Abstract
Objective
To understand the relationship between the timing of initiation of nutritional support in children with severe traumatic brain injury (TBI) and outcomes.
Design
Secondary analysis of a randomized, controlled trial of therapeutic hypothermia (Pediatric Traumatic Brain Injury Consortium: Hypothermia, also known as the Cool Kids Trial (NCT 00222742).
Setting
Fifteen clinical sites in the US, Australia and New Zealand.
Subjects
Inclusion criteria included: (i) age < 18 y, (ii) post-resuscitation Glasgow coma scale (GCS) ≤ 8, (iii) GCS motor score < 6 and (iv) available to be randomized within 6 h after injury. Exclusion criteria included: normal head CT, GCS = 3, hypotension for > 10 min (<5th% for age), uncorrectable coagulopathy, hypoxia (SaO2 < 90% for > 30 min), pregnancy, penetrating injury and unavailability of a parent or guardian to consent at centers without emergency waiver of consent.
Interventions
Therapeutic hypothermia (32 – 33°C for 48 h) followed by slow rewarming for the primary study. For this analysis, the only intervention was the extraction of data regarding nutritional support from the existing database.
Measurements and Main Results
Timing of initiation of nutritional support was determined and patients stratified into 4 groups (Group 1 – no nutritional support over first 7 days; Group 2 – nutritional support initiated < 48 h after injury; Group 3 – nutritional support initiated 48 h - < 72 h after injury; Group 4 – nutritional support initiated 72 h – 168 h after injury). Outcomes were also stratified (mortality and Glasgow Outcomes Scale-Extended for Pediatrics [GOS-E Peds; 1–4, 5–7, 8] at 6 m and 12 m. Mixed-effects models were performed to define the relationship between nutrition and outcome. Children (n = 90, 77 randomized, 13 run-in) were enrolled (mean GCS= 5.8); the mortality rate was 13.3%. 57.8% of subjects received hypothermia Initiation of nutrition before 72 h was associated with survival (p = 0.01), favorable 6 m GOS-E Peds (p = 0.03) and favorable 12 m GOS-E Peds (p = 0.04). Specifically, groups 2 and 3 had favorable outcomes versus Group 1.
Conclusions
Initiation of nutritional support before 72 hours after TBI was associated with decreased mortality and favorable outcome in this secondary analysis. While this provides a rationale to initiate nutritional support early after TBI, definitive studies that control for important co-variates (severity of injury, clinical site, calories delivered, parenteral/enteral routes and other factors) are needed to provide definitive evidence on the optimization of the timing of nutritional support after severe TBI in children.
Keywords: nutritional support, severe traumatic brain injury, mortality, functional outcome, hypothermia
Introduction
According to the Centers for Disease Control, more than 73,000 children (age ≤ 19 y) died of traumatic brain injury (TBI) between 1997 and 2007 (1) and more than 145,000 children were living with a TBI-related disability in 2005 (2). Despite this burden of disease, specific treatments or therapeutic maneuvers to improve outcomes – mortality or neuropsychological health – have proven elusive.
For decades, evidenced-based guidelines for the management of severe TBI have been published (3, 4) and all editions – for both adults and children - have emphasized the importance of nutritional support. In the earliest editions of the guidelines, literature regarding caloric requirements after TBI (5–9) and the role of hyperglycemia (10–12) led to initial recommendations regarding these parameters. Contemporary guidelines for adults with severe TBI emphasize the timing/quantity of nutritional support (Level IIA recommendation that patients should be fed to attain basal caloric replacement at least by the 5th day and, at most, the 7th day after injury) and method of feeding (Level IIB recommendation that transgastric jejunal feeding should be initiated to reduce the incidence of ventilator-associated pneumonia) (13). For children, current guidelines state only that an immune-enhanced diet is not recommended due to lack of efficacy (14, 15). Newer studies have begun to address the issue of timing of nutritional support (16–18), yet these have not been incorporated into guidelines to date. In summary, while there has been some evidence emerging regarding the timing of nutritional support for children, concrete recommendations regarding nutritional support have not yet been established.
