Abstract
Purpose
Limited information is available on the impact of chemotherapy (CTX)-induced neurotoxicity on adult survivors’ symptom experience and quality of life (QOL). Purposes were to describe occurrence of hearing loss and tinnitus and evaluate for differences in phenotypic characteristics and measures of sensation, balance, perceived stress, symptom burden, and QOL between survivors who received neurotoxic CTX and did (i.e., neurotoxicity group) and did not (i.e., no neurotoxicity group) develop neurotoxicity. Neurotoxicity was defined as the presence of chemotherapy-induced neuropathy (CIN), hearing loss, and tinnitus. Survivors in the no neurotoxicity group had none of these conditions.
Methods
Survivors (n=609) completed questionnaires that evaluated hearing loss, tinnitus, stress, symptoms, and QOL. Objective measures of sensation and balance were evaluated.
Results
Of the 609 survivors evaluated, 68.6% did and 31.4% did not have CIN. Of the survivors without CIN, 42.4% reported either hearing loss and/or tinnitus and 48.1% of the survivors with CIN reported some form of ototoxicity. Compared to the no neurotoxicity group (n=110), survivors in the neurotoxicity group (n=85) were older, were less likely to be employed, had a higher comorbidity burden, and a higher symptom burden, higher levels of perceived stress, and poorer QOL (all p<.05).
Conclusions
Findings suggest that CIN, hearing loss, and tinnitus are relatively common conditions in survivors who received neurotoxic CTX.
Implications for Cancer Survivors
Survivors need to be evaluated for these neurotoxicities and receive appropriate interventions. Referrals to audiologists and physical therapists are warranted to improve survivors’ hearing ability, functional status, and QOL.
Keywords: chemotherapy, peripheral neuropathy, hearing loss, tinnitus, balance, survivor
INTRODUCTION
Given that by January 2024, the number of cancer survivors in the United States will total 19 million [1], organizations like the National Comprehensive Cancer Network (NCCN) developed guidelines for adult cancer survivors [2]. While pain is addressed in these guidelines, little information is provided on the assessment and management of chemotherapy-induced neuropathy (CIN). In addition, the guideline is silent on any evaluation of the deleterious effects of neurotoxic chemotherapy (CTX) on the audiovestibular system (i.e., hearing loss, tinnitus, disturbances in balance).
Research on hearing loss associated with neurotoxic CTX has focused primarily on pediatric patients who received platinum [3–5]. The limited amount of work in adults focused on hearing loss in patients receiving platinum for testicular or head and neck cancer. Across these studies, platinum induced a bilateral and symmetrical sensorineural hearing loss [6]. Compared to testicular [7–10] and head and neck [11, 12] cancer, only a few case reports and small studies provide inconclusive findings on hearing loss in patients with breast, gastrointestinal (GI), gynecological (GYN), or lung cancer [13–18]. Even less is known about the occurrence and impact of hearing loss associated with taxanes. In fact, no large scale study has evaluated the impact of hearing loss in adult survivors of breast, GI, GYN, or lung cancer who received a platinum and/or taxane containing CTX regimen.
Tinnitus is the awareness of an auditory percept in the absence of an external stimulus. Typically, these percepts are described as hissing, buzzing, and ringing [19]. In patients with testicular cancer, who received cisplatin, tinnitus rates ranged from 19% to 42% [20]. In the one study of 41 ovarian cancer patients who received platinum, tinnitus was reported by 27% of the women [18]. Again, no studies have described the occurrence or impact of tinnitus in adult survivors of breast, GI, or lung cancer who received a platinum and/or a taxane compound.
Stress may be a common underlying mechanism for CIN [21] and tinnitus [22, 23]. Increased stress exacerbates both conditions and evidence suggests that patients with pain [24, 25] and tinnitus [26, 27] have alterations in autonomic processing. However, no studies were found that evaluated associations between perceived stress and any of the neurotoxic effects (i.e., CIN, hearing loss, tinnitus) of CTX.
Given that breast, GI, GYN, and lung cancers are the four most common oncologic diagnoses in adults and platinum and taxane compounds are the mainstay of treatment for these cancers, an evaluation of the occurrence and impact of hearing loss and tinnitus in adult cancer survivors is warranted at the present time. Therefore, the purposes of this study were to describe the occurrence of hearing loss and tinnitus and to evaluate for differences in demographic and clinical characteristics, as well as measures of sensation, balance, perceived stress, symptom burden, and quality of life (QOL) between survivors who received a platinum and/or a taxane compound and did (i.e., neurotoxicity group) and did not (i.e., no neurotoxicity group) develop neurotoxicity. For the purposes of this paper, neurotoxicity is defined as the presence of CIN, hearing loss, and tinnitus. Survivors in the no neurotoxicity group had none of these conditions.
METHODS
Survivors and Settings
The methods for this larger study are described in detail elsewhere [28]. In brief, a convenience sample of survivors was recruited from throughout the San Francisco Bay area using the following strategies: direct referral from clinicians; direct mailing to survivors who were identified through targeted searches of our medical center’s electronic health record; newspaper advertisements; emails to participants in the Dr. Susan Love Research Foundation’s Army of Women® Program; emails to support group members; postings on survivorship websites; postings on ClinicalTrials.gov; presentations at support group meetings; and snowball sampling through referrals from survivors. Survivors with CIN met the following inclusion criteria: were ≥18 years of age; had received a platinum and/or a taxane compound; had completed their course of CTX ≥3 months prior to enrollment; had changes in sensation and/or pain in their feet and/or hands of ≥3 months duration following the completion of CTX; had a rating of ≥3 on a 0 to 10 numeric rating scale (NRS) for any one of the following sensations from the Pain Qualities Assessment Scale (i.e., numb, tender, shooting, sensitive, electrical, tingling radiating, throbbing, cramping, itchy, unpleasant) [29]; if they had pain associated with the CIN, had an average pain intensity score in their feet and/or hands of ≥3 on a 0 to 10 NRS; had a Karnofsky Performance Status (KPS) score of ≥50; and were able to read, write, and understand English.
Survivors without CIN met the following inclusion criteria: were ≥18 years of age; had received a platinum and/or a taxane compound; had completed their course of CTX ≥3 months prior to enrollment; did not have persistent changes in sensation and/or pain in their hands or feet at the time of enrollment; had a KPS score of ≥50; and were able to read, write, and understand English.
Survivors with and without CIN were excluded if they had: peripheral vascular disease, vitamin B12 deficiency, thyroid dysfunction, HIV neuropathy, another painful condition that was difficult for them to distinguish from their CIN, a hereditary sensory or autonomic neuropathy, and/or a hereditary mitochondrial disorder. Of the 1450 survivors who were screened, 754 were enrolled, and 609 completed the self-report questionnaires and the study visit. This paper focuses on a subset of this sample (i.e., those without neurotoxicity (n=110) and those with all three neurotoxicities (n=85)).
Study procedures
Research nurses screened and consented the survivors over the phone; sent and asked them to complete the self-report questionnaires prior to their study visit; and scheduled the in person assessment. At this assessment, written informed consent was obtained, questionnaires were reviewed for completeness, and objective measurements were done.
Study Measures
Demographic and Clinical Characteristics
Survivors provided information on demographic characteristics and completed the Karnofsky Performance Status (KPS) scale [30–32] and the Self-Administered Comorbidity Questionnaire (SCQ) [33, 34].
Hearing Loss and Tinnitus
Two items from the Functional Assessment of Therapy/Gynecologic Oncology Group Neurotoxicity (FACT/GOG-Ntx) subscale were used to evaluate hearing loss (i.e., I have trouble hearing) and tinnitus (i.e., I get ringing or buzzing in my ears) [35]. Each item was rated on a 0 (not at all) to 4 (very much) scale. Survivors who reported a 0 for either of these questions were classified as not having that neurotoxic effect.
