Abstract
Proliferative responsiveness of hepatocytes to epidermal growth factor (EGF) declines during aging. The role of EGF receptors in mediating age-dependent changes of EGF-induced mitogenic signaling in liver remains incompletely understood. We assessed EGF receptor expression levels in whole liver specimens as well as in freshly isolated and cultured hepatocytes from young adult and senescent Fischer 344 male rats. Hepatic EGF receptor messenger RNA and protein levels, and the number of high- and low-affinity receptor binding sites, decreased with aging. Ligand-induced EGF receptor activation, determined by receptor dimerization and tyrosine phosphorylation, was reduced in old animals in parallel with the age-related decline in receptor expression. Stimulation of the extracellular signal-regulated kinase pathway by EGF was also attenuated in hepatocytes from old animals. Our results implicate decreased expression of EGF receptors as a key determinant of reduced mitogenic signaling responsive to EGF stimulation of liver during aging.
Keywords: Extracellular signal-regulated kinase, Hepatocytes, Receptor dimerization, Receptor tyrosine kinase
Cellular proliferation is triggered by a variety of intracellular events responsive to growth factors binding to their cognate receptors. A number of growth factors, including epidermal growth factor (EGF), exert their mitogenic effects by binding to receptor tyrosine kinases coupled to stimulation of the mitogen-activated protein kinase (MAP kinase)/extracellular signal-regulated kinase (ERK) signal transduction pathway (1–3). The EGF receptor (ErbB1/HER1), a member of a family of receptor tyrosine kinases that also includes ErbB2/HER2, ErbB3/HER3, and ErbB4/HER4, is a ;170 kd transmembrane glycoprotein comprising an extracellular ligand-binding region, a single-transmembrane helix, and a cytoplasmic domain containing intrinsic tyrosine kinase activity (2,4,5). Activation of the EGF receptor occurs via a process of ligand-induced receptor dimerization, facilitating autophosphorylation of tyrosine residues in the cytoplasmic domain (4–6). The phosphorylation sites of the activated receptor recruit adapter proteins such as Shc and Grb2, which in turn activate downstream signals leading to stimulation of the MAP kinase/ERK cascade (1–3,5). Previous studies have revealed high- and low-affinity states of the EGF receptor and have suggested that high-affinity binding is associated with the dimerized form of the receptor (7,8).
The proliferative capacity of several tissues, including the liver, declines with age (9–13). Regeneration of rat liver following partial hepatectomy or other liver damage is reduced during senescence (12,14–16). Although liver regeneration in rats has been widely used as a model of age-related alterations in proliferative responsiveness, the molecular mechanisms underlying decreased hepatocellular proliferation or regeneration with age are incompletely understood. EGF receptor activation plays a critical role in liver regeneration (17–19). In hepatocytes from senescent rats, EGF-induced activation of DNA synthesis and mitogenic signaling via the ERK pathway is markedly lower than in cells from young adult animals (12,20,21). Previous studies of hepatic EGF receptor expression during aging have been limited and have yielded conflicting results (11,12,14,20). In the present work, we have used a variety of measures to assess EGF receptor expression and activation in whole liver specimens and in freshly isolated and cultured hepatocytes from young and old Fischer 344 male rats. Our results demonstrate an age-related loss of EGF receptor expression linked to decreased receptor activation and mitogenic signaling.
MATERIALS AND METHODS
Materials
All tissue culture reagents were obtained from Gibco-BRL (Gaithersburg, MD). BioCoat collagen-coated plates were purchased from Becton Dickinson (Franklin Lakes, NJ). TRI Reagent was obtained from Molecular Research Center (Cincinnati, OH). DNAse I, random hexamer primers, and Complete Mini tablets were obtained from Roche Diagnostics (Indianapolis, IN). Superscript RTIII, 5× First strand buffer, dithiothreitol (DTT), and recombinant human EGF were purchased from Invitrogen (Carlsbad, CA). TaqMan Gene Expression Assays (EGF receptor, 18S), TaqMan Universal Master Mix, nucleotides, magnesium chloride, and plasticware required for performing real-time reverse transcription–polymerase chain reaction (RT–PCR) were obtained from Applied Biosystems (ABI, Foster City, CA). The bicinchoninic acid (BCA) assay and enhanced chemiluminescence (ECL) kit were obtained from Pierce (Rockford, IL). Polyvinylidene fluoride (PVDF) membranes were obtained from GE Osmonics (Minnetonka, MN). Bradford protein assay reagents were purchased from BioRad Laboratories (Hercules, CA). [125I]EGF (50 μCi/mL) and the ECL Advance kit were purchased from Amersham Biosciences (Piscataway, NJ). Whatman glass fiber filters (GF/C) were obtained from Brandel (Gaithersburg, MD). Antibodies specific for EGF receptor and phospho-EGF receptor (pEGF receptor) (p1173) were ordered from Santa Cruz Biotechnology (Santa Cruz, CA); ERK and phospho-ERK (pERK) antibodies were obtained from Cell Signaling Technology (Danvers, MA). Appropriate peroxidase-labeled secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Animals
Young adult (4–7 months) and senescent (24–26 months) specific pathogen-free (SPF) Fischer 344 male rats were obtained from the National Institute on Aging. Upon receipt, the animals were housed singly in an SPF barrier facility within the Veterinary Medical Unit of the Audie L. Murphy Memorial Veterans Hospital (ALMMVH), San Antonio, Texas; rats were maintained for at least 1 week prior to use. Animals were treated in accordance with the guidelines approved by the Institutional Animal Care and Use Committee at the ALMMVH.
Methods
Preparation of liver samples
Rats were killed by exsanguination after anesthesia as previously described (22). The livers were rapidly removed and cut into pieces, which were quick-frozen in liquid nitrogen and stored at −80°C until use.
