obstet Gynecol. 2010;202(04):3530–3.53E8. doi: 10.1016/j.ajog.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Rath W H. Postpartum hemorrhage–update on problems of definitions and diagnosis. Acta Obstet Gynecol Scand. 2011;90(05):421–428. doi: 10.1111/j.1600-0412.2011.01107.x. [DOI] [PubMed] [Google Scholar]
- 13.Kavle J A, Khalfan S S, Stoltzfus R J, Witter F, Tielsch J M, Caulfield L E. Measurement of blood loss at childbirth and postpartum. Int J Gynaecol Obstet. 2006;95(01):24–28. doi: 10.1016/j.ijgo.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Stafford I, Dildy G A, Clark S L, Belfort M A. Visually estimated and calculated blood loss in vaginal and cesarean delivery. Am J Obstet Gynecol. 2008;199(05):5190–5.19E9. doi: 10.1016/j.ajog.2008.04.049. [DOI] [PubMed] [Google Scholar]
- 15.Schorn M N. Measurement of blood loss: review of the literature. J Midwifery Womens Health. 2010;55(01):20–27. doi: 10.1016/j.jmwh.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Conner S N, Tuuli M G, Colvin R, Shanks A L, Macones G A, Cahill A G. Accuracy of estimated blood loss in predicting need for transfusion after delivery. Am J Perinatol. 2015;32(13):1225–1230. doi: 10.1055/s-0035-1552940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tita A T, Szychowski J M, Rouse D Jet al. Higher-dose oxytocin and hemorrhage after vaginal delivery: a randomized controlled trial Obstet Gynecol 2012119(2, Pt 1):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunningham F, Leveno K J, Bloom S L . New York, NY: McGraw-Hill; 2013. Obstetrical hemorrhage; pp. 780–828. [Google Scholar]
- 19.Ganguli S, Stecker M S, Pyne D, Baum R A, Fan C M. Uterine artery embolization in the treatment of postpartum uterine hemorrhage. J Vasc Interv Radiol. 2011;22(02):169–176. doi: 10.1016/j.jvir.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 20.Bonnar J. Massive obstetric haemorrhage. Best Pract Res Clin Obstet Gynaecol. 2000;14(01):1–18. doi: 10.1053/beog.1999.0060. [DOI] [PubMed] [Google Scholar]
- 21.Flood K M, Said S, Geary M, Robson M, Fitzpatrick C, Malone F D. Changing trends in peripartum hysterectomy over the last 4 decades. Am J Obstet Gynecol. 2009;200(06):6320–6.32E8. doi: 10.1016/j.ajog.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Brown B J, Heaston D K, Poulson A M, Gabert H A, Mineau D E, Miller F J., Jr Uncontrollable postpartum bleeding: a new approach to hemostasis through angiographic arterial embolization. Obstet Gynecol. 1979;54(03):361–365. [PubMed] [Google Scholar]
- 23.Gonsalves M, Belli A. The role of interventional radiology in obstetric hemorrhage. Cardiovasc Intervent Radiol. 2010;33(05):887–895. doi: 10.1007/s00270-010-9864-4. [DOI] [PubMed] [Google Scholar]
- 24.Abada H T, Golzarian J, Sun S. Berlin, Germany: Springer; 2006. Interventional management of postpartum hemorrhage. In: Vascular Embolotherapy: A Comprehensive Approach, Vol 1; pp. 107–118. [Google Scholar]
- 25.Bick R L. Syndromes of disseminated intravascular coagulation in obstetrics, pregnancy, and gynecology. Objective criteria for diagnosis and management. Hematol Oncol Clin North Am. 2000;14(05):999–1044. doi: 10.1016/s0889-8588(05)70169-6. [DOI] [PubMed] [Google Scholar]
- 26.Combs C A, Murphy E L, Laros R K., Jr Factors associated with postpartum hemorrhage with vaginal birth. Obstet Gynecol. 1991;77(01):69–76. [PubMed] [Google Scholar]