Sensation
Light touch was evaluated using Semmes Weinstein monofilaments [36]. Cold sensation was evaluated using the Tiptherm Rod [37, 38]. Pain sensation was evaluated using the Neurotip [38]. Vibration threshold was assessed using a vibrometer [39]. For all of the measures of sensation, both the upper and lower extremities on the dominant side were tested.
Balance
Self-report questions from the Chemotherapy-Induced Peripheral Neuropathy Assessment Tool (CIPNAT) were used to assess balance [40]. The objective measures of balance were the timed get up and go test (TUG) [41] and the Fullerton Advanced Balance (FAB) test [42, 43]. The TUG test is a timed test of a person’s ability to stand from an armed chair, walk 10 feet, turn, and return to a seated position [41]. Survivors were instructed to walk as quickly as possible, without running. The time needed to perform the test was recorded.
The FAB is a measure of balance that includes ten tasks: standing with feet together and eyes closed, reaching forward to retrieve a pencil held at shoulder height, turning 360° in a right then in a left direction, stepping up and over a 15.2 cm (6 in) bench, tandem walking, standing on one leg, standing on foam with eyes closed, 2-footed jumping for a distance, walking with head turns, and responding to an unexpected trunk perturbation [42, 43]. The FAB was chosen because the tasks challenge the sensory systems (i.e., visual, somatosensory, vestibular) used for postural control that may be more sensitive to balance problems in individuals with CIN, a primary sensory neuropathy. The quality of the performance of each task is scored using standardized ordinal scoring criteria. Total scores can range from 0 to 40. Higher scores indicate a better performance.
Symptom Burden
Survivors completed self-report questionnaires that evaluated trait and state anxiety [44], depressive symptoms [45], diurnal variations in fatigue and energy [46], sleep disturbance [47], and changes in attentional function [48].
The Spielberger State-Trait Anxiety Inventories (STAI-S and STAI-T) each have 20 items that are rated from 1 to 4. The summed scores for each scale can range from 20 to 80. The STAI-T measures a person’s predisposition to anxiety as part of one’s personality. The STAI-S measures a person’s temporary anxiety response to a specific situation or how anxious or tense a person is “right now” in a specific situation. Cutoff scores of 31.8 and 32.2 indicate high levels of trait and state anxiety, respectively. The STAI-T and STAI-S inventories have well established validity and reliability [44]. In our study, the Cronbach’s alphas for the STAI-T and STAI-S were 0.91 and 0.95, respectively.
The Center for Epidemiological Studies-Depression (CES-D) scale consists of 20 items selected to represent the major symptoms in the clinical syndrome of depression. A total score can range from 0 to 60, with scores of ≥16 indicating the need for individuals to seek clinical evaluation for major depression. The CES-D has well established validity and reliability [45]. In our study, the Cronbach’s alpha for the CES-D total score was 0.86.
The 18-item Lee Fatigue Scale (LFS) assessed physical fatigue and energy [46]. Each item was rated on a 0 to 10 numeric rating scale (NRS). Total fatigue and energy scores were calculated as the mean of the 13 fatigue items and the 5 energy items, respectively. Higher scores indicate greater fatigue severity and higher levels of energy. Using separate LFSs, patients rated each item based on how they felt within 30 minutes of awakening (i.e., morning fatigue, morning energy) and prior to going to bed (i.e., evening fatigue, evening energy). The LFS has established cut-off scores for clinically meaningful levels of fatigue (i.e., ≥3.2 for morning fatigue, ≥5.6 for evening fatigue) [49] and energy (i.e., ≥6.2 for morning energy, ≥3.5 for evening energy) [49]. In our study, the Cronbach’s alphas were 0.96 for morning and 0.94 for evening fatigue and 0.95 for morning and 0.93 for evening energy.
The General Sleep Disturbance Scale (GSDS) consists of 21 items designed to assess the quality of sleep in the past week. Each item was rated on a 0 (never) to 7 (everyday) NRS. A GSDS total score of ≥43 indicates a significant level of sleep disturbance [49]. The GSDS has well established validity and reliability [47, 50]. In our study, the Cronbach’s alpha for the GSDS total score was 0.85.
The Attentional Function Index (AFI) consists of 16 items designed to measure attentional function [48]. A higher total mean score on a 0 to 10 NRS indicates greater capacity to direct attention [48]. Total scores are grouped into categories of attentional function (i.e., <5.0 low function, 5.0 to 7.5 moderate function, >7.5 high function) [51]. The AFI has well established validity and reliability [48]. In our study, the Cronbach’s alpha for the total AFI score was 0.92.
Stress
Survivors completed the Perceived Stress Scale [52] and the Impact of Event Scale – Revised [53, 54].
The Perceived Stress Scale (PSS) is a 14-item instrument that provides a global evaluation of perceived stress due to life circumstances appraised as stressful over the course of the past week.[52] Each item was rated on a 0 to 4 Likert scale (i.e., 0 = never, 1 = almost never, 2 = sometimes, 3 = fairly often, 4 = very often). Seven out of the fourteen items of PSS-14 are considered negative and the remaining seven are positive. Total scores are calculated after reversing the positive items’ scores and then summing up all scores. Total scores for PSS-14 can range from 0 to 56. A higher score indicates greater stress. The PSS has well established validity and reliability [55]. In this study, its Cronbach’s alpha was 0.91.
The Impact of Event Scale-Revised (IES-R) is a 22 item instrument that was used to measure distress associated with cancer and its treatment [53, 54]. Patients rated each item based on how distressing each potential difficulty was for them during the past week ‘with respect to their cancer and its treatment’. Each item was rated on a 0 to 4 Likert scale (i.e., 0 = not at all, 1 = a little bit, 2 = moderately, 3 = quite a bit, 4 = extremely). Three subscales are created using the mean of the responses. These mean scores allow the user to identify the degree of symptomatology because the subscale scores are presented on the same metric as the item responses. A total score is created by summing the responses to the 22 items. The three subscales evaluate the level of intrusion (8 items), avoidance (8 items), and hyperarousal (6 items) perceived by patient. The total score can range from 0 to 88. For the total score, a cut-off is set at 33, while a score between 24 and 29 is taken as a sign of a partial PTSD and a score of ≥37 indicates a high presence of post-traumatic symptomatology) [56]. The IES-R has well established validity and reliability [56–58]. In this study, the Cronbach’s alpha for the IES-R total score was 0.92.
QOL
A generic evaluation of QOL was done using the Medical Outcomes Study-Short Form (SF12) [59]. The disease specific measure of QOL was the QOL Scale-Patient Version (QOL-PV) [60–63].
The SF-12 consists of 12 questions about physical and mental health as well as overall health status. The SF-12 was scored into two components that measure physical (i.e., physical component summary (PCS)) and psychological (mental component summary (MCS)) function. These scores can range from 0 to 100. Higher PCS and MCS scores indicate better physical and psychological functioning, respectively. The individual items on the SF-12 were used to evaluate generic aspects of QOL. The SF-12 has well established validity and reliability [59].
The QOL-PV consists of 41-items that measure four domains of QOL (i.e., physical, psychological, social, and spiritual well-being) in oncology patients, as well as a total QOL score. Each item is rated on a 0 to 10 NRS with higher scores indicating a better QOL. The QOL-PV has well established validity and reliability [60–63]. In the current study, the Cronbach’s alpha for the QOL-PV total score was 0.92.