Isolation of hepatocytes
Hepatocytes were isolated from young and old rats, as described previously (23) with modification. Briefly, the animals were anesthetized using pentobarbital sodium (65 mg intraperitoneal injection per kg body weight); the livers were perfused in situ with calcium-free Earle’s Balanced Salt Solution (EBSS), pH 7.4, followed by calcium-free EBSS containing 0.05% collagenase (Type I), pH 7.4. Hepatocytes from collagenase-perfused livers were suspended in calcium-containing EBSS, filtered through a nylon mesh, and washed twice by low-speed centrifugation (52 g at 4°C for 2 minutes; Sorvall RT7 centrifuge). Freshly isolated hepatocytes were then suspended in Williams’ Medium E. Cell viability and yield were determined by trypan blue dye exclusion.
Cell culture
Freshly isolated hepatocytes were resuspended in Williams’ Medium E containing 1% glutamine and 1% penicillin/streptomycin and were plated on collagen-coated dishes in the absence or presence of 5% fetal bovine serum (FBS) and 10−9 M dexamethasone. The cells were plated at a density of 3 × 106 cells/100-mm dish at 37°C in a humidified 5% CO2 atmosphere. Two hours after plating, the cells were washed and fresh William’s Medium E containing glutamine and antibiotics was added to the plates. Cells that were previously exposed to FBS and dexamethasone were continued in culture in the presence of 10−9 M dexamethasone but without serum. The cells were cultured for an additional 24–72 hours prior to use.
Real-time RT–PCR
Total RNA was isolated from frozen liver pieces, freshly isolated hepatocytes, or cultured hepatocytes using TRI Reagent according to the manufacturer’s instructions. The RNA samples were treated with DNAse I (RNAse-free) in the presence of 4.2 mM MgCl2 in a standard thermocycler (37°C/30 minutes, 75°C/10 minutes, 4°C). The RNA samples were then reverse transcribed in the presence of 5× First strand buffer, 10 mM DTT, random hexamer primers, and SuperScript RTIII. The complementary DNA (cDNA) synthesis was carried out in a thermocycler at 25°C/10 minutes, 42°C/50 minutes, 72°C/10 minutes, and 4°C. Real-time RT–PCR was then performed with the TaqMan Gene Expression Assay for rat EGF receptor using an ABI 7700 Sequence Detection System. In each experiment, messenger RNA (mRNA) levels were normalized to 18S RNA, which did not change with age (data not shown).
Western blotting
Frozen liver pieces or hepatocytes (freshly isolated or cultured) were homogenized in lysis buffer (50 mM NaCl, 1% Nonidet P-40 [NP-40], 50 mM Tris-HCl, pH 7.4) containing protease and phosphatase inhibitors. The homogenates were rocked at 4°C for 30 minutes, followed by centrifugation at 10,000 × g for 2 minutes at 4°C. The supernatant protein was estimated by BCA; protein concentration (μg/μL) from cultured cells was unaffected by culture conditions. Protein samples (40–70 μg) were added to 10 μL of 4× sample buffer (150 mM Tris-HCl, pH 8.8, 1% sodium dodecyl sulfate [SDS], 40% glycerol) and β-mercaptoethanol and then diluted with lysis buffer to a total volume of 40 μL. The samples were fractionated on 8% SDS–polyacrylamide gel electrophoresis (PAGE) gels and electroblotted overnight onto 0.45 μm PVDF membranes. The membranes were immunoblotted with a primary antibody (1:500 dilution for EGF receptor, ERK, and phospho-ERK; 1:250 dilution for phospho-EGF receptor) and a secondary horseradish peroxidase–conjugated antibody (1:10,000). Specific proteins were visualized using an ECL kit, and immunoblots were quantified with Scion Image analysis software (Scion Corporation, Frederick, MD).
Measurement of EGF receptor binding
EGF receptor binding in liver membrane preparations was measured by an equilibrium binding assay using [125I]EGF as the radio-ligand, as described by Earp and O’Keefe (18) with modifications. Briefly, frozen liver pieces were placed in 20 vol (wet wt/vol) of tissue buffer (0.154 M NaCl, 20 mM Na-HEPES, pH 7.5) and homogenized in a Brinkmann polytron (Brinkmann Instruments, Westbury, NY) using two 10-second bursts at setting 6. The homogenates were centrifuged at 5000 g for 15 minutes at 4°C, and the pellets were resuspended in 40 vol (original wet wt/vol) tissue buffer. Protein estimation was carried out by the method of Bradford. For binding studies, tissue suspensions (70–160 μg of protein) were incubated with [125I]EGF in 250 μL of reaction buffer (0.116 M NaCl, 15.2 mM Na-HEPES, pH 7.5) for 30 minutes at 37°C with shaking. Reactions were terminated by adding 5 mL of wash buffer (0.154 M NaCl, 0.01 M Tris-HCl, pH 7.5) at room temperature, and membrane-bound radioligand was collected on Whatman glass fiber filters (GF/C) with a Brandel Cell Harvester (Biomedical Research and Development Laboratories, Gaithersburg, MD). Nonspecific binding of [125I]EGF was determined as the amount of radioligand bound in the presence of excess (10−4 M) EGF. Saturation binding curves were constructed by measuring specific binding of [125I]EGF at 11 concentrations of radioligand over the range of 0.1–2 nM.
Native gel electrophoresis
Frozen liver tissue pieces were homogenized in lysis buffer in the absence of NP-40 (50 mM NaCl, 50 mM Tris-HCl, pH 7.4 with protease and phosphatase inhibitors). The homogenates were incubated in the absence or presence of EGF (100 ng/mL) at 37°C for 10 minutes, followed by centrifugation at 15,294 g for 2 minutes at 4°C (Eppendorf centrifuge 5417R). The supernatant proteins (100 μg) were fractionated by electrophoresis on a 5% polyacrylamide gel lacking SDS. The gels were then electroblotted overnight onto 0.45-μm PVDF membranes. Blots were incubated with antibodies against EGF receptor and reactive bands detected by an ECL Advance kit after incubation with peroxidase-labeled secondary antibody.