In addition to limitations of evidenced-based guidelines, it has become apparent that randomized, controlled trials have significant limitations in the field of severe TBI. Lingsma and colleagues found that significant inter-center variations in outcomes existed between hundreds of international studies that likely diminished the ability of the studies to prove their primary endpoint (19) and Clifton has previously argued that such variation played a role in a failed trial of therapeutic hypothermia (20). We have recently completed a randomized, controlled trial of therapeutic hypothermia for children with severe TBI that failed to show an effect on mortality (Pediatric Traumatic Brain Injury Consortium: Hypothermia, also known as the Cool Kids Trial, NCT 00222742)(21). We chose to perform a secondary analysis of this clinical study to determine the potential impact of the timing of initiation of nutritional support on important outcomes that were prospectively measured by the study. Specifically, we hypothesized that early institution of nutritional support would be associated with reduced mortality and a favorable effect on functional outcome (as measured by Glasgow Outcome Scale score Extended for Pediatrics (GOS-E Peds) at 6 months and 12 months after injury.
Methods
The University of Pittsburgh Institutional Review Board reviewed and approved the study. At participating institutions, principal investigators obtained informed consent from parents/guardians for randomization to receive hypothermia or normothermia treatment as well as data collection from medical records. In one center, emergency waiver of consent for randomization followed by obtaining consent from the parents was permitted and one subject was enrolled in this manner. As a part of the overall study, details regarding a number of medical treatments, including the management of metabolic support, were collected. This is a secondary analysis of this information.
The Pediatric Traumatic Brain Injury Consortium: Hypothermia (also known as the Cool Kids Trial, NCT 00222742) was a phase 3, multi-national, randomized, controlled trial designed to assess the efficacy of early, moderate hypothermia (32 – 33°C) with slow rewarming on mortality after pediatric traumatic brain injury (21). Specifically, children were randomized to receive hypothermia for 48 hours or controlled normothermia – with randomization required within the first 6 hours after injury with the primary outcome of improving outcomes at 6 months after injury. Children were eligible for inclusion if they were less than 18 years of age, had a Glasgow coma scale (GCS) ≤ 8, a GCS motor score < 6 after resuscitation and were available to be randomized within 6 hours after injury. Exclusion criteria included a normal head CT, Glasgow coma score of 3, hypotension for > 10 minutes (defined as <5th percentile for age), uncorrectable coagulopathy, hypoxia (oxygen saturation <90% for >30 min after resuscitation), pregnancy, penetrating injury and unavailability of a parent or guardian to consent at centers without emergency waiver of consent. During the performance of the study, the decision was made to allow enrollment of children with GCS = 3 with reactive pupils. Randomization and masking procedures have been previously described and children were enrolled at a total of 17 study hospitals in the USA, New Zealand, and Australia (21).
A clinical protocol was suggested for all study sites participating in the trial, including treatment of intracranial hypertension and other clinical parameters. Study sites were given specific instructions regarding cooling and rewarming of subjects that were mandated by the Coordinating Center and monitored by a Medical Monitor and the Data Safety Monitoring Board (DSMB). An intracranial hypertension algorithm consistent with evidenced-based guidelines was provided to the sites and compliance with this algorithm was strongly encouraged. However, all sites were free to use their clinical standards of care regarding decisions regarding intracranial hypertension as well as institution of basic aspects of care such as nutritional support. In particular, sites were free to provide nutritional support in the quantity, timing and mode (enteral/parenteral) in accordance with their local standards of care.
Data Collection on Metabolic Support and Statistical Analysis
As part of the routine study procedures, clinical sites recorded information regarding metabolic support within the study database. Specifically, sites recorded the amount of enteral or parenteral nutritional support administered every hour for the first 7 days. These procedures were instituted to monitor the fluid balance of subjects because previous studies have demonstrated that fluid balance may have an effect on the safety and/or effectiveness of hypothermia as a therapeutic modality (20). Importantly, specific aspects of nutritional support – caloric density of enteral nutrition, components of parenteral prescriptions, and dextrose concentrations of maintenance intravenous fluids - were not available since they were not associated with the primary hypothesis of the study regarding therapeutic hypothermia.