- 27.Chichakli L O, Atrash H K, MacKay A P, Musani A S, Berg C J.Pregnancy-related mortality in the United States due to hemorrhage: 1979–1992 Obstet Gynecol 199994(5, Pt 1):721–725. [DOI] [PubMed] [Google Scholar]
- 28.Jackson K W, Jr, Allbert J R, Schemmer G K, Elliot M, Humphrey A, Taylor J. A randomized controlled trial comparing oxytocin administration before and after placental delivery in the prevention of postpartum hemorrhage. Am J Obstet Gynecol. 2001;185(04):873–877. doi: 10.1067/mob.2001.117363. [DOI] [PubMed] [Google Scholar]
- 29.Stones R W, Paterson C M, Saunders N J. Risk factors for major obstetric haemorrhage. Eur J Obstet Gynecol Reprod Biol. 1993;48(01):15–18. doi: 10.1016/0028-2243(93)90047-g. [DOI] [PubMed] [Google Scholar]
- 30.Blomberg M. Maternal obesity and risk of postpartum hemorrhage. Obstet Gynecol. 2011;118(03):561–568. doi: 10.1097/AOG.0b013e31822a6c59. [DOI] [PubMed] [Google Scholar]
- 31.Mavrides E, Allard S, Chandraharan E et al. Prevention and management of postpartum haemorrhage. BJOG. 2017;124(05):e106–e149. doi: 10.1111/1471-0528.14178. [DOI] [PubMed] [Google Scholar]
- 32.Magann E F, Evans S, Hutchinson M, Collins R, Howard B C, Morrison J C. Postpartum hemorrhage after vaginal birth: an analysis of risk factors. South Med J. 2005;98(04):419–422. doi: 10.1097/01.SMJ.0000152760.34443.86. [DOI] [PubMed] [Google Scholar]
- 33.Ohkuchi A, Onagawa T, Usui R et al. Effect of maternal age on blood loss during parturition: a retrospective multivariate analysis of 10,053 cases. J Perinat Med. 2003;31(03):209–215. doi: 10.1515/JPM.2003.028. [DOI] [PubMed] [Google Scholar]
- 34.Horng H C, Hu W M, Tseng H S, Chang W H, Chao K C, Yang M J. Uterine arterial embolization in the management of severe post-partum hemorrhage: a successful rescue method to avoid peripartum hysterectomy. J Chin Med Assoc. 2011;74(06):255–258. doi: 10.1016/j.jcma.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Begley C M, Gyte G M, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2015;(03):CD007412. doi: 10.1002/14651858.CD007412.pub4. [DOI] [PubMed] [Google Scholar]
- 36.Shakur H, Roberts I, Fawole Bet al. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trialLancet. Published online, April 26, 2017. Available at:http://thelancet.com/pdfs/journals/lancet/PIIS0140-6736(17)30638-4. Accessed April 27, 2017 [DOI] [PMC free article] [PubMed]
- 37.Franchini M, Franchi M, Bergamini V et al. The use of recombinant activated FVII in postpartum hemorrhage. Clin Obstet Gynecol. 2010;53(01):219–227. doi: 10.1097/GRF.0b013e3181cc4378. [DOI] [PubMed] [Google Scholar]
- 38.Laas E, Bui C, Popowski T, Mbaku O M, Rozenberg P. Trends in the rate of invasive procedures after the addition of the intrauterine tamponade test to a protocol for management of severe postpartum hemorrhage. Am J Obstet Gynecol. 2012;207(04):2810–2.81E9. doi: 10.1016/j.ajog.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 39.Chan L L, Lo T K, Lau W L et al. Use of second-line therapies for management of massive primary postpartum hemorrhage. Int J Gynaecol Obstet. 2013;122(03):238–243. doi: 10.1016/j.ijgo.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 40.Ferrazzani S, Iadarola R, Perrelli A et al. Use of an intrauterine inflated catheter balloon in massive post-partum hemorrhage: a series of 52 cases. J Obstet Gynaecol Res. 2014;40(06):1603–1610. doi: 10.1111/jog.12404. [DOI] [PubMed] [Google Scholar]