Cross-linking experiments
Freshly isolated hepatocytes suspended in William’s Medium E were incubated at 37°C in the absence or presence of EGF (100 ng/mL) for 10 minutes with shaking. At the end of the incubation period, the cells were exposed to the cross-linking reagent BS3 (1.3 mM) for 30 minutes at room temperature. Cells were then washed three times in ice-cold phosphate-buffered saline and homogenized to prepare samples for Western blotting as described above.
Data analysis
Data from multiple experiments are expressed as means ± standard error (SE). Statistical significance of single comparisons was determined by Student’s t test. Multiple comparisons were performed by analysis of variance (ANOVA) followed by Newman–Keuls Multiple Comparison Test. [125I]EGF saturation binding data were plotted according to the method of Scatchard, and were analyzed by a weighted, least-squares curve-fitting program (24) to calculate the number and affinity of [125I]EGF binding sites.
RESULTS
EGF Receptor Expression in Rat Liver During Aging
EGF receptor expression, as assessed by levels of mRNA, immunoreactive protein, and radioligand binding, was determined in liver tissue specimens from young and old Fischer 344 male rats. Real-time RT–PCR revealed that steady-state levels of EGF receptor mRNA declined by 40% with age (p = .006; Figure 1). Consistent with this finding, EGF receptor protein levels measured by Western blotting of liver tissue lysates decreased by 54% in old rats (p < .001; Figure 2A). Protein levels of ERKs were used as loading controls in Western blots, as ERK levels were found to remain unchanged with age (Figure 2B). Additionally, immunoblotting experiments using liver specimens from rats over the entire adult life span indicated that the decline in EGF receptor protein levels with age occurred primarily during senescence, i.e., between 18 and 24 months of age (Figure 2C).
Figure 1.

Epidermal growth factor (EGF) receptor messenger RNA (mRNA) expression decreases with age in rat liver. A, EGF receptor mRNA levels in liver specimens from individual young (n = 10) and old (n = 9) Fischer 344 male rats were determined by real-time reverse transcription–polymerase chain reaction (RT–PCR), as described in Materials and Methods. mRNA levels in each specimen (mean values ± standard error [SE] from triplicate determinations) were normalized to 18S in the same sample and expressed as fold change relative to the normalized value from a single animal (calibrator). B, Values represent mean EGF receptor mRNA levels ± SE from 10 young and 9 old rats (individual values shown in A). *p = .006 vs young rats.
Figure 2.
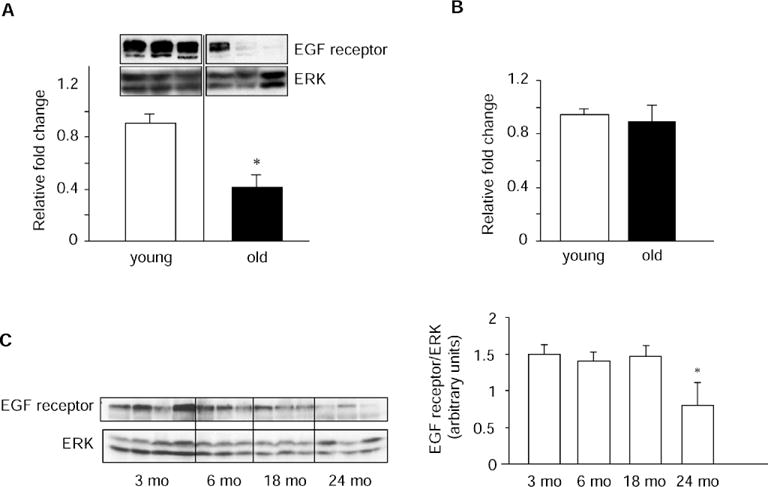
Epidermal growth factor (EGF) receptor protein levels in rat liver decline during senescent aging. A, EGF receptor protein levels were measured by Western blotting of liver tissue lysates from young (n =11) and old (n = 10) rats, as described in Materials and Methods. Immunoblots were quantified by densitometric analysis, and EGF receptor values were normalized to loading control (extracellular signal-regulated kinase [ERK]) for each sample. Bar graphs represent means ± standard error (SE) of normalized EGF receptor/ERK values expressed as fold change relative to the normalized value from a single animal (calibrator). *p < .001 vs young rats. Inset: A representative immunoblot showing EGF receptor and ERK protein levels in three young and three old animals. In our experiments, immunoreactive EGF receptors migrated on denaturing gels as one or two bands of about 170 kd; migration of EGF receptors as doublets has been observed by others using Western blot analysis (5,26). B, Mean (±SE) ERK protein levels in liver lysates used for EGF receptor protein determinations. C, Immunoreactive EGF receptor and ERK protein levels were measured in liver lysates from 3-, 6-, 18-, and 24-month-old rats (n = 3–4 rats in each age group). Bar graphs represent mean values ± SE of EGF receptor normalized to ERK at each age; *p < .05 vs younger ages.
Radioligand binding experiments revealed greater [125I]EGF binding to liver membrane preparations from young rats compared to that to membranes from old rats (Figure 3, A and B). Scatchard analysis of [125I]EGF binding to liver membranes from young animals revealed concave-upward curvilinear plots, indicative of the presence of both high- and low-affinity EGF receptor binding sites (with dissociation constants KH and KL of about 50 pm and 2 nM, respectively), with 93% of the sites displaying low-affinity binding. In contrast, in liver preparations from old rats, high-affinity binding was undetectable and the number of low-affinity binding sites declined by 74% (Figure 3, C and D). The apparent binding affinity of the EGF receptor population remaining in the liver preparations from old rats (KL < 1 nM) was significantly increased (p <.001) in comparison with the low-affinity receptor population from the young rats (Figure 3D).