Data Analysis
Data were analyzed using SPSS version 23 [64]. Descriptive statistics and frequency distributions were calculated for survivors’ demographic and clinical characteristics. For the four measures of sensation (i.e., light touch, cold, pain, vibration), composite scores, over all of the sites that were tested on the dominant upper and lower extremities, were created. For light touch, cold, and pain, the number of sites with loss of each sensation were summed. For vibration, the mean score across the sites was calculated. Differences between the neurotoxicity and no neurotoxicity groups in phenotypic characteristics, balance, and levels of perceived stress, symptom burden, and QOL were evaluated through bivariate analyses using Independent sample t-tests, Chi square analyses, and Mann-Whitney U tests. A p-value of <0.05 was considered statistically significant.
RESULTS
Occurrence of Neurotoxicity
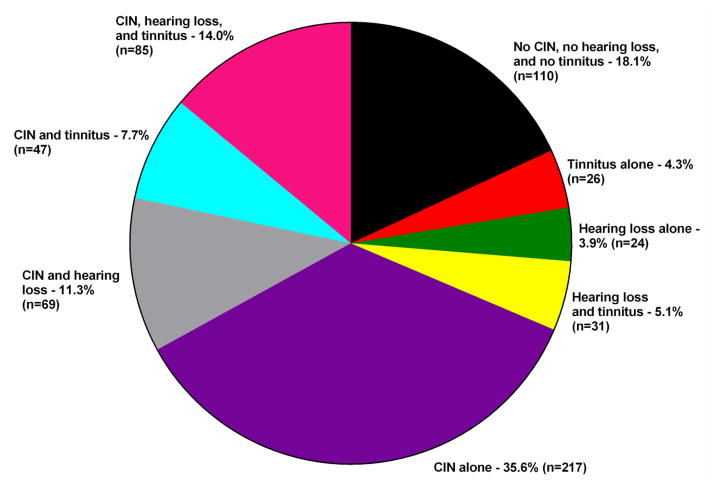
In this sample of 609 survivors, 68.6% did and 31.4% did not have CIN. The distribution of hearing loss and tinnitus among the 609 survivors is illustrated in Figure 1. For this paper, differences between the 110 survivors without neurotoxicity (i.e., neither CIN, nor hearing loss, nor tinnitus; 18.1% of the total sample) and the 85 survivors with neurotoxicity (i.e., CIN, hearing loss, and tinnitus; 14.0% of the total sample) were evaluated.
Figure 1.
Distribution of neurotoxicities in a sample of adult cancer survivors (n=609).
Differences in Demographic and Clinical Characteristics
As shown in Table 1, survivors with neurotoxicity were significantly older (p=.001) and were more likely to be unemployed (p=.006); more likely to report a lower annual household income (p<.001); and more likely not to have child care responsibilities (p=.001). In terms of clinical characteristics (see Table 2), survivors with neurotoxicity had: a higher BMI (p=.011); a higher number of comorbidities (p<.001) and worse comorbidity profile (p<.001); a lower KPS score (p<.001), and received fewer cancer treatments. In addition, these survivors were more likely to report osteoarthritis (p<.001), back pain (p<.001), depression (p=.006), and kidney disease (p=.003); were more likely to have had a dose reduction or delay due to CIN (p=.011); and were less likely to exercise on a regular basis (p=.003). Of note, no between group differences were found in the years since the cancer diagnosis, number of metastatic sites, type of CTX regimen administered, and doses of platinum and/or taxane compounds received.
Table 1.
Differences in Demographic Characteristics Between Cancer Survivors With (n=85) and Without (n=110) Chemotherapy-Induced Neurotoxicity*
| Characteristic | No Neurotoxicity 56.4% (n=110) |
Neurotoxicity 43.6% (n=85) |
Test, p-value |
|---|---|---|---|
|
| |||
| Mean (SD) | Mean (SD) | ||
|
| |||
| Age (years) | 57.3 (11.9) | 62.5 (9.8) | t = −3.34, p = .001 |
|
| |||
| Education (years) | 16.6 (2.5) | 15.9 (2.5) | t = 1.91, p = .057 |
|
| |||
| % (n) | % (n) | ||
|
| |||
| Female | 86.4 (95) | 75.3 (64) | FE, p = .062 |
|
| |||
| Married/partnered | 65.7 (71) | 62.2 (51) | FE, p = .649 |
|
| |||
| Lives alone | 27.5 (30) | 28.6 (24) | FE, p = .873 |
|
| |||
| Employed | 57.3 (63) | 36.5 (31) | FE, p = .006 |
|
| |||
| Ethnicity | |||
| White | 87.3 (96) | 77.6 (66) | |
| Asian/Pacific Islander | 5.5 (6) | 4.7 (4) | χ2 = 5.16, p = .161 |
| Black | 3.6 (4) | 7.1 (6) | |
| Hispanic/Mixed/Other | 3.6 (4) | 10.6 (9) | |
|
| |||
| Annual household income | |||
| <$30,000 | 8.7 (9) | 33.3 (27) | |
| $30,000 – $69,999 | 20.4 (21) | 23.5 (19) | U, p <.001 |
| $70,000 – $99,999 | 20.4 (21) | 11.1 (9) | |
| >$100,000 | 50.5 (52) | 32.1 (26) | |
|
| |||
| Child care responsibilities | 24.1 (26) | 6.0 (5) | FE, p = .001 |
|
| |||
| Adult care responsibilities | 3.8 (4) | 0.0 (0) | FE, p = .141 |
Chemotherapy-induced neurotoxicity = chemotherapy-induced neuropathy and hearing loss and tinnitus
Abbreviations: FE = Fisher’s Exact test, U = Mann-Whitney U test, SD = standard deviation
Table 2.
Differences in Clinical Characteristics Between Cancer Survivors With (n=85) and Without (n=110) Chemotherapy-Induced Neurotoxicity*
| Characteristic | No Neurotoxicity 56.4% (n=110) |
Neurotoxicity 43.6% (n=85) |
Test, p-value |
|---|---|---|---|
|
| |||
| Mean (SD) | Mean (SD) | ||
|
| |||
| Karnofsky Performance Status score | 92.2 (8.5) | 80.7 (10.0) | t = 8.36, p <.001 |
|
| |||
| Body mass index (kg/m2) | 24.9 (4.6) | 26.8 (5.6) | t = −2.55, p = .011 |
|
| |||
| Number of comorbidities | 1.3 (1.3) | 2.4 (1.5) | t = −5.04, p <.001 |
|
| |||
| Self-Administered Comorbidity Questionnaire score | 2.7 (3.0) | 5.1 (3.7) | t = −4.90, p <.001 |
|
| |||
| Years since cancer diagnosis | 4.6 (4.8) | 5.6 (5.8) | t = −1.32, p = .188 |
|
| |||
| Number of prior cancer treatments | 3.3 (1.0) | 3.0 (1.0) | t = 2.29, p = .023 |
|
| |||
| Number of current cancer treatments | 0.5 (0.6) | 0.4 (0.6) | t = 1.43, p = .154 |
|
| |||
| Number of metastatic sites (out of 7) | 0.7 (0.7) | 0.7 (0.7) | t = −0.15, p = .879 |
|
| |||
| Number of metastatic sites without lymph node involvement | 0.1 (0.4) | 0.2 (0.5) | t = −0.38, p = .708 |
|
| |||
| % (n) | % (n) | ||
|
| |||
| Smoker (ever) | 30.6 (33) | 41.7 (35) | FE, p = .129 |
|
| |||
| Exercise on a regular basis (% yes) | 92.6 (100) | 77.6 (66) | FE, p = .003 |
|
| |||
| Born prematurely (% yes) | 1.9 (2) | 6.3 (5) | FE, p = .142 |
|
| |||
| Comorbid conditions (% yes) | |||
| Cancer | 40.9 (45) | 47.1 (40) | FE, p = .467 |
| Osteoarthritis | 11.8 (13) | 36.5 (31) | FE, p <.001 |
| Back pain | 22.7 (25) | 47.1 (40) | FE, p <.001 |
| Depression | 15.5 (17) | 32.9 (28) | FE, p = .006 |
| High blood pressure | 20.0 (22) | 29.4 (25) | FE, p = .133 |
| Heart disease | 3.6 (4) | 7.1 (6) | FE, p = .337 |
| Diabetes | 3.6 (4) | 3.5 (3) | FE, p = 1.000 |
| Lung disease | 6.4 (7) | 7.1 (6) | FE, p = 1.000 |
| Anemia or blood disease | 3.6 (4) | 2.4 (2) | FE, p = .698 |
| Ulcer or stomach disease | 2.7 (3) | 5.9 (5) | FE, p = .299 |
| Kidney disease | 0.0 (0) | 8.2 (7) | FE, p = .003 |
| Liver disease | 0.0 (0) | 3.5 (3) | FE, p = .081 |
| Rheumatoid arthritis | 0.9 (1) | 5.9 (5) | FE, p = .088 |
|
| |||
| Type of cancer | |||