For this study, we defined the time of institution of nutritional support as the hour after injury when enteral nutrition (via NG tube or equivalent) or parenteral nutrition was started in each subject. We did not consider administration of maintenance solutions of intravenous fluids that contain glucose as nutritional support. We stratified the timing of nutritional support into 4 groups – Group 1 – no nutritional support over the 7 days of acute data collection; Group 2 – nutritional support initiated < 48 h after injury; Group 3 – nutritional support initiated 48 h - < 72 h after injury; Group 4 – nutritional support initiated 72 h – 168 h after injury. We chose these strata because recent evidence suggests that initiation of nutritional support within 72 h was associated with improved outcomes (18). Outcome measures addressed both mortality and functional outcome (Glasgow Outcome Scale score extended for Pediatrics [GOS-E Peds] at 6 m and 12 m after injury. For this analysis, GOS-E Peds was stratified into three groups; favorable outcome (GOS-E Peds = 1 – 4); unfavorable outcome (GOS-E Peds = 5 – 7) and dead (GOS-E Peds = 8).
Descriptive statistics are presented as means ± SEM for continuous variables and percentages for discrete variables. An alpha level of 0.05 was used to test for statistical significance. Data were analyzed with SAS v9.2 (SAS Institute, Inc., Cary, NC). Chi-square tests were used to compare discrete measures across groups. First, logistic regression models with nutrition time as independent variables and mortality as the dependent variable were generated to examine the outcome in an unadjusted manner. Further analyses were conducted with adjustment for baseline characteristics that were independently associated with nutrition time in an adjusted logistic regression model. For GOS-E Peds outcomes, bivariate multinomial regression models were used to assess the association of patient characteristics. The dependent variable was the GOS-E Peds group and the independent variables were the nutrition time and other significant factors from the bivariate model. To determine the independent effect, multinomial regression models were fit for each variable controlling for Hispanic ethnicity with GOS-E Peds group with favorable outcome (GOS-E Peds score 1 – 4) as the reference group. For the discrete characteristics of nutrition time with more than two levels that were statistically significant in the bivariate analysis, pair-wise distributions were carried to assess the association of each level. Bonferroni corrections were used to adjust for multiple comparisons.
Results
For this analysis, a total of 90 children were included – 77 children were randomized to receive either hypothermia or normothermia and 13 children were run-in patients who all received hypothermia as a part of site start-up procedures to ensure compliance with the hypothermia protocol. In the cohort, the mean GCS was 5.8 ± 0.1 and the population was predominantly male (63.3%) and Caucasian (74.4%)(see Table 1). Due to the run-in subjects, a slight majority of subjects received hypothermia (57.8%) and the overall mortality rate was 13.3%. Stratification of the timing of nutritional support generated groups of varying sizes (Group 1 – no nutritional support over the study period, n = 5; Group 2 – nutritional support initiated < 48 h after injury, n = 32; Group 3 – nutritional support initiated 48 h - < 72 h after injury, n = 36; Group 4 – nutritional support initiated 72 h – 168 h after injury, n = 17). Most demographic variables were similar between these 4 groups, except that a disproportionate number of children with Hispanic ethnicity were in Group 1 (p = 0.005). Thus, models to determine associations between the 4 groups and outcomes were performed with and without correction for Hispanic ethnicity.
Table 1.