Figure 3.

Epidermal growth factor (EGF) receptor binding in rat liver decreases with age. EGF receptor binding in liver membrane preparations from young and old rats was measured using [125I]EGF as the radioligand. A, Representative experiment in which [125I]EGF saturation binding curves were generated using liver membrane preparations from one young and one old rat. Scatchard plots of saturation binding data from the representative experiment are shown in B. C and D, Curve-fitting analysis of Scatchard plots was used to calculate the density (number) and affinity of [125I]EGF binding sites in liver membranes from young and old rats. EGF receptor binding was best fit by a two-site model in livers from young rats and a one-site model in livers from old rats. Values are means ± standard error (SE) from nine rats in each age group. RH and RL (C), number of sites binding [125I]EGF with high and low affinity, respectively; KH and KL (D), dissociation constants of high- and low-affinity binding sites. ND = not detected.
EGF Receptor Expression in Freshly Isolated and Cultured Hepatocytes From Aging Rats
To extend the results obtained with liver tissue, we compared EGF receptor expression in hepatocytes from young and old rats. Receptor protein and mRNA levels were determined in freshly isolated hepatocytes and, for comparison with earlier studies of hepatic EGF receptors during aging, in primary hepatocyte cultures. Cells were cultured in the absence and presence of FBS and dexamethasone, which are frequently used to promote proliferation, survival, and function of hepatocytes.
Immunoreactive EGF receptor protein levels in freshly isolated hepatocytes and cells cultured with or without serum and dexamethasone exhibited a 40%–60% decline with age (Figure 4), a change equivalent to that observed in liver tissue homogenates (Figure 2A). In other experiments directly comparing immunoreactive EGF receptor content over time in culture, we observed that EGF receptor levels in hepatocytes from both young and old rats declined by about 50% at 24 hours (without serum and dexamethasone) relative to levels in freshly isolated cells. Culturing for longer periods (up to 72 hours) did not result in any further decline in immunoreactive receptor content at either age. Real-time RT–PCR measurements revealed a 30%–40% decrease of EGF receptor mRNA levels in freshly isolated and cultured hepatocytes from old rats compared to cells from young animals (data not shown). Taken together, the results of the receptor protein and mRNA determinations in liver tissue homogenates and hepatocytes, and radioligand binding assays in liver tissue preparations, all strongly implicate an age-related decrease of EGF receptor expression in rat liver.
Figure 4.

Epidermal growth factor (EGF) receptor protein levels in freshly isolated and cultured rat hepatocytes decrease with age. Freshly isolated and 24-hour cultured (±5% fetal bovine serum [FBS], 10−9 M dexamethasone [Dex]) hepatocytes from young (n = 3) and old (n = 3) rats were used to prepare lysates for determination of EGF receptor and extracellular signal-regulated kinase (ERK) protein levels by Western blotting, as described in Materials and Methods. A, Immunoblots showing EGF receptor and ERK protein levels in freshly isolated and cultured hepatocytes. B, The immunoblots shown in (A) were subjected to densitometric analysis, and EGF receptor values were normalized to ERK for each sample. Bar graphs represent means ± standard error (SE) of EGF receptor/ERK protein values. *p = .047; **p = .014 vs corresponding values from young rats.
Ligand-Induced EGF Receptor Dimerization and Phosphorylation in Rat Liver During Aging
Upon ligand binding the EGF receptor is activated by a process of receptor dimerization leading to autophosphorylation of tyrosine residues in the cytoplasmic domain (4–6). The dimerized, active form of the receptor is generally considered to represent the state of the receptor binding EGF with high affinity (7,8). Because we observed an age-dependent loss of high-affinity EGF receptor binding sites in rat liver membrane preparations (Figure 3), we performed experiments to determine the effects of age on EGF receptor dimerization and phosphorylation (Figure 5). Liver homogenates from young and old rats were incubated in the absence or presence of EGF (100 ng/mL), subjected to native gel electrophoresis, and then immunoblotted using an EGF receptor-specific antibody. As shown in Figure 5A, ligand-induced receptor dimerization was readily apparent in liver homogenates from both young and old rats. Densitometric analysis indicated that the percentage of total receptor protein in the dimerized form was equivalent in EGF-treated homogenates from young and old rats (37.13 ± 0.03% in young, n = 5; 36.65 ± 0.04% in old, n = 5). However, the levels of receptor dimers induced by EGF were lower in preparations from old rats (Figure 5A), consistent with the age-related decline in receptor expression observed in other experiments (Figures 1–4). A small amount of receptor dimerization was also observed in untreated homogenates, perhaps reflecting the effect of endogenous EGF levels.
Figure 5.

Epidermal growth factor (EGF) causes dimerization and phosphorylation of hepatic EGF receptors in young and old rats. A, Liver tissue homogenates from young and old rats were treated with EGF (100 ng/mL) or vehicle for 10 minutes at 37°C and then subjected to native gel electrophoresis as described in Materials and Methods. Representative gels from one young and one old rat are shown. Extracellular signal-regulated kinase (ERK) protein levels in the homogenates were used as loading controls. B, Freshly isolated hepatocytes from young and old rats were incubated with or without EGF (100 ng/mL) for 10 minutes at 37°C, followed by addition of the cell-impermeable cross-linker BS3 (1.3 mM) or vehicle for 30 minutes at room temperature. EGF receptor, phospho-EGF receptor (pEGF receptor) (p1173), and ERK protein levels in cell lysates were determined by Western blotting, as described in Materials and Methods. In both tissue homogenates and hepatocyte lysates, EGF receptor monomers and dimers were detected at about 170 kd and 360 kd, respectively.