- 41.Evans S, McShane P. The efficacy of internal iliac artery ligation in obstetric hemorrhage. Surg Gynecol Obstet. 1985;160(03):250–253. [PubMed] [Google Scholar]
- 42.Clark S L, Phelan J P, Yeh S Y, Bruce S R, Paul R H. Hypogastric artery ligation for obstetric hemorrhage. Obstet Gynecol. 1985;66(03):353–356. [PubMed] [Google Scholar]
- 43.Kayem G, Kurinczuk J J, Alfirevic Z, Spark P, Brocklehurst P, Knight M. Specific second-line therapies for postpartum haemorrhage: a national cohort study. BJOG. 2011;118(07):856–864. doi: 10.1111/j.1471-0528.2011.02921.x. [DOI] [PubMed] [Google Scholar]
- 44.Kayem G, Kurinczuk J J, Alfirevic Z, Spark P, Brocklehurst P, Knight M; U.K. Obstetric Surveillance System (UKOSS).Uterine compression sutures for the management of severe postpartum hemorrhage Obstet Gynecol 20111170114–20. [DOI] [PubMed] [Google Scholar]
- 45.Doumouchtsis S K, Papageorghiou A T, Arulkumaran S. Systematic review of conservative management of postpartum hemorrhage: what to do when medical treatment fails. Obstet Gynecol Surv. 2007;62(08):540–547. doi: 10.1097/01.ogx.0000271137.81361.93. [DOI] [PubMed] [Google Scholar]
- 46.Sentilhes L, Gromez A, Clavier E, Resch B, Verspyck E, Marpeau L. Predictors of failed pelvic arterial embolization for severe postpartum hemorrhage. Obstet Gynecol. 2009;113(05):992–999. doi: 10.1097/AOG.0b013e3181a114f7. [DOI] [PubMed] [Google Scholar]
- 47.Sentilhes L, Vayssière C, Deneux-Tharaux C et al. Postpartum hemorrhage: guidelines for clinical practice from the French College of Gynaecologists and Obstetricians (CNGOF): in collaboration with the French Society of Anesthesiology and Intensive Care (SFAR) Eur J Obstet Gynecol Reprod Biol. 2016;198:12–21. doi: 10.1016/j.ejogrb.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 48.Lee H J, Jeon G S, Kim M D, Kim S H, Lee J T, Choi M J. Usefulness of pelvic artery embolization in cesarean section compared with vaginal delivery in 176 patients. J Vasc Interv Radiol. 2013;24(01):103–109. doi: 10.1016/j.jvir.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 49.Kim Y J, Yoon C J, Seong N J et al. Failed pelvic arterial embolization for postpartum hemorrhage: clinical outcomes and predictive factors. J Vasc Interv Radiol. 2013;24(05):703–709. doi: 10.1016/j.jvir.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 50.Lee H Y, Shin J H, Kim J et al. Primary postpartum hemorrhage: outcome of pelvic arterial embolization in 251 patients at a single institution. Radiology. 2012;264(03):903–909. doi: 10.1148/radiol.12111383. [DOI] [PubMed] [Google Scholar]
- 51.Hwang S M, Jeon G S, Kim M D, Kim S H, Lee J T, Choi M J. Transcatheter arterial embolisation for the management of obstetric haemorrhage associated with placental abnormality in 40 cases. Eur Radiol. 2013;23(03):766–773. doi: 10.1007/s00330-012-2612-1. [DOI] [PubMed] [Google Scholar]
- 52.Fargeaudou Y, Morel O, Soyer P et al. Persistent postpartum haemorrhage after failed arterial ligation: value of pelvic embolisation. Eur Radiol. 2010;20(07):1777–1785. doi: 10.1007/s00330-010-1713-y. [DOI] [PubMed] [Google Scholar]