Baseline characteristics based on nutrition initiation time
| Baseline variables | Total n = 90, (%) |
Groups | p value |
|||
|---|---|---|---|---|---|---|
| 1 n=5 |
2 n=32 |
3 n=36 |
4 n=17 |
|||
| Gender | 0.26 | |||||
| Boys | 57,(63.3) | 4,(80) | 19,(59.4) | 26,(72.2) | 8,(47.1) | |
| Girls | 33,(36.7) | 1,(20) | 13,(40.6) | 10,(27.8) | 9,(52.9) | |
| Race | 0.34 | |||||
| White | 67,(74.4) | 5,(100) | 24,(75) | 25,(69.4) | 13,(76.5) | |
| Black | 15,(16.7) | 0 | 6,(18.7) | 5,(13.9) | 4,(23.5) | |
| Other | 8,(8.9) | 0 | 2,(6.3) | 6,(16.7) | 0 | |
| Hispanic | 0.005* | |||||
| Yes | 19,(21.1) | 4,(80) | 7,(21.9) | 4,(11.1) | 4,(23.5) | |
| No | 71,(78.9) | 1,(20) | 25,(78.1) | 32,(88.9) | 13,(76.5) | |
| Treatment | 0.89 | |||||
| Hypothermia | 52,(57.8) | 3,(60) | 17,(53.1) | 21,(58.3) | 11,(64.7) | |
| Normothermia | 38,(42.2) | 2,(40) | 15,(46.9) | 15,(41.7) | 6,(35.3) | |
| Cause | 0.34 | |||||
| Fall | 16,(17.8) | 2,(40) | 4,(25) | 8,(22.2) | 2,(11.8) | |
| Motor vehicle collision | 61,(67.8) | 3,(60) | 20,(62.5) | 24,(66.7) | 14,(82.3) | |
| Assault | 1,(1.1) | 0 | 0 | 1,(2.8) | 0 | |
| Other | 12,(13.3) | 0 | 8,(25) | 3,(8.3) | 1,(5.9) | |
| GCS score | 0.69 | |||||
| 4 | 17,(18.9) | 1,(20) | 10,(31.3) | 6,(16.7) | 0 | |
| 5 | 16,(17.8) | 1,(20) | 6,(18.7) | 6,(16.7) | 3,(17.6) | |
| 6 | 29,(32.2) | 2,(40) | 8,(25) | 11,(30.5) | 8,(47.1) | |
| 7 | 20,(22.2) | 1,(20) | 6,(18.7) | 9,(25) | 4,(23.5) | |
| 8 | 8,(8.9) | 0 | 2,(6.3) | 4,(11.1) | 2,(11.8) | |
| GCS scores (mean ± SEM) | 5.8,(0.1) | 5.6,(0.5) | 5.5,(0.2) | 6,(0.2) | 6.3,(0.2) | 0.14 |
| AIS (Median, IQR) | ||||||
| Head | 4(1) | 4(1) | 4(1) | 4(1) | 4(2) | 0.99 |
| Face | 1(2) | 0(1) | 1(2) | 1(2) | 1(1) | 0.69 |
| Neck | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) | 0.38 |
| Thorax | 0(1) | 0(0) | 0(1) | 0(2) | 0(1) | 0.63 |
| Abdomen | 0(0) | 0(0) | 0(0) | 0(0) | 0(2) | 0.22 |
| Spine | 0(0) | 0(0) | 0(0) | 0(0) | 0(1) | 0.47 |
| Upper Extremities | 0(1) | 0(0) | 0(0) | 0(1) | 1(1) | 0.009* |
| Lower Extremities | 0(1) | 1(1) | 0(1.5) | 0(1) | 1(2) | 0.12 |
| External | 0(1) | 0(1) | 0(1) | 0(0) | 0(0) | 0.25 |
| AIS-Severity Score | 26.5(16) | 18(14) | 25.5(16.5) | 28.5(16) | 27(17) | 0.54 |
Group 1 – no nutritional support administered during study period; Group 2 – nutritional support initiated < 48 h after injury; Group 3 – nutritional support initiated 48 h – < 72 h after injury; Group 4 – nutritional support initiated 72 h – 168 h after injury; GCS – Glasgow Coma Scale score at the time of enrollment into the study
The distribution of the time to initiation of nutritional support of the cohort is shown in Figure 1. Overall, duration of time until nutrition was initiated was associated with mortality (mortality rates: Group 1 = 60%, Group 2 = 6.3%, Group 3 = 11.1%, Group 4 = 17.6%, p = 0.01; Table 2), with earlier initiation of support associated with improved survival. In post-hoc comparisons, differences between Group 1 and Groups 2 and 3 were statistically significant (p = 0.001 and 0.006, respectively) while Groups 1 and 4 tended to be different (p = 0.06). Similarly, time to nutrition initiation was associated with GOS-E Peds at 6 m and 12 m (p = 0.03 and 0.04, respectively, Tables 3 and 4). At both time points, Group 1 had lower GOS-E Peds scores compared to Groups 2 and 3 (6 m: p = 0.007 and 0.02, respectively; 12 m: p = 0.005 and p = 0.03, respectively) indicating that earlier nutrition initiation was associated with improved GOS-E Peds compared to the group that did not receive nutrition during the study period. Other comparisons between groups were not associated with differences in outcomes.