To confirm and extend these observations in liver homogenates, we performed experiments in which hepatocytes from young and old rats were incubated with or without EGF followed by addition of the cell-impermeable cross-linking reagent BS3 or vehicle (8). Figure 5B shows that, at both ages, EGF-induced formation of receptor dimers was detected by Western blot analysis in cells treated with the cross-linker, although levels of receptor dimers in hepatocytes from old rats were lower than in cells from young animals. As in tissue homogenates (Figure 5A), densitometric analysis revealed an equivalent ratio of dimers to total receptor protein (30%–36%) in EGF-treated hepatocytes from young and old animals (Figure 5B). In cells untreated with BS3, no dimers were detected even in the presence of EGF, presumably reflecting separation of dimers into monomers upon SDS electrophoresis. EGF treatment also induced marked phosphorylation of the EGF receptor, as detected by an anti-phospho-EGF receptor antibody (p1173), in hepatocytes from both young and old animals; phosphorylated dimers were apparent in the BS3-treated samples (Figure 5B). The degree of ligand-induced receptor phosphorylation measured by densitometric analysis decreased by nearly 50% with age, in parallel with the age-related decline in total receptor levels. Interestingly, low levels of EGF receptor dimerization were detected in hepatocytes incubated with BS3 in the absence of EGF (Figure 5B). This finding is in agreement with other studies in cell lines showing that EGF receptor dimerization can occur in the absence of ligand (8).
EGF Receptor-Induced ERK Activation in Rat Hepatocytes During Aging
Ligand-induced activation of the EGF receptor leads to stimulation of the downstream MAP kinase/ERK cascade (1–3,5). We performed immunoblotting experiments to evaluate levels of activated, that is, phosphorylated ERK1/2 upon EGF treatment of freshly isolated and cultured hepatocytes from young and old rats (Figure 6). Densitometric analysis of the immunoblots demonstrated that EGF-induced ERK activation declined markedly (by 70%–90%) with age in all hepatocyte preparations tested. The results therefore suggest that reduced EGF receptor expression during aging (Figures 1–4) contributes to a decrease in mitogenic signaling by EGF in livers of old rats.
Figure 6.

Epidermal growth factor (EGF)-induced activation of extracellular signal-regulated kinase (ERK) in rat hepatocytes decreases with age. Hepatocytes were isolated from young and old rats and either used immediately (Fresh) or cultured in the absence or presence of fetal bovine serum (FBS, 5%) and dexamethasone (Dex, 10−9 M) for 48 hours, as described in Materials and Methods. Freshly isolated or cultured hepatocytes were treated with EGF (3 ng/mL) for 10 minutes at 37°C, and phospho-ERK (pERK) and ERK protein levels were determined in cell lysates by Western blotting. Representative immunoblots from freshly isolated and cultured hepatocytes are shown.
DISCUSSION
EGF receptor activation in the liver is a hallmark of hepatocellular regenerative capacity, which is impaired with aging (12,14,21). In the present study we have demonstrated for the first time that EGF receptor expression as measured by levels of receptor mRNA, protein, and radioligand binding decreases with senescent aging in rat liver homogenates and hepatocytes. EGF receptors from livers of old rats undergo ligand-induced dimerization and phosphorylation, although the extent of these processes measurable in liver preparations from aged animals reflects the decrease in receptor expression occurring with senescence. Furthermore, EGF receptor-induced activation of downstream signaling through the mitogenic ERK pathway is also reduced with aging in rat liver.
Our results extend earlier observations by others of EGF receptor expression and function in rat liver during aging. Using liver tissue homogenates and both freshly isolated and cultured hepatocytes from young adult and senescent Fischer 344 male rats, we determined that EGF receptor mRNA and immunoreactive protein levels decline by 40%–50% with age (Figures 1, 2, and 4). To our knowledge, hepatic EGF receptor mRNA levels have not been studied previously as a function of age, nor has densitometric analysis of immunoblots been used to quantify age-related changes of EGF receptor expression in whole liver specimens or hepatocytes. In earlier studies of cultured rat hepatocytes, other investigators reported no observable change of immunoreactive EGF receptor protein levels with age (12,14). However, these investigators used rat strains other than the Fischer 344 model as well as culture conditions that included variable concentrations of hormone, that is, glucocorticoid with or without insulin (12,14,20). High concentrations of glucocorticoid (equivalent to >10−8 M dexamethasone) are known to increase and/or stabilize EGF receptor numbers in hepatocytes maintained over time in culture (25,26). Whether addition of glucocorticoid or insulin to culture medium exerts different effects on EGF receptor expression in hepatocytes obtained from young versus old animals has not been evaluated. In our experiments, EGF receptor protein levels in hepatocytes decreased with age to about the same extent irrespective of whether cells were freshly isolated or cultured in the absence or presence of low concentrations of dexamethasone (10−9 M) and serum. The results of this study therefore indicate that primary hepatocyte cultures are a valid model system for further studies of altered hepatic expression of EGF receptors during aging.
Consistent with the age-related decline in EGF receptor mRNA and protein levels, we also found that receptor numbers determined by radioligand binding in liver membrane preparations are markedly reduced with age. Quantitative binding experiments in numerous tissues and cell types reveal the existence of EGF receptors with two distinct affinities for ligand—a minority of receptors binding EGF with high affinity (KH = 10–100 pM) and a majority with low-affinity binding (KL = 2–5 nM) (6). In the present study, both high- and low-affinity forms of the EGF receptor (with KH and KL values of about 50 pM and 2 nM, respectively) were evident in liver membranes from young rats, whereas in old rats only low-affinity receptors were detected albeit in decreased numbers (Figure 3). Our binding results are generally in accord with earlier work by Marti (11), who observed an age-related decline of EGF binding in rat liver plasma membranes; in this previous study binding data in both young and old animals were best fit to a one-site model by curve-fitting analysis. The findings in liver membranes are in apparent contrast to a previous report by other investigators, in which EGF receptor binding in cultured rat hepatocytes did not change with age (20). In this latter study, hepatocytes from Wistar rats were cultured in the presence of low concentrations of serum and dexamethasone, after which radioligand binding to whole cells revealed a single class of low-affinity binding sites (KD ; 1 nM) in young and old animals. Interestingly, under a variety of culture conditions, hepatocytes from young rats exhibit a time-dependent loss of high-affinity EGF receptors, accompanied in some reports by a decrease of low-affinity receptors as well (27–29). It remains to be determined whether age-dependent changes of binding observed in liver membrane preparations but not in some studies of cultured hepatocytes may reflect altered binding characteristics occurring during prolonged culture.