| Breast | 60.0 (66) | 48.2 (41) | χ2 = 9.72, p = .045 No significant pair wise contrasts by type of cancer |
| Colon | 3.6 (4) | 10.6 (9) | |
| Lung | 6.4 (7) | 1.2 (1) | |
| Ovarian | 6.4 (7) | 5.9 (5) | |
| Other | 23.6 (26) | 34.1 (29) | |
|
| |||
| Chemotherapy regimen | |||
| Only a platinum compound | 25.7 (28) | 36.5 (31) | χ2 = 2.99, p = .224 |
| Only a taxane compound | 53.2 (58) | 42.4 (36) | |
| Both a platinum and a taxane compound | 21.1 (23) | 21.2 (18) | |
|
| |||
| Total dose of platinum compound for patients who received only a platinum** | 455.8 (269.4) n=23 |
633.0 (585.1) n=29 |
t = −1.34, p = .186 |
|
| |||
| Total dose of taxane compound for patients who received only a taxane** | 658.3 (277.3) n=51 |
649.5 (290.0) n=30 |
t = 0.14, p = .893 |
|
| |||
| Total dose of drugs for patients who received both a platinum and a taxane compound** | |||
| Platinum dose | 1568.3 (522.9) | 1721.7 (517.2) | t = −0.88, p = .387 |
| Taxane dose | 711.9 (290.2) n=21 |
699.3 (345.8) n=17 |
t = 0.12, p = .902 |
|
| |||
| Patients who had a dose reduction or delay due to neuropathy (% (n)) | 1.9 (2) | 11.1 (9) | FE, p = .011 |
Chemotherapy-induced neurotoxicity = chemotherapy-induced neuropathy and hearing loss and tinnitus
Doses are reported as milligrams per meter squared
Abbreviations: FE = Fisher’s Exact test, kg = kilograms, m2 = meters squared, mg = milligrams, U = Mann-Whitney U test, SD = standard deviation
Differences in Sensation
Survivors in the neurotoxicity group had a higher number of upper and lower extremity sites with loss of light touch, cold, and pain sensations (all, p<.001). For both the upper and lower extremities, vibration thresholds were significantly higher in the neurotoxicity group (both, p<.001, Table 3).
Table 3.
Differences in Sensation Measures, Balance Measures, Symptom Severity Scores, Stress Measures, and Quality of Life Outcomes Between Cancer Survivors With (n=85) and Without (n=110) Chemotherapy-Induced Neurotoxicity*
| Characteristic** | No Neurotoxicity 56.4% (n=110) | Neurotoxicity 43.6% (n=85) | Statistic; p-value |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Sensation Measures+ | |||
| Light touch – upper extremity sites (out of 7)a | 0.04 (0.2) | 0.4 (1.1) | t = −2.82, p <.001 |
| Light touch – lower extremity sites (out of 9)b | 0.6 (1.2) | 2.6 (2.5) | t = −6.94, p <.001 |
| Cold – upper extremity sites out of 4c | 0.5 (0.8) | 1.0 (1.0) | t = −3.87, p <.001 |
| Cold – lower extremity sites out of 4d | 1.6 (1.2) | 2.4 (1.2) | t = −4.69, p <.001 |
| Pain – upper extremity sites (out of 7)e | 0.6 (1.1) | 1.4 (1.6) | t = −4.20, p <.001 |
| Pain – lower extremity sites (out of 9)f | 1.8 (1.9) | 3.8 (1.9) | t = −7.54, p <.001 |
| Vibration – upper extremity sites (volts)g | 7.0 (2.8) | 10.4 (4.3) | t = −5.89, p <.001 |
| Vibration – lower extremity sites (volts)h | 19.7 (9.4) | 30.3 (13.5) | t = −6.19, p <.001 |
| Balance Measures | |||
| Trouble with balance (% yes (n))i | 14.0 (15) | 76.2 (64) | FE, p <.001 |
| Severity of balance trouble (0 to 10)k | 2.9 (2.4) | 5.6 (2.5) | t = −3.83, p <.001 |
| Frequency of balance trouble (0 to 10)l | 2.4 (1.8) | 5.1 (2.6) | t = −4.81, p <.001 |
| Distress from balance trouble (0 to 10)m | 3.1 (2.8) | 5.9 (3.0) | t = −3.25, p = .002 |
| Timed get up and go test (>13.5 seconds = higher risk for falls) | 6.4 (1.5) | 8.4 (3.4) | t = −4.95, p <.001 |
| Fullerton Advanced Balance test (≤25 is associated with a higher risk of falls) | 36.2 (5.0) | 32.2 (7.6) | t = 4.18, p <.001 |
| Symptom Severity Scores | |||
| Trait anxiety (STAI-T score ≥31.8) | 31.8 (8.5) | 38.5 (11.1) | t = −4.60, p <.001 |
| State anxiety (STAI-S score ≥32.2) | 28.4 (8.0) | 35.5 (14.0) | t = −4.22, p <.001 |
| Depressive symptoms (CES-D score ≥16) | 6.7 (6.9) | 13.5 (11.3) | t = −4.84, p <.001 |
| Morning fatigue (LFS score ≥3.2) | 2.5 (2.0) | 3.5 (2.4) | t = −3.04, p = .003 |
| Evening fatigue (LFS score ≥5.6) | 5.3 (1.9) | 5.4 (1.7) | t = −0.20, p = .841 |
| Morning energy (LFS score ≤6.2) | 5.4 (2.3) | 4.4 (2.4) | t = 2.97, p = .003 |
| Evening energy (LFS score ≤3.5) | 4.1 (2.0) | 3.4 (1.9) | t = 2.39, p = .018 |
| Sleep disturbance (GSDS score ≥43) | 39.2 (17.8) | 51.4 (19.9) | t = −4.52, p <.001 |
| Attentional function (AFI score <5 is low function, 5.0 to 7.5 is moderate function, >7.5 is high function) | 7.5 (1.4) | 6.2 (1.7) | t = 6.03, p <.001 |
| Stress Measures | |||
| IES-R Avoidance mean subscale score | 0.5 (0.5) | 0.7 (0.8) | t = −1.78, p = .077 |
| IES-R Intrusion mean subscale score | 0.4 (0.5) | 0.8 (0.9) | t = −3.67, p <.001 |
| IES-R Hyperarousal mean subscale score | 0.2 (0.3) | 0.7 (0.9) | t = −4.83, p <.001 |
| IES-R Total mean score | 0.4 (0.4) | 0.7 (0.8) | t = −3.52, p = .001 |
| IES-R Total score (≥33) | 8.2 (8.3) | 15.4 (17.4) | t = −3.52, p = .001 |
| Perceived Stress Scale score | 14.0 (7.6) | 19.4 (10.0) | t = −4.08, p <.001 |
| MOS - SF12 Scores | |||
| Physical functioning | 81.9 (24.8) | 54.5 (35.8) | t = 6.00, p <.001 |
| Role physical | 79.1 (25.9) | 51.5 (30.6) | t = 6.62, p <.001 |
| Bodily pain | 86.7 (21.1) | 60.1 (31.6) | t = 6.60, p <.001 |
| General health | 75.2 (22.1) | 56.9 (23.4) | t = 5.46, p <.001 |
| Vitality | 59.3 (24.6) | 40.8 (24.8) | t = 5.18, p <.001 |
| Social functioning | 89.0 (18.1) | 69.0 (31.6) | t = 5.16, p <.001 |
| Role emotional | 87.2 (20.1) | 72.5 (28.1) | t = 4.06, p <.001 |
| Mental health | 75.8 (17.1) | 65.8 (22.4) | t = 3.41, p <.001 |
| Physical component summary score (≥50.0) | 50.6 (9.2) | 39.7 (11.2) | t = 7.08, p <.001 |
| Mental component summary score (≥50.0) | 51.8 (8.9) | 47.5 (10.7) | t = 2.96, p = .004 |
| Multidimensional Quality of Life (QOL) Scale – Cancer | |||
| Physical well-being | 8.2 (1.3) | 6.8 (1.8) | t = 6.17, p <.001 |
| Psychological well-being | 6.3 (1.5) | 5.2 (1.7) | t = 4.58, p <.001 |
| Social well-being | 6.9 (2.0) | 5.3 (2.2) | t = 5.13, p <.001 |
| Spiritual well-being | 5.1 (1.8) | 5.2 (2.3) | t = −0.40, p = .690 |
| Total QOL score | 6.6 (1.3) | 5.6 (1.5) | t = 4.95, p <.001 |
Chemotherapy-induced neurotoxicity = chemotherapy-induced neuropathy and hearing loss and tinnitus
When available, the clinically meaningful cut-point score is provided in parentheses next to the characteristic.