Figure 1.
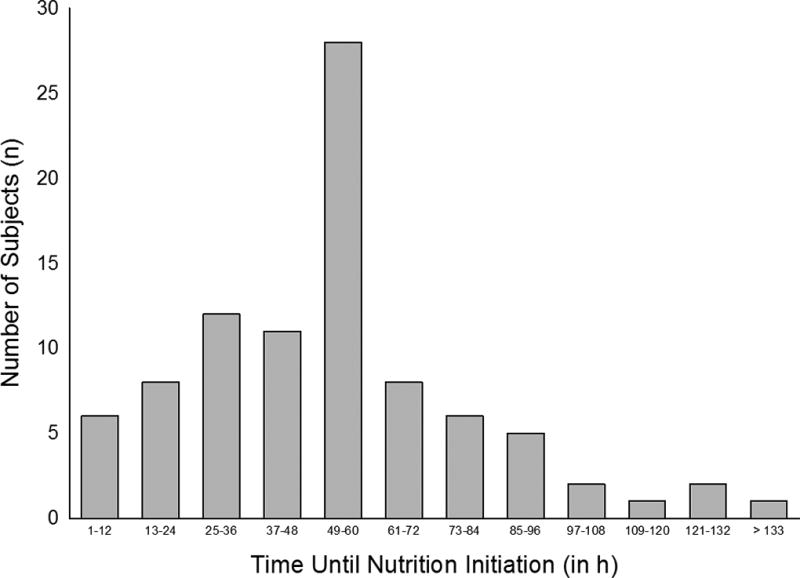
The distribution of the time to initiation of nutritional support.
Table 2.
Relationship between nutrition initiation time group and mortality
| n = 90, (%) | Mortality | p value | ||
|---|---|---|---|---|
| No (n = 78, 86.7%) |
Yes (n = 12, 13.3%) |
|||
| Nutrition Group | 0.01* | |||
| Group 1 | 5,(5.6) | 2,(40) | 3,(60) | |
| Group 2 | 32,(35.5) | 30,(93.7) | 2,(6.3) | |
| Group 3 | 36,(40) | 32,(88.9) | 4,(11.1) | |
| Group 4 | 17,(18.9) | 14,(82.4) | 3,(17.6) | |
Pairwise comparisons: Group 1 vs. 2, p = 0.001*; Group 1 vs. 3, p = 0.006*; Group 1 vs. 4, p = 0.06; Group 2 vs. 3, p = 0.48; Group 2 vs. 4, p = 0.21; Group 3 vs. 4, p = 0.51.
Group 1 – no nutritional support administered during study period; Group 2 – nutritional support initiated < 48 h after injury; Group 3 – nutritional support initiated 48 h – < 72 h after injury; Group 4 – nutritional support initiated 72 h – 168 h after injury
Table 3.