Functional and crystallographic analyses of the wild-type EGF receptor and receptor mutants have demonstrated that EGF receptor dimerization is linked to high-affinity binding and receptor tyrosine kinase activation (5,7,8). Using native gel electrophoresis and cross-linking experiments, we observed ligand-induced dimer formation in liver homogenates and hepatocytes from young and old rats, although in old animals the level of dimerization was decreased commensurate with reduced receptor expression during aging (Figure 5). In view of these results, the failure to detect any high-affinity binding sites in liver from old rats (Figure 3) may have been due to a decline of the small fraction of high-affinity receptors to a level below the detection limit of the radioligand binding assay. The KD of the single class of sites detected at advanced age was intermediate between the apparent binding affinities of the high- and low-affinity forms of the receptor in young rats, suggesting the retention of some high-affinity binding with age. Decreased ligand-induced EGF receptor dimerization with age was also accompanied by a decline in receptor phosphorylation at tyrosine 1173. Recently others have reported an age-dependent loss of high-affinity EGF receptor binding in cultured hepatocytes in association with reduced receptor dimerization and tyrosine phosphorylation (30). Our work using rat liver tissue homogenates as well as freshly isolated and cultured hepatocytes is the first to link age-related decreases in the critical elements of EGF responsive receptor activation—induction of high-affinity binding, dimerization, and phosphorylation—to a reduction of receptor expression.
Phosphorylated tyrosine residues in the cytoplasmic domain of the activated EGF receptor serve as high-affinity binding sites for a number of intracellular signaling proteins (e.g., Shc) containing Src homology 2 (SH2) or phosphotyrosine binding (PTB) motifs. These “adapter” proteins then trigger activation of mitogenic signaling pathways such as the MAP kinase/ERK cascade. The 1173 phosphotyrosine residue in the carboxy terminal tail of the activated EGF receptor has been identified as a binding site for both PTB and SH2 domains of the Shc adapter protein (31,32). Our present results (Figure 6) and work by others (12,14,21) demonstrate that EGF-induced activation of ERK in rat liver declines with age. Moreover, in earlier studies of cultured rat hepatocytes, phosphorylation at tyrosine residue 1173 and association of Shc with the EGF receptor after EGF stimulation were found to be reduced during aging (12,14). Previous investigators reporting no age-related decrease in EGF receptor expression attributed the decline in EGF signaling with age to altered phosphorylation of specific receptor tyrosine site(s) involved in Shc binding (12,14). Our own findings in liver tissue and hepatocyte preparations implicate an alternative mechanism, that is, an age-related decrease in the expression of hepatic EGF receptors available for ligand-induced activation and interaction with intracellular signaling proteins. It should be noted here that in our study EGF-induced ERK activation declined with age to a greater extent than did EGF receptor expression or activation, suggesting that additional factors also influence changes in hepatic ERK activity during aging. In this context, increased activity of MAP kinase phosphatases and decreased phospho-ERK nuclear translocation have been reported to play a role in reducing ERK signaling during aging and cellular senescence (21,33–35).
Summary
We show for the first time that decreased expression of EGF receptors is a primary determinant of reduced mitogenic signaling and cellular proliferation responsive to EGF stimulation of liver during aging. Liver regeneration following partial hepatectomy or other hepatic injury is delayed and decreased during senescent aging (12,15,16). Because ligands (e.g., EGF and transforming growth factor-α [TGF-α]) binding to the EGF receptor appear to play a major regulatory role in hepatocellular regeneration (17,19), our results suggest that reduced EGF receptor expression may contribute to the depressed regenerative response at advanced age. Ligand binding and activation of EGF receptors in regenerating liver induce receptor endocytosis and down-regulation (18,36). Whereas no changes in circulating levels of EGF or TGF-α have been consistently observed over the adult life span, alterations of hepatic processing of EGF (11) or EGF availability in the portal circulation could conceivably reduce EGF receptor numbers in the liver of aged animals through modification of receptor trafficking and degradation. However, this mechanism probably does not account for the age-related decline in hepatic EGF receptor expression observed in our study, in which decreased receptor mRNA levels with age suggest regulation at the transcriptional level. In recent microarray analyses using RNA from livers of young and old rats (data not shown), we have observed age-related changes in a number of transcription factors (RARα, c-jun, egr-1) that regulate EGF receptor expression (37–39); the same analyses confirmed the results of the current study showing a decrease in EGF receptor expression with age. Interestingly, a decline in EGF receptor content, as occurs during aging, is demonstrable not only in regenerating liver but also under a number of other conditions including hepatocarcinogenesis (40), cholestasis (41), hypothyroidism (42), and early postnatal development (43). Additional investigations into the regulation of hepatic EGF receptor expression are currently being performed to elucidate the mechanisms underlying the control of hepatocellular proliferation during normal aging and in response to pathological and physiological perturbations.
Acknowledgments
This work was supported by a University Research Council Grant from The University of Texas Health Science Center at San Antonio (to A. Kamat), medical research funds from the Department of Veterans Affairs (to C.-K. Yeh, G. Ghosh Choudhury, and M.S. Katz), grants from the National Institutes of Health (RO1 DK 50190) and the Juvenile Diabetes Research Foundation (to G. Ghosh Choudhury), and a research grant from the Kronos Longevity Research Institute (to M. S. Katz). G. Ghosh Choudhury is a Research Career Scientist in the Department of Veteran Affairs.