Changes in sensation are reported for the dominant extremity
Upper extremity sites for light touch were: pad of thumb, thumb webspace, tip of index finger, tip of little finger, midway base of palm, one third up anterior arm, two thirds up anterior arm
Lower extremity sites for light touch were: pad of great toe, pad of 3rd toe, pad of 5th toe, base of heel, metocarpophalangeal (MP) joint of great toe, MP joint of 3rd toe, MP joint of 5th toe, midway along tibia, patella
Upper extremity sites for cold were: pad of index finger, pad of little finger, dorsal MP area of the hand, wrist
Lower extremity sites for cold were: top of great toe at 1st MP joint, pad of great toe, dorsum of foot midpoint, medial malleolus
Upper extremity sites for pain were: pad of thumb, thumb webspace, tip of index finger, tip of little finger, midway base of palm, one third up anterior arm, two thirds up anterior arm
Lower extremity sites for pain were: pad of great toe, pad of 3rd toe, pad of 5th toe, base of heel, metocarpophalangeal (MP) joint of great toe, MP joint of 3rd toe, MP joint of 5th toe, midway along tibia, patella
Upper extremity sites for vibration were: dorsal interphalangeal (IP) joint of thumb, dorsal IP joint of index finger, ulnar prominence, lateral epicondyle
Lower extremity sites for vibration were: dorsal IP joint of great toe, medial malleolus, patella
Since your chemotherapy, have you had trouble with your balance?
Have you had any falls since starting chemotherapy?
At its worst, how severe is the trouble with your balance (0 = not at all severe to 10 = extremely severe)?
How often do you have trouble with your balance (0 = never to 10 = always)?
At its worst, how distressing is the trouble with your balance (0 = not at all distressing to 10 = extremely distressing)?
Abbreviations: AFI = Attentional Function Index, CES-D = Center for Epidemiological Studies-Depression Scale, LFS = Lee Fatigue Scale, GSDS = General Sleep Disturbance Scale, IES-R = Impact of Event Scale-Revised, MOS-SF-12 = Medical Outcomes Study-Short Form 12, QOL = quality of life, SD = standard deviation
Differences in Balance
Survivors in the neurotoxicity group were more likely to report trouble with balance (p<.001) as well as higher severity (p<.001), frequency (p<.001), and distress (p=.002) scores associated with balance problems (see Table 3). In addition, these survivors reported worse TUG (p<.001) and worse FAB (p<.001) scores.
Differences in Symptom Burden
Except for evening fatigue, survivors in the neurotoxicity group reported higher scores for trait and state anxiety (both p<.001), depressive symptoms (p<.001), morning fatigue (p=.003), and sleep disturbance (p<.001). In addition, these survivors reported lower levels of morning (p=.003) and evening (p=.018) energy, and worse attentional function scores (p<.001).
Differences in Perceived Stress
Except for the IES-R avoidance subscale, survivors in the neurotoxicity group reported higher IES-R subscale (both p<.001) and total (p=.001) scores and a higher PSS (p<.001) score.
Differences in QOL
For all of the SF-12 subscale scores, as well as for the PCS and MCS scores, survivors in the neurotoxicity group reported lower scores (all p<.001, except for the MCS score (p=.004)). Except for the spiritual well-being subscale, the same group differences were seen for the subscale and total scores on the QOLS-PV (all p<.001).
DISCUSSION
This study is the first to provide occurrence data on ototoxicity (i.e., hearing loss and tinnitus) in adult cancer survivors with and without CIN and to evaluate for differences in demographic and clinical characteristics, as well as important outcomes, in survivors with and without neurotoxicity. In this large, convenience sample, 81 of the 191 adult survivors without CIN (42.4%) reported hearing loss and/or tinnitus. In the 418 survivors with CIN, 201 (48.1%) reported some form of ototoxicity. While previous studies of survivors with testicular [10] and ovarian [18] cancer evaluated only individuals who received a platinum compound and did not include CIN in their phenotyping, their occurrence rates for hearing and tinnitus were relatively similar to our findings. While CIN is assessed by most oncologists, these data suggest that ongoing audiologic evaluations for hearing and tinnitus are warranted during and following neurotoxic CTX.
In terms of differences in demographic and clinical characteristics, survivors in the neurotoxicity group were more likely to be older, not employed, and have a lower annual household income. While no studies of adult cancer survivors were identified, the association between increasing age in the general population and hearing loss is known [65].
In terms of clinical characteristics, while findings from previous studies suggest that ototoxicity occurs in a dose dependent manner [6], no between group differences were found in the distribution of the CTX regimens or in the total doses of the platinum and/or taxane compounds administered (Table 2). However, while the absolute numbers in this sample were relatively small, a higher percentage of survivors in the neurotoxicity group had a dose reduction or delay in their CTX treatment. In a similar manner, 8.2% of the survivors in the neurotoxicity group reported kidney disease as a concurrent comorbidity. Overall, survivors in the neurotoxicity group had a worse comorbidity profile and a poorer functional status. Again, cross-sectional studies of the general population found that adults with hearing loss and/or tinnitus report a more severe comorbidity profile, particularly anxiety and depression, as well as poorer functional outcomes [66–69].
As expected, all of the measures of sensation were significantly worse in the neurotoxicity group. Overall decrements in all sensations were worse in the lower extremity than in the upper extremity. Clinicians need to assess for losses in protective sensations associated with CIN and educate patients to employ strategies to prevent injuries to their hands and feet.
Recent evidence suggests that balance problems and falls are a significant problem for survivors with CIN [70]. Of note, all of the self-report data and objective measures of balance were significantly worse in the neurotoxicity group. The impact and consequences of these balance problems warrant investigation in future studies.
As shown in Table 3, with the exception of evening fatigue, all of the other symptom severity scores were higher in the neurotoxicity group. In addition, except for depressive symptoms, all of the symptom severity scores in the neurotoxicity group were above the clinically meaningful cutoff scores. While the relative contribution of CIN versus hearing loss versus tinnitus to the symptom burden of these survivors warrants additional investigation, our findings suggest that clinicians need to assess for multiple symptoms and initiate appropriate symptom management interventions.