Relationship between nutrition initiation time group and 6 month GOS-E Peds score
| n = 87 (%) | 6 month GOS-E Peds | p value | |||
|---|---|---|---|---|---|
| 1 – 4 (n = 43, 49.4%) |
5 – 7 (n=32, 36.8%) |
8 (12, 13.8%) |
|||
| Nutrition Group | 0.03* | ||||
| Group 1 | 5,(5.7) | 1,(20) | 1,(20) | 3,(60) | |
| Group 2 | 30,(34.5) | 16,(53.3) | 12,(40) | 2,(6.7) | |
| Group 3 | 36,(41.4) | 21,(58.3) | 11,(30.6) | 4,(11.1) | |
| Group 4 | 16,(18.4) | 5,(31.2) | 8,(50) | 3,(18.8) | |
Pairwise comparisons: Group 1 vs. 2, p = 0.007*; Group 1 vs. 3, p = 0.02*; Group 1 vs. 4, p = 0.20; Group 2 vs. 3, p = 0.65; Group 2 vs. 4, p = 0.25; Group 3 vs. 4, p = 0.20.
GOS-E Peds, Glasgow Outcome Scale extended for Pediatrics; Group 1 – no nutritional support administered during study period; Group 2 – nutritional support initiated < 48 h after injury; Group 3 – nutritional support initiated 48 h – < 72 h after injury; Group 4 – nutritional support initiated 72 h – 168 h after injury
Table 4.
Relationship between nutrition initiation time group and 12 month GOS-E Peds score
| n = 86 (%) | 12 month GOS-E Peds | p value | |||
|---|---|---|---|---|---|
| 1 – 4 (n = 49, 57%) |
5 – 7 (n = 25, 29.1%) |
8 (n = 12, 13.9%) |
|||
| Nutrition Group | 0.04* | ||||
| Group 1 | 5,(5.8) | 1,(20) | 1,(20) | 3,(60) | |
| Group 2 | 32,(37.2) | 19,(59.4) | 11,(34.4) | 2,(6.2) | |
| Group 3 | 33,(38.4) | 22,(66.7) | 7,(21.2) | 4,(12.1) | |
| Group 4 | 16,(18.6) | 7,(43.7) | 6,(37.5) | 3,(18.8) | |
Pairwise comparisons: Group 1 vs. 2, p = 0.005*; Group 1 vs. 3, p = 0.03*; Group 1 vs. 4, p = 0.20; Group 2 vs. 3, p = 0.41; Group 2 vs. 4, p = 0.35; Group 3 vs. 4, p = 0.31
GOS-E Peds, Glasgow Outcome Scale extended for Pediatrics; Group 1 – no nutritional support administered during study period; Group 2 – nutritional support initiated < 48 h after injury; Group 3 – nutritional support initiated 48 h – < 72 h after injury; Group 4 – nutritional support initiated 72 h – 168 h after injury
Time to initiation of nutrition was associated with mortality (p = 0.05) with Group 1 having a 22-fold greater odds of mortality when compared to Group 2 (Table 5). When adjusted for Hispanic ethnicity, this relationship failed to reach statistical significance (p = 0.10). There were trends toward an association between initiation of nutrition and GOS-E Peds at 6 m and 12 m (p = 0.11 and p = 0.13, Table 6) but these failed to reach statistical significance. We attempted to control for AIS score for upper extremity because this variable was different between groups. However, addition of this variable to the model resulted in failure of the model to converge.
Table 5.
Logistic regression analysis (unadjusted and adjusted for Hispanic ethnicity) to determine the association between nutrition initiation time group and mortality
| OR (95% CI) | P value | |
|---|---|---|
| Nutrition time group (Ref = Group 2) | 0.05 | |
| Group 1 | 22.5(2.3–222.5) | |
| Group 3 | 1.9(0.32–10.9) | |
| Group 4 | 3.2(0.48–21.5) | |
| Adjusted for Hispanic ethnicity | ||
| Nutrition time group (Ref = Group 2) | 0.10 | |
| Group 1 | 22.4(1.8–271.7) | |
| Group 3 | 1.9(0.31–11.1) | |
| Group 4 | 3.2(0.48–21.4) |
GOS-E Peds, Glasgow Outcome Scale extended for Pediatrics; Group 1 – no nutritional support administered during study period; Group 2 – nutritional support initiated < 48 h after injury; Group 3 – nutritional support initiated 48 h – < 72 h after injury; Group 4 – nutritional support initiated 72 h – 168 h after injury
Table 6.