We gratefully acknowledge Gertrude Kokkonen, Lenin Mahimainathan, and Balachander Venkatesan for their expert technical advice. We also thank YuFei Huang and Jianqiu Zhang for conducting the microarray analyses.
References
- 1.Pierce KL, Luttrell LM, Lefkowitz RJ. New mechanisms in heptahelical receptor signaling to mitogen activated protein kinase cascades. Oncogene. 2001;20:1532–1539. doi: 10.1038/sj.onc.1204184. [DOI] [PubMed] [Google Scholar]
- 2.Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–672. doi: 10.1016/s0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- 3.Skarpen E, Oksvold MP, Grosvik H, Widnes C, Huitfeldt HS. Altered regulation of EGF receptor signaling following a partial hepatectomy. J Cell Physiol. 2005;202:707–716. doi: 10.1002/jcp.20171. [DOI] [PubMed] [Google Scholar]
- 4.Hubbard SR. EGF receptor activation: push comes to shove. Cell. 2006;125:1029–1031. doi: 10.1016/j.cell.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;25:1137–1149. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Klein P, Mattoon D, Lemmon MA, Schlessinger J. A structure-based model for ligand binding and dimerization of EGF receptors. Proc Natl Acad Sci U S A. 2004;101:929–934. doi: 10.1073/pnas.0307285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorokin A, Lemmon MA, Ulrich A, Schlessinger J. Stabilization of an active dimeric form of the epidermal growth factor receptor by introduction of an inter-receptor disulfide bond. J Biol Chem. 1994;269:9752–9759. [PubMed] [Google Scholar]
- 8.Walker F, Orchard SG, Jorissen RN, et al. CR1/CR2 interactions modulate the functions of the cell surface epidermal growth factor receptor. J Biol Chem. 2004;279:22387–22398. doi: 10.1074/jbc.M401244200. [DOI] [PubMed] [Google Scholar]
- 9.Atadja PW, Stringer KF, Riabowol KT. Loss of serum response element-binding activity and hyperphosphorylation of serum response factor during cellular aging. Mol Cell Biol. 1994;14:4991–4999. doi: 10.1128/mcb.14.7.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maedler K, Schumann DM, Schulthess F, et al. Aging correlates with decreased b-cell proliferative capacity and enhanced sensitivity to apoptosis: a potential role for Fas and pancreatic duodenal homeobox-1. Diabetes. 2006;55:2455–2462. doi: 10.2337/db05-1586. [DOI] [PubMed] [Google Scholar]
- 11.Marti U. Handling of epidermal growth factor and number of epidermal growth factor receptors are changed in aged male rats. Hepatology. 1993;18:1432–1436. [PubMed] [Google Scholar]
- 12.Palmer HJ, Tuzon CT, Paulson KE. Age-dependent decline in mitogenic stimulation of hepatocytes. Reduced association between Shc and the epidermal growth factor receptor is coupled to decreased activation of Raf and extracellular signal-regulated kinases. J Biol Chem. 1999;274:11424–11430. doi: 10.1074/jbc.274.16.11424. [DOI] [PubMed] [Google Scholar]
- 13.Riabowol K, Schiff J, Gilman MZ. Transcription factor AP-1 activity is required for initiation of DNA synthesis and is lost during cellular aging. Proc Natl Acad Sci U S A. 1992;89:157–161. doi: 10.1073/pnas.89.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutter D, Yo Y, Chen W, et al. Age-related decline in Ras/ERK mitogen-activated protein kinase cascade is linked to a reduced association between Shc and EGF receptor. J Gerontol. 2000;55A:B125–B134. doi: 10.1093/gerona/55.3.b125. [DOI] [PubMed] [Google Scholar]
- 15.Timchenko NA, Wilde M, Kosai KI, et al. Regenerating livers of old rats contain high levels of C/EBPa that correlate with altered expression of cell cycle associated proteins. Nucleic Acids Res. 1998;13:3293–3299. doi: 10.1093/nar/26.13.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Krupezak-Hollis K, Tan Y, Dennewitz MB, Adami GR, Costa RH. Increased hepatic forkhead box M1B (FoxM1B) levels in old-aged mice stimulated liver regeneration through diminished p27Kip1 protein levels and increased Cdc25B expression. J Biol Chem. 2002;277:44310–44316. doi: 10.1074/jbc.M207510200. [DOI] [PubMed] [Google Scholar]
- 17.Berasain C, Garcia-Trevijano ER, Castillo J, et al. Amphiregulin: an early trigger of liver regeneration in mice. Gastroenterology. 2005;128:424–432. doi: 10.1053/j.gastro.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Earp HS, O’Keefe EJ. Epidermal growth factor receptor number decreases during rat liver regeneration. J Clin Invest. 1981;67:1580–1583. doi: 10.1172/JCI110190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michalopolous GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 20.Ishigami A, Reed TD, Roth GS. Effect of aging on EGF stimulated DNA synthesis and EGF receptor levels in primary cultured rat hepatocytes. Biochem Biophys Res Commun. 1993;196:181–186. doi: 10.1006/bbrc.1993.2232. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Guyton KZ, Gorospe M, et al. Age-related decline in mitogen-activated protein kinase activity in epidermal growth factor-stimulated rat hepatocytes. J Biol Chem. 1996;271:3604–3607. [PubMed] [Google Scholar]
- 22.Katz MS. Food restriction modulates β-adrenergic-sensitive adenylate cyclase in rat liver during aging. Am J Physiol Endocrinol Metab. 1988;254:E54–E62. doi: 10.1152/ajpendo.1988.254.1.E54. [DOI] [PubMed] [Google Scholar]