- 53.Kirby J M, Kachura J R, Rajan D K et al. Arterial embolization for primary postpartum hemorrhage. J Vasc Interv Radiol. 2009;20(08):1036–1045. doi: 10.1016/j.jvir.2009.04.070. [DOI] [PubMed] [Google Scholar]
- 54.Bros S, Chabrot P, Kastler A et al. Recurrent bleeding within 24 hours after uterine artery embolization for severe postpartum hemorrhage: are there predictive factors? Cardiovasc Intervent Radiol. 2012;35(03):508–514. doi: 10.1007/s00270-011-0181-3. [DOI] [PubMed] [Google Scholar]
- 55.Worthington-Kirsch R L. Anatomy of the uterine artery. AJR Am J Roentgenol. 2000;174(01):258. doi: 10.2214/ajr.174.1.1740258. [DOI] [PubMed] [Google Scholar]
- 56.Pelage J P, Le Dref O, Soyer P et al. Arterial anatomy of the female genital tract: variations and relevance to transcatheter embolization of the uterus. AJR Am J Roentgenol. 1999;172(04):989–994. doi: 10.2214/ajr.172.4.10587133. [DOI] [PubMed] [Google Scholar]
- 57.Razavi M K, Wolanske K A, Hwang G L, Sze D Y, Kee S T, Dake M D. Angiographic classification of ovarian artery-to-uterine artery anastomoses: initial observations in uterine fibroid embolization. Radiology. 2002;224(03):707–712. doi: 10.1148/radiol.2243011513. [DOI] [PubMed] [Google Scholar]
- 58.Wang M Q, Liu F Y, Duan F, Wang Z J, Song P, Song L. Ovarian artery embolization supplementing hypogastric-uterine artery embolization for control of severe postpartum hemorrhage: report of eight cases. J Vasc Interv Radiol. 2009;20(07):971–976. doi: 10.1016/j.jvir.2009.04.049. [DOI] [PubMed] [Google Scholar]
- 59.Saraiya P V, Chang T C, Pelage J P, Spies J B.Uterine artery replacement by the round ligament artery: an anatomic variant discovered during uterine artery embolization for leiomyomata J Vasc Interv Radiol 200213(9, Pt 1):939–941. [DOI] [PubMed] [Google Scholar]
- 60.Wi J Y, Kim H C, Chung J W, Jun J K, Jae H J, Park J H. Importance of angiographic visualization of round ligament arteries in women evaluated for intractable vaginal bleeding after uterine artery embolization. J Vasc Interv Radiol. 2009;20(08):1031–1035. doi: 10.1016/j.jvir.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Leleup G, Fohlen A, Dohan A et al. Value of round ligament artery embolization in the management of postpartum hemorrhage. J Vasc Interv Radiol. 2017;28(05):696–701. doi: 10.1016/j.jvir.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 62.Waltman A C, Courey W R, Athanasoulis C, Baum S. Technique for left gastric artery catheterization. Radiology. 1973;109(03):732–734. doi: 10.1148/109.3.732. [DOI] [PubMed] [Google Scholar]
- 63.Naydich M, Friedman A, Aaron G, Silberzweig J. Arterial embolization of vaginal arterial branches for severe postpartum hemorrhage despite hysterectomy. J Vasc Interv Radiol. 2007;18(08):1047–1050. doi: 10.1016/j.jvir.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 64.Salomon L J, deTayrac R, Castaigne-Meary V et al. Fertility and pregnancy outcome following pelvic arterial embolization for severe post-partum haemorrhage. A cohort study. Hum Reprod. 2003;18(04):849–852. doi: 10.1093/humrep/deg168. [DOI] [PubMed] [Google Scholar]
- 65.Deux J F, Bazot M, Le Blanche A F et al. Is selective embolization of uterine arteries a safe alternative to hysterectomy in patients with postpartum hemorrhage? AJR Am J Roentgenol. 2001;177(01):145–149. doi: 10.2214/ajr.177.1.1770145. [DOI] [PubMed] [Google Scholar]