Logistic regression analysis to determine the association between nutrition initiation time group and 6- and 12-month GOS-E Peds score
| 6 Month GOS-E Peds OR(95% CI) | p value | ||
| 5 – 7 | 8 | ||
| Nutrition time group (Ref = Group 2) | 0.11 | ||
| Group 1 | 1.1(0.1–9.3) | 6.6(1.1–39.6) | |
| Group 3 | 0.6(0.2–1.5) | 0.4(0.1–1.3) | |
| Group 4 | 1.8(0.6–5.5) | 1.3(0.4–4.8) | |
| 12 Month GOS-E Peds | |||
| 5 – 7 | 8 | ||
| Nutrition time group (Ref = Group 2) | 0.13 | ||
| Group 1 | 1.59(0.2–13.2) | 7.57(1.3–45.3) | |
| Group 3 | 0.50(0.2–1.37) | 0.46(0.2–1.4) | |
| Group 4 | 1.36(0.5–4.12) | 1.08(0.3–3.8) | |
For 6 month GOS-E Peds, p = 0.19 after adjustment for Hispanic ethnicity; for 12 month GOS-E Peds, p = 0.20 after adjustment for Hispanic Ethnicity.
GOS-E Peds, Glasgow Outcome Scale extended for Pediatrics; Group 1 – no nutritional support administered during study period; Group 2 – nutritional support initiated < 48 h after injury; Group 3 – nutritional support initiated 48 h – < 72 h after injury; Group 4 – nutritional support initiated 72 h – 168 h after injury
Discussion
In our secondary analysis of this randomized, controlled trial of therapeutic hypothermia, we have found an association between early initiation of nutritional support and survival and have found trends toward an association between this nutritional parameter and functional outcomes as measured by the GOS-E Peds at 6 m and 12 m after the injury. Specifically, these findings support the hypothesis that earlier institution of nutrition may be superior to not instituting such support during the first week after injury. However, due to the nature of the study design, a cause and effect cannot be established with our data. Of interest, we also observed an effect on Hispanic ethnicity on nutritional support which has not been previously described.
As outlined earlier, the current recommendations for nutritional support for children with severe TBI are limited to the avoidance of an immune-enhanced diet (14, 15). Since publication of these recommendations, Taha and colleagues have performed a retrospective study of 109 children with severe TBI (median age = 13 y) (17). In that study, the median time to initiate nutritional support was quite early – 1.49 d [0.02 d – 11.88 d] and early initiation of nutritional support was associated with (i) decreased length of stay in the intensive care unit (p < 0.01) and (ii) improved functional outcome at hospital discharge (p < 0.05, as measured by the Pediatric Cerebral Performance Category [PCPC]). Our findings are largely consistent with these - in that we also found that earlier initiation of nutritional support was associated with improved mortality and trends toward improvements in functional outcomes at times after the injury. However, important differences exist between the two studies. First, their study included a majority of children with GCS = 3 at admission, while ours excluded these children. Second, their study measured the outcomes at hospital discharge and did not examine the relationship between timing of nutritional support and mortality. Lastly, several aspects of their study population is quite different from ours – 11.9% with normal CT scans and “mortality/coma” rate of 24.7% and earlier initiation of nutritional support – which may have significant effects on the interpretation of the two studies.
In another single center study, Malakouti and colleagues studied the effect of nutritional support on important clinical outcomes of 101 children with severe TBI over an 11-year period (16). In this comprehensive assessment, the authors found that nutrition (all enteral and some supplemented with parenteral) was initiated within 53 h ± 20 [12 h – 162 h] with 52% of subjects initiating nutritional support within 48 h and 82% initiated within the first 72 h after injury. Since their study design included a focused review of many nutritional parameters, they were able to determine that children who were able to meet their caloric and protein goals had an earlier initiation of nutritional support (goals met vs. not met: calories - 44 h ± 23 vs. 67 h ± 31, p < 0.001; protein – 43 h ± 17 vs. 65 h ± 29, p = 0.001). These findings are also generally consistent with our study, in that earlier institution of nutritional support was associated with benefit to children. However, there are important differences between the two studies. First, our multi-institutional study exhibited much more variation in clinical practices regarding nutrition – and included the test intervention of hypothermia which was the primary reason for the initiation of the study. Second, their study did not directly test our hypotheses that timing of initiation of nutritional support would be associated with mortality or functional outcome at times after hospital discharge. Their overall mortality rate was quite low – 4% - so it appears unlikely that any mortality effect could be detectable in this patient population. Lastly, they were able to compare the effect of early feeding on surrogate markers that were not collected in our study.