- 23.Katz MS, McNair CL, Hymer TK, Boland SR. Emergence of beta adrenergic-responsive hepatic glycogenolysis in male rats during post-maturational aging. Biochem Biophys Res Commun. 1987;147:724–730. doi: 10.1016/0006-291x(87)90990-9. [DOI] [PubMed] [Google Scholar]
- 24.Munson PJ, Rodbard D. LIGAND: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 25.Gladhaug IP, Christoffersen T. n-Butyrate and dexamethasone synergistically modulate the surface expression of epidermal growth factor receptors in cultured rat hepatocytes. FEBS Lett. 1989;243:21–24. doi: 10.1016/0014-5793(89)81209-8. [DOI] [PubMed] [Google Scholar]
- 26.Scheving LA, Buchanan R, Krause MA, Zhang X, Stevenson MC, Russell WE. Dexamethasone modulates ErbB tyrosine kinase expression and signaling through multiple and redundant mechanisms in cultured rat hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2007;293:G552–G559. doi: 10.1152/ajpgi.00140.2007. [DOI] [PubMed] [Google Scholar]
- 27.Gladhaug IP, Refsnes M, Sand TE, Christoffersen T. Effects of butyrate on epidermal growth factor receptor binding, morphology, and DNA synthesis in cultured rat hepatocytes. Cancer Res. 1988;48:6560–6564. [PubMed] [Google Scholar]
- 28.O’Connor-McCourt M, Soley M, Hayden LJ, Hollenberg MD. Receptors for epidermal growth factor (urogastrone) and insulin in primary cultures of rat hepatocytes maintained in serum-free medium. Biochem Cell Biol. 1986;64:803–810. doi: 10.1139/o86-108. [DOI] [PubMed] [Google Scholar]
- 29.Wollenberg GK, Harris L, Farber E, Hayes MA. Inverse relationship between epidermal growth factor induced proliferation and expression of high affinity surface epidermal growth factor receptors in rat hepatocytes. Lab Invest. 1989;60:254–259. [PubMed] [Google Scholar]
- 30.Ohtake Y, Maruko A, Ohishi N, Fukumoto M, Ohkubo Y. Effect of aging on EGF-induced proliferative response in primary cultured periportal and perivenous hepatocytes. J Hepatol. 2007 doi: 10.1016/j.jhep.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Batzer AG, Rotin D, Urena JM, Skolnik EY, Schlessinger J. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol Cell Biol. 1994;14:5192–5201. doi: 10.1128/mcb.14.8.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakaguchi K, Okabayashi Y, Kido Y, et al. Shc phosphotyrosine-binding domain dominantly interacts with epidermal growth factor receptors and mediates Ras activation in intact cells. Mol Endocrinol. 1998;12:536–543. doi: 10.1210/mend.12.4.0094. [DOI] [PubMed] [Google Scholar]
- 33.Kim HS, Song MC, Kwak IH, Park TJ, Lim IK. Constitutive induction of pERK1/2 accompanied by reduced activities of protein phosphatases 1 and 2A and MKP3 due to reactive oxygen species during cellular senescence. J Biol Chem. 2003;278:37497–37510. doi: 10.1074/jbc.M211739200. [DOI] [PubMed] [Google Scholar]
- 34.Tresini M, Lorenzini A, Torres C, Cristofalo VJ. Modulation of replicative senescence of diploid human cells by nuclear ERK signaling. J Biol Chem. 2007;282:4136–4151. doi: 10.1074/jbc.M604955200. [DOI] [PubMed] [Google Scholar]
- 35.Kim-Kaneyama J, Nose K, Shibanuma M. Significance of nuclear relocalization of ERK1/2 in reactivation of c-fos transcription and DNA synthesis in senescent fibroblasts. J Biol Chem. 2000;275:20685–20692. doi: 10.1074/jbc.M908723199. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee S, Tessema M, Wandinger-Ness A. Vesicular trafficking of tyrosine kinase receptors and associated proteins in the regulation of signaling and vascular function. Circ Res. 2006;98:743–756. doi: 10.1161/01.RES.0000214545.99387.e3. [DOI] [PubMed] [Google Scholar]
- 37.Hudson LG, Santon JB, Glass CK, Gill GN. Ligand-activated thyroid hormone and retinoic acid receptors inhibit growth factor receptor promoter expression. Cell. 1990;62:1165–1175. doi: 10.1016/0092-8674(90)90393-s. [DOI] [PubMed] [Google Scholar]
- 38.Nishi H, Nishi KH, Johnson AC. Early growth response-1 gene mediates up-regulation of epidermal growth factor receptor expression during hypoxia. Cancer Res. 2002;62:827–834. [PubMed] [Google Scholar]
- 39.Hou X, Johnson AC, Rosner MR. Induction of epidermal growth factor receptor gene transcription by transforming growth factor b1: association with loss of protein binding to a negative regulatory element. Cell Growth Differ. 1994;5:801–809. [PubMed] [Google Scholar]
- 40.DeCicco LA, Panzeter PL, Cashman RE, Ringer DP. Changes in the binding capacity of hepatic membranes for epidermal growth factor during multistage hepatocarcinogenesis in rats. Biochem Biophys Res Commun. 1996;228:69–74. doi: 10.1006/bbrc.1996.1617. [DOI] [PubMed] [Google Scholar]
- 41.Oguey D, Marti U, Reichen J. Epidermal growth factor receptor in chronic bile duct obstructed rats: implication for maintenance of hepatocellular mass. Eur J Cell Biol. 1992;59:187–195. [PubMed] [Google Scholar]
- 42.Mukku VR. Regulation of epidermal growth factor receptor levels by thyroid hormone. J Biol Chem. 1984;259:6543–6547. [PubMed] [Google Scholar]
- 43.De BK, Brown TL, Suchy FJ. Ontogeny of epidermal growth factor receptor tyrosine kinase in rat liver. Am J Physiol Gastrointest Liver Physiol. 1991;260:G290–G298. doi: 10.1152/ajpgi.1991.260.2.G290. [DOI] [PubMed] [Google Scholar]