While previous research demonstrates positive associations between stress and pain [24, 25], as well as tinnitus [26, 27], our study is the first to suggest that survivors with neurotoxicity have higher levels of both generic and disease/treatment-related stress. The PSS is a widely used measure that evaluates non-specific stress that exceeds a person’s coping abilities [52]. While no clinically meaningful cutoff score for the PSS exists, the patients with neurotoxicity had significantly higher scores. Our PSS scores are comparable to those reported by breast (i.e., 11.6 (±7.9)) [71] and prostate (i.e., 17.9 (±8.1)) [72] cancer survivors. In terms of the IES-R, which is designed to measure an individual’s response to a specific traumatic event (i.e., cancer and its treatment) [54], while neither group exceeded the clinically meaningful cutoff score of ≥33, survivors with neurotoxicity reported higher scores for the intrusion and hyperarousal subscales as well as for the total IES-R score. In addition, while only one survivor (0.01%) in the no neurotoxicity group reported a total IES-R score above the cutoff, fourteen survivors (16.7%) in the neurotoxicity group reported total IES-R scores that ranged from 33 to 76.
Recent reviews on cancer survivorship suggest that a higher symptom burden is associated with significant decrements in the various domains of QOL [73–75]. However, no studies were identified that evaluated for differences in QOL outcomes between survivors with and without CTX-induced neurotoxicity. In our study, except for the spiritual well-being subscale of the QOLS-PV, the neurotoxicity group reported not only statistically significant but clinically meaningful (i.e., Cohen’s d = 0.43 to 0.96) decrements in the physical, psychological, and social domains of QOL (see Table 3) [76, 77]. It should be noted that for both the PCS and MCS subscales of the SF-12, the neurotoxicity group had scores of below 50 which is the normative score for the general United States population [59]. Future studies need to evaluate the relative contribution of each neurotoxicity to these significant decrements in QOL.
A number of limitations warrant consideration. While pretreatment hearing loss and tinnitus were not assessed and a detailed clinical evaluation of hearing loss and tinnitus was not performed, our data suggest that survivors’ self-reports of these two conditions can assist clinicians to determine the need for additional tests. While only survivors who received a platinum and/or a taxane compound were evaluated, other CTX drugs produce neurotoxicity. Therefore, our findings may not generalize to survivors who received other types of neurotoxic CTX.
Despite these limitations, our findings suggest that CIN, hearing loss, and tinnitus are relatively common conditions in adult cancer survivors who received a platinum and/or a taxane compound. In addition, survivors with all three of these conditions experience an extremely high symptom burden and significant decrements in QOL. Additional research is warranted to evaluate the common and distinct mechanisms associated with these three conditions; their mechanistic relationships with stressful life events; as well as the impact of these three conditions on balance, risk for falls, and physical activity. In the interim, clinicians who care for adult survivors who received neurotoxic CTX need to assess for these three conditions and initiate appropriate referrals for a more complete audiometric evaluation of hearing loss and tinnitus. In addition, referrals to physical and occupational therapists are warranted to assist survivors to deal with the loss of protective sensations and problems with balance.
Acknowledgments
This study was funded by the National Cancer Institute (NCI, CA151692). Dr. Miaskowski is supported by a grant from the American Cancer Society and NCI (CA168960). This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR000004. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Recruitment was facilitated by Dr. Susan Love Research Foundation’s Army of Women® Program.
References
- 1.Sogaard M, Thomsen RW, Bossen KS, Sorensen HT, Norgaard M. The impact of comorbidity on cancer survival: a review. Clin Epidemiol. 2013;5(Suppl 1):3–29. doi: 10.2147/CLEP.S47150clep-5-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denlinger CS, Ligibel JA, Are M, Baker KS, Demark-Wahnefried W, Dizon D, et al. Survivorship: screening for cancer and treatment effects, version 2.2014. J Natl Compr Canc Netw. 2014;12(11):1526–31. doi: 10.6004/jnccn.2014.0152. 12/11/1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellman SC. Monitoring chemotherapy-induced hearing loss in children. Eur J Cancer. 1996;32A(7):1185–8. doi: 10.1016/0959-8049(96)00068-8. [DOI] [PubMed] [Google Scholar]
- 4.Brock PR, Knight KR, Freyer DR, Campbell KC, Steyger PS, Blakley BW, et al. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J Clin Oncol. 2012;30(19):2408–17. doi: 10.1200/JCO.2011.39.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freyer DR, Sung L, Reaman GH. Prevention of hearing loss in children receiving cisplatin chemotherapy. J Clin Oncol. 2009;27(2):317–8. doi: 10.1200/JCO.2008.20.1160. [DOI] [PubMed] [Google Scholar]
- 6.Landier W. Ototoxicity and cancer therapy. Cancer. 2016;122(11):1647–8. doi: 10.1002/cncr.29779. [DOI] [PubMed] [Google Scholar]
- 7.Biro K, Noszek L, Prekopp P, Nagyivanyi K, Geczi L, Gaudi I, et al. Characteristics and risk factors of cisplatin-induced ototoxicity in testicular cancer patients detected by distortion product otoacoustic emission. Oncology. 2006;70(3):177–84. doi: 10.1159/000093776. 93776. [DOI] [PubMed] [Google Scholar]
- 8.Bokemeyer C, Berger CC, Hartmann JT, Kollmannsberger C, Schmoll HJ, Kuczyk MA, et al. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer. 1998;77(8):1355–62. doi: 10.1038/bjc.1998.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oldenburg J, Kraggerud SM, Cvancarova M, Lothe RA, Fossa SD. Cisplatin-induced long-term hearing impairment is associated with specific glutathione s-transferase genotypes in testicular cancer survivors. J Clin Oncol. 2007;25(6):708–14. doi: 10.1200/JCO.2006.08.9599. JCO.2006.08.9599. [DOI] [PubMed] [Google Scholar]
- 10.Frisina RD, Wheeler HE, Fossa SD, Kerns SL, Fung C, Sesso HD, et al. Comprehensive audiometric analysis of hearing impairment and tinnitus after cisplatin-based chemotherapy in survivors of adult-onset cancer. J Clin Oncol. 2016;34(23):2712–20. doi: 10.1200/JCO.2016.66.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheraghi S, Nikoofar P, Fadavi P, Bakhshandeh M, Khoie S, Gharehbagh EJ, et al. Short-term cohort study on sensorineural hearing changes in head and neck radiotherapy. Med Oncol. 2015;32(7):646. doi: 10.1007/s12032-015-0646-3. [DOI] [PubMed] [Google Scholar]
- 12.Madasu R, Ruckenstein MJ, Leake F, Steere E, Robbins KT. Ototoxic effects of supradose cisplatin with sodium thiosulfate neutralization in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 1997;123(9):978–81. doi: 10.1001/archotol.1997.01900090094014. [DOI] [PubMed] [Google Scholar]