- 66.Pelage J P, Le Dref O, Mateo J et al. Life-threatening primary postpartum hemorrhage: treatment with emergency selective arterial embolization. Radiology. 1998;208(02):359–362. doi: 10.1148/radiology.208.2.9680559. [DOI] [PubMed] [Google Scholar]
- 67.Poujade O, Ceccaldi P F, Davitian C et al. Uterine necrosis following pelvic arterial embolization for post-partum hemorrhage: review of the literature. Eur J Obstet Gynecol Reprod Biol. 2013;170(02):309–314. doi: 10.1016/j.ejogrb.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 68.Cottier J P, Fignon A, Tranquart F, Herbreteau D.Uterine necrosis after arterial embolization for postpartum hemorrhage Obstet Gynecol 2002100(5, Pt 2):1074–1077. [DOI] [PubMed] [Google Scholar]
- 69.Hardeman S, Decroisette E, Marin B et al. Fertility after embolization of the uterine arteries to treat obstetrical hemorrhage: a review of 53 cases. Fertil Steril. 2010;94(07):2574–2579. doi: 10.1016/j.fertnstert.2010.02.052. [DOI] [PubMed] [Google Scholar]
- 70.Matsubara S, Sato T, Nakata M. Vaginal artery embolization with a permanent embolic agent for intractable postpartum hemorrhage. J Obstet Gynaecol Res. 2011;37(04):377–378. doi: 10.1111/j.1447-0756.2011.01527.x. [DOI] [PubMed] [Google Scholar]
- 71.Coulange L, Butori N, Loffroy R et al. Uterine necrosis following selective embolization for postpartum hemorrhage using absorbable material. Acta Obstet Gynecol Scand. 2009;88(02):238–240. doi: 10.1080/00016340802596041. [DOI] [PubMed] [Google Scholar]
- 72.Tseng J J, Ho J Y, Wen M C, Hwang J I. Uterine necrosis associated with acute suppurative myometritis after angiographic selective embolization for refractory postpartum hemorrhage. Am J Obstet Gynecol. 2011;204(06):e4–e6. doi: 10.1016/j.ajog.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 73.Park K J, Shin J H, Yoon H K, Gwon D I, Ko G Y, Sung K B. Postpartum hemorrhage from extravasation or pseudoaneurysm: efficacy of transcatheter arterial embolization using N-butyl cyanoacrylate and comparison with gelatin sponge particle. J Vasc Interv Radiol. 2015;26(02):154–161. doi: 10.1016/j.jvir.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 74.Igarashi S, Izuchi S, Ishizuka B, Yoshimatu M, Takizawa K. A case of pregnancy and childbirth after uterine artery embolization with a permanent embolic agent. Fertil Steril. 2011;95(01):2.9E11–2.9E13. doi: 10.1016/j.fertnstert.2010.04.081. [DOI] [PubMed] [Google Scholar]
- 75.Pardo M I, Trujillo M V, Campos S et al. Successful uterine artery embolization for intractable postpartum hemorrhage using a novel embolic agent: ONYX. J Gynecol Surg. 2012;28(04):309–311. [Google Scholar]
- 76.Sathe N A, Likis F E, Young J L, Morgans A, Carlson-Bremer D, Andrews J. Procedures and uterine-sparing surgeries for managing postpartum hemorrhage: a systematic review. Obstet Gynecol Surv. 2016;71(02):99–113. doi: 10.1097/OGX.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 77.Cheong J Y, Kong T W, Son J H, Won J H, Yang J I, Kim H S. Outcome of pelvic arterial embolization for postpartum hemorrhage: a retrospective review of 117 cases. Obstet Gynecol Sci. 2014;57(01):17–27. doi: 10.5468/ogs.2014.57.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamasaki Y, Morita H, Miyahara Y et al. The factors associated with the failure of transcatheter pelvic arterial embolization for intractable postpartum hemorrhage. J Perinat Med. 2014;42(03):359–362. doi: 10.1515/jpm-2013-0242. [DOI] [PubMed] [Google Scholar]