Most recently, the Pediatric Guideline Adherence and Outcomes Study (also known as the PEGASUS study) was performed to determine the acute care clinical indicators that are associated with outcomes after severe TBI in children (18). These indicators – based on the pediatric guidelines published in 2003 – were gathered from information from the prehospital, emergency department, operating room and intensive care unit. A total of 236 children were enrolled in 5 centers within the US and they found that early start of nutritional support – defined as start of nutritional support within 72 h after injury - was associated with decreased mortality (hazard ratio 0.06, CI, 0.01 – 0.26) but not discharge GOS scale score. As with the previous studies, our findings are largely consistent with these data, in that we found that children who had nutritional support initiated within the first 72 h had a higher survival rate than children who did not receive nutritional support within the first 7 days after injury. However, again, this study did not test the relationship between nutrition initiation and functional outcomes at times after hospital discharge.
Our study has significant limitations. Since it is a secondary analysis of a randomized, controlled trial for therapeutic hypothermia, the database was not designed to gather information necessary to explore more detailed analyses of nutritional parameters of interest. In particular, we could not determine the quantity (caloric density or protein content) or quality (percentage of carbohydrate, fat and protein administered) of nutritional support that was provided that might have significant effects on outcomes. It seems likely that the timing of nutritional support may be a marker of these other factors and a more detailed exploration may be informative regarding how to initiate nutrition to optimize outcomes in these children. Moreover, since more than half of the children in our study received therapeutic hypothermia, there could be important effects of hypothermia on nutritional requirements or the decision to initiate nutritional support that we were not able to measure with our study design. Lastly, we had a relatively small sample size that limited our ability to control for potential confounders including but not limited to the effect of the clinical site, the effect of post-TBI rehabilitation and the mode of nutritional support (enteral vs. parenteral) on outcomes. We observed a statistically significant difference in time to initiate nutritional support between various ethnicities, with those of Hispanic ethnicity overrepresented within the group that did not receive support within the first 7 days after injury. We speculate that this may be related to differences in the practices across the clinical sites. Although this would be an important finding, the limited numbers of subjects restricts our ability to test this hypothesis. Injury severity may also act as a patient confounder as more severe TBI as well as severe abdominal injuries may lead to decisions that alter the timing of nutritional support. We attempted to mitigate this confounder by controlling for both AIS and GCS scores, but our analysis would obviously be more robust with a larger sample size.
There are practical implications of this study. Our study adds to the literature that suggests that earlier institution of nutritional support can be beneficial to children with severe TBI and other critical illnesses. However, our findings need to be interpreted with caution. Recently, a randomized controlled trial of 1440 critically-ill children – with some children with severe TBI but the vast majority with other critical illnesses – surprisingly demonstrated that withholding nutritional support for > 7 days was associated with decreased infection rates, shorter ICU stays, decreased number of days on mechanical ventilation with equal mortality rates when compared to those where nutrition was initiated within the first 24 h (22). Given these conflicting findings, we believe that testing the various nutritional strategies that are already in clinical use – while controlling for important co-variates (severity of injury, clinical site, calories delivered, route of administration among many other factors) is a vital next step in our understanding how nutrition should be administered to children with severe TBI.
Acknowledgments
The Pediatric Traumatic Brain Injury Consortium: Hypothermia was funded by NINDS (NS052478; NCT 00222742).
Dr. Meinert’s institution received funding from the National Institute