- 13.Salvinelli F, Casale M, Vincenzi B, Santini D, Di Peco V, Firrisi L, et al. Bilateral irreversible hearing loss associated with the combination of carboplatin and paclitaxel chemotherapy: a unusual side effect. J Exp Clin Cancer Res. 2003;22(1):155–8. [PubMed] [Google Scholar]
- 14.Sarafraz M, Ahmadi K. Paraclinical evaluation of side-effects of Taxanes on auditory system. Acta Otorhinolaryngol Ital. 2008;28(5):239–42. [PMC free article] [PubMed] [Google Scholar]
- 15.Ozguroglu M, Sari O, Turna H. Devastating effects of chemotherapy: deafness and acute renal failure in a patient with epithelial ovarian cancer. Int J Gynecol Cancer. 2006;16(Suppl 1):394–6. doi: 10.1111/j.1525-1438.2006.00214.x. IJG214. [DOI] [PubMed] [Google Scholar]
- 16.Bacon M, James K, Zee B. A comparison of the incidence, duration, and degree of the neurologic toxicities of cisplatin-paclitaxel (PT) and cisplatin-cyclophosphamide (PC) Int J Gynecol Cancer. 2003;13(4):428–34. doi: 10.1046/j.1525-1438.2003.13320.x. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins V, Low R, Mitra S. Hearing sensitivity in women following chemotherapy treatment for breast cancer: results from a pilot study. Breast. 2009;18(5):279–83. doi: 10.1016/j.breast.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Skalleberg J, Solheim O, Fossa SD, Smastuen MC, Osnes T, Gundersen PO, et al. Long-term ototoxicity in women after cisplatin treatment for ovarian germ cell cancer. Gynecol Oncol. 2017 doi: 10.1016/j.ygyno.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Baguley D, McFerran D, Hall D. Tinnitus. Lancet. 2013;382(9904):1600–7. doi: 10.1016/S0140-6736(13)60142-7. [DOI] [PubMed] [Google Scholar]
- 20.Travis LB, Fossa SD, Sesso HD, Frisina RD, Herrmann DN, Beard CJ, et al. Chemotherapy-induced peripheral neurotoxicity and ototoxicity: new paradigms for translational genomics. J Natl Cancer Inst. 2014;106(5) doi: 10.1093/jnci/dju044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson AC, Greenwood-Van Meerveld B. Stress-induced pain: a target for the development of novel therapeutics. J Pharmacol Exp Ther. 2014;351(2):327–35. doi: 10.1124/jpet.114.218065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Betz LT, Muhlberger A, Langguth B, Schecklmann M. Stress Reactivity in Chronic Tinnitus. Sci Rep. 2017;7:41521. doi: 10.1038/srep41521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinecke K, Weise C, Schwarz K, Rief W. Physiological and psychological stress reactivity in chronic tinnitus. J Behav Med. 2008;31(3):179–88. doi: 10.1007/s10865-007-9145-0. [DOI] [PubMed] [Google Scholar]
- 24.Woda A, Picard P, Dutheil F. Dysfunctional stress responses in chronic pain. Psychoneuroendocrinology. 2016;71:127–35. doi: 10.1016/j.psyneuen.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 25.Thieme K, Turk DC, Gracely RH, Maixner W, Flor H. The relationship among psychological and psychophysiological characteristics of fibromyalgia patients. J Pain. 2015;16(2):186–96. doi: 10.1016/j.jpain.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Ylikoski J, Lehtimaki J, Pirvola U, Makitie A, Aarnisalo A, Hyvarinen P, et al. Non-invasive vagus nerve stimulation reduces sympathetic preponderance in patients with tinnitus. Acta Otolaryngol. 2017:1–9. doi: 10.1080/00016489.2016.1269197. [DOI] [PubMed] [Google Scholar]
- 27.Vanneste S, De Ridder D. Brain areas controlling heart rate variability in tinnitus and tinnitus-related distress. PLoS One. 2013;8(3):e59728. doi: 10.1371/journal.pone.0059728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miaskowski C, Mastick J, Paul SM, Topp K, Smoot B, Abrams G, et al. Chemotherapy-induced neuropathy in cancer survivors. J Pain Symptom Manage. 2017 doi: 10.1016/j.jpainsymman.2016.12.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Victor TW, Jensen MP, Gammaitoni AR, Gould EM, White RE, Galer BS. The dimensions of pain quality: factor analysis of the Pain Quality Assessment Scale. Clin J Pain. 2008;24(6):550–5. doi: 10.1097/AJP.0b013e31816b1058. [DOI] [PubMed] [Google Scholar]
- 30.Karnofsky D. Factors that influence the therapeutic response in cancer: a comprehensive treatise. New York: Plenum Press; 1977. Performance scale. [Google Scholar]
- 31.Karnofsky D, Abelmann WH, Craver LV, Burchenal JH. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634–56. [Google Scholar]
- 32.Schnadig ID, Fromme EK, Loprinzi CL, Sloan JA, Mori M, Li H, et al. Patient-physician disagreement regarding performance status is associated with worse survivorship in patients with advanced cancer. Cancer. 2008;113(8):2205–14. doi: 10.1002/cncr.23856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunner F, Bachmann LM, Weber U, Kessels AG, Perez RS, Marinus J, et al. Complex regional pain syndrome 1--the Swiss cohort study. BMC Musculoskelet Disord. 2008;9:92. doi: 10.1186/1471-2474-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cieza A, Geyh S, Chatterji S, Kostanjsek N, Ustun BT, Stucki G. Identification of candidate categories of the International Classification of Functioning Disability and Health (ICF) for a Generic ICF Core Set based on regression modelling. BMC Med Res Methodol. 2006;6:36. doi: 10.1186/1471-2288-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang HQ, Brady MF, Cella D, Fleming G. Validation and reduction of FACT/GOG-Ntx subscale for platinum/paclitaxel-induced neurologic symptoms: a gynecologic oncology group study. Int J Gynecol Cancer. 2007;17(2):387–93. doi: 10.1111/j.1525-1438.2007.00794.x. IJG794. [DOI] [PubMed] [Google Scholar]
- 36.Bell-Krotoski JA. Sensibility testing with Semmes-Weinstein monofilaments. In: Hunter JM, Mackin EJ, Callahan ED, editors. Rehabilitation of the Hand and Upper Extremity. 5. St. Louis: Mosby, Inc; 2002. [Google Scholar]
- 37.Viswanathan V, Snehalatha C, Seena R, Ramachandran A. Early recognition of diabetic neuropathy: evaluation of a simple outpatient procedure using thermal perception. Postgrad Med J. 2002;78(923):541–2. doi: 10.1136/pmj.78.923.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papanas N, Ziegler D. New diagnostic tests for diabetic distal symmetric polyneuropathy. Journal of Diabetes and Its Complications. 2011;25(1):44–51. doi: 10.1016/j.jdiacomp.2009.09.006. S1056-8727(09)00097-X. [DOI] [PubMed] [Google Scholar]
- 39.Duke J, McEvoy M, Sibbritt D, Guest M, Smith W, Attia J. Vibrotactile threshold measurement for detecting peripheral neuropathy: defining variability and a normal range for clinical and research use. Diabetologia. 2007;50(11):2305–12. doi: 10.1007/s00125-007-0813-y. [DOI] [PubMed] [Google Scholar]
- 40.Tofthagen CS, McMillan SC, Kip KE. Development and psychometric evaluation of the chemotherapy-induced peripheral neuropathy assessment tool. Cancer Nurs. 2011;34(4):E10–20. doi: 10.1097/NCC.0b013e31820251de. [DOI] [PubMed] [Google Scholar]
- 41.Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the “get-up and go” test. Arch Phys Med Rehabil. 1986;67(6):387–9. [PubMed] [Google Scholar]
- 42.Hernandez D, Rose DJ. Predicting which older adults will or will not fall using the Fullerton Advanced Balance scale. Arch Phys Med Rehabil. 2008;89(12):2309–15. doi: 10.1016/j.apmr.2008.05.020. S0003-9993(08)00832-0. [DOI] [PubMed] [Google Scholar]