- 79.Mohan P P, Hamblin M H, Vogelzang R L. Uterine artery embolization and its effect on fertility. J Vasc Interv Radiol. 2013;24(07):925–930. doi: 10.1016/j.jvir.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 80.Sentilhes L, Gromez A, Marpeau L. Fertility after pelvic arterial embolization, stepwise uterine devascularization, hypogastric artery ligation, and B-Lynch suture to control postpartum hemorrhage. Int J Gynaecol Obstet. 2010;108(03):249. doi: 10.1016/j.ijgo.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 81.Poggi S H, Yaeger A, Wahdan Y, Ghidini A. Outcome of pregnancies after pelvic artery embolization for postpartum hemorrhage: retrospective cohort study. Am J Obstet Gynecol. 2015;213(04):5760–5.76E7. doi: 10.1016/j.ajog.2015.06.063. [DOI] [PubMed] [Google Scholar]
- 82.Gizzo S, Saccardi C, Patrelli T S et al. Fertility rate and subsequent pregnancy outcomes after conservative surgical techniques in postpartum hemorrhage: 15 years of literature. Fertil Steril. 2013;99(07):2097–2107. doi: 10.1016/j.fertnstert.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 83.Doumouchtsis S K, Nikolopoulos K, Talaulikar V, Krishna A, Arulkumaran S. Menstrual and fertility outcomes following the surgical management of postpartum haemorrhage: a systematic review. BJOG. 2014;121(04):382–388. doi: 10.1111/1471-0528.12546. [DOI] [PubMed] [Google Scholar]
- 84.Holub Z, Mara M, Kuzel D, Jabor A, Maskova J, Eim J. Pregnancy outcomes after uterine artery occlusion: prospective multicentric study. Fertil Steril. 2008;90(05):1886–1891. doi: 10.1016/j.fertnstert.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 85.Niola R, Giurazza F, Nazzaro G et al. Uterine artery embolization before delivery to prevent postpartum hemorrhage. J Vasc Interv Radiol. 2016;27(03):376–382. doi: 10.1016/j.jvir.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 86.Diop A N, Chabrot P, Bertrand A et al. Placenta accreta: management with uterine artery embolization in 17 cases. J Vasc Interv Radiol. 2010;21(05):644–648. doi: 10.1016/j.jvir.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 87.Lee J S, Shepherd S M. Endovascular treatment of postpartum hemorrhage. Clin Obstet Gynecol. 2010;53(01):209–218. doi: 10.1097/GRF.0b013e3181ce09f5. [DOI] [PubMed] [Google Scholar]
- 88.Angstmann T, Gard G, Harrington T, Ward E, Thomson A, Giles W. Surgical management of placenta accreta: a cohort series and suggested approach. Am J Obstet Gynecol. 2010;202(01):380–3.8E10. doi: 10.1016/j.ajog.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 89.Li Q, Yang Z Q, Mohammed W, Feng Y L, Shi H B, Zhou X. Prophylactic uterine artery embolization assisted cesarean section for the prevention of intrapartum hemorrhage in high-risk patients. Cardiovasc Intervent Radiol. 2014;37(06):1458–1463. doi: 10.1007/s00270-014-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mok M, Heidemann B, Dundas K, Gillespie I, Clark V. Interventional radiology in women with suspected placenta accreta undergoing caesarean section. Int J Obstet Anesth. 2008;17(03):255–261. doi: 10.1016/j.ijoa.2007.11.010. [DOI] [PubMed] [Google Scholar]