- 43.Rose DJ, Lucchese N, Wiersma LD. Development of a multidimensional balance scale for use with functionally independent older adults. Arch Phys Med Rehabil. 2006;87(11):1478–85. doi: 10.1016/j.apmr.2006.07.263. S0003-9993(06)00868-9. [DOI] [PubMed] [Google Scholar]
- 44.Spielberger CG, Gorsuch RL, Suchene R, Vagg PR, Jacobs GA. Manual for the State-Anxiety (Form Y): Self Evaluation Questionnaire. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 45.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 46.Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 1991;36(3):291–8. doi: 10.1016/0165-1781(91)90027-m. [DOI] [PubMed] [Google Scholar]
- 47.Lee KA. Self-reported sleep disturbances in employed women. Sleep. 1992;15(6):493–8. doi: 10.1093/sleep/15.6.493. [DOI] [PubMed] [Google Scholar]
- 48.Cimprich B, Visovatti M, Ronis DL. The Attentional Function Index--a self-report cognitive measure. Psychooncology. 2011;20(2):194–202. doi: 10.1002/pon.1729. [DOI] [PubMed] [Google Scholar]
- 49.Fletcher BS, Paul SM, Dodd MJ, Schumacher K, West C, Cooper B, et al. Prevalence, severity, and impact of symptoms on female family caregivers of patients at the initiation of radiation therapy for prostate cancer. J Clin Oncol. 2008;26(4):599–605. doi: 10.1200/JCO.2007.12.2838. [DOI] [PubMed] [Google Scholar]
- 50.Miaskowski C, Lee KA. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot study. J Pain Symptom Manage. 1999;17(5):320–32. doi: 10.1016/s0885-3924(99)00008-1. S0885-3924(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 51.Cimprich B, So H, Ronis DL, Trask C. Pre-treatment factors related to cognitive functioning in women newly diagnosed with breast cancer. Psychooncology. 2005;14(1):70–8. doi: 10.1002/pon.821. [DOI] [PubMed] [Google Scholar]
- 52.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. [PubMed] [Google Scholar]
- 53.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41(3):209–18. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Weiss DS, Marmar CR. Impact of Event Scale - Revised. New York: Guilford Press; 1997. The Impact of Event Scale - Revised. [Google Scholar]
- 55.Cuevas BT, Hughes DC, Parma DL, Trevino-Whitaker RA, Ghosh S, Li R, et al. Motivation, exercise, and stress in breast cancer survivors. Support Care Cancer. 2014;22(4):911–7. doi: 10.1007/s00520-013-2038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Creamer M, Bell R, Failla S. Psychometric properties of the Impact of Event Scale - Revised. Behav Res Ther. 2003;41(12):1489–96. doi: 10.1016/j.brat.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 57.Civilotti C, Castelli L, Binaschi L, Cussino M, Tesio V, Di Fini G, et al. Dissociative symptomatology in cancer patients. Front Psychol. 2015;6:118. doi: 10.3389/fpsyg.2015.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sundin EC, Horowitz MJ. Impact of Event Scale: psychometric properties. Br J Psychiatry. 2002;180:205–9. doi: 10.1192/bjp.180.3.205. [DOI] [PubMed] [Google Scholar]
- 59.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Padilla GV, Ferrell B, Grant MM, Rhiner M. Defining the content domain of quality of life for cancer patients with pain. Cancer Nurs. 1990;13(2):108–15. [PubMed] [Google Scholar]
- 61.Padilla GV, Presant C, Grant MM, Metter G, Lipsett J, Heide F. Quality of life index for patients with cancer. Res Nurs Health. 1983;6(3):117–26. doi: 10.1002/nur.4770060305. [DOI] [PubMed] [Google Scholar]
- 62.Ferrell BR, Dow KH, Grant M. Measurement of the quality of life in cancer survivors. Qual Life Res. 1995;4(6):523–31. doi: 10.1007/BF00634747. [DOI] [PubMed] [Google Scholar]
- 63.Ferrell BR. The impact of pain on quality of life. A decade of research. Nurs Clin North Am. 1995;30(4):609–24. [PubMed] [Google Scholar]
- 64.SPSS. IBM SPSS for Windows (Version 23) Armonk, NY: SPSS, Inc; 2015. [Google Scholar]
- 65.Bainbridge KE, Wallhagen MI. Hearing loss in an aging American population: extent, impact, and management. Annu Rev Public Health. 2014;35:139–52. doi: 10.1146/annurev-publhealth-032013-182510. [DOI] [PubMed] [Google Scholar]
- 66.Hsu WT, Hsu CC, Wen MH, Lin HC, Tsai HT, Su P, et al. Increased risk of depression in patients with acquired sensory hearing loss: A 12-year follow-up study. Medicine. 2016;95(44):e5312. doi: 10.1097/md.0000000000005312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holgers KM, Erlandsson SI, Barrenas ML. Predictive factors for the severity of tinnitus. Audiology. 2000;39(5):284–91. doi: 10.3109/00206090009073093. [DOI] [PubMed] [Google Scholar]
- 68.Pattyn T, Van Den Eede F, Vanneste S, Cassiers L, Veltman DJ, Van De Heyning P, et al. Tinnitus and anxiety disorders: A review. Hear Res. 2016;333:255–65. doi: 10.1016/j.heares.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 69.Tseng CC, Hu LY, Liu ME, Yang AC, Shen CC, Tsai SJ. Risk of depressive disorders following sudden sensorineural hearing loss: A nationwide population-based retrospective cohort study. J Affect Disord. 2016;197:94–9. doi: 10.1016/j.jad.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 70.Gewandter JS, Fan L, Magnuson A, Mustian K, Peppone L, Heckler C, et al. Falls and functional impairments in cancer survivors with chemotherapy-induced peripheral neuropathy (CIPN): a University of Rochester CCOP study. Support Care Cancer. 2013;21(7):2059–66. doi: 10.1007/s00520-013-1766-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiao C, Miller AH, Felger J, Mister D, Liu T, Torres MA. Depressive symptoms and inflammation are independent risk factors of fatigue in breast cancer survivors. Psychol Med. 2017:1–11. doi: 10.1017/s0033291717000150. [DOI] [PubMed] [Google Scholar]
- 72.Penedo FJ, Benedict C, Zhou ES, Rasheed M, Traeger L, Kava BR, et al. Association of stress management skills and perceived stress with physical and emotional well-being among advanced prostrate cancer survivors following androgen deprivation treatment. J Clin Psychol Med Settings. 2013;20(1):25–32. doi: 10.1007/s10880-012-9308-1. [DOI] [PubMed] [Google Scholar]
- 73.Mayer DK, Nasso SF, Earp JA. Defining cancer survivors, their needs, and perspectives on survivorship health care in the USA. Lancet Oncol. 2017;18(1):e11–e8. doi: 10.1016/s1470-2045(16)30573-3. [DOI] [PubMed] [Google Scholar]
- 74.Mols F, Vingerhoets AJ, Coebergh JW, van de Poll-Franse LV. Quality of life among long-term breast cancer survivors: a systematic review. Eur J Cancer. 2005;41(17):2613–9. doi: 10.1016/j.ejca.2005.05.017. S0959-8049(05)00726-4. [DOI] [PubMed] [Google Scholar]
- 75.Jacobs LA, Shulman LN. Follow-up care of cancer survivors: challenges and solutions. Lancet Oncol. 2017;18(1):e19–e29. doi: 10.1016/s1470-2045(16)30386-2. [DOI] [PubMed] [Google Scholar]
- 76.Sloan JA, Frost MH, Berzon R, Dueck A, Guyatt G, Moinpour C, et al. The clinical significance of quality of life assessments in oncology: a summary for clinicians. Support Care Cancer. 2006;14(10):988–98. doi: 10.1007/s00520-006-0085-y. [DOI] [PubMed] [Google Scholar]
- 77.Osoba D. Interpreting the meaningfulness of changes in health-related quality of life scores: lessons from studies in adults. Int J Cancer Suppl. 1999;12:132–7. doi: 10.1002/(SICI)1097-0215(1999)83:12+<132::AID-IJC23>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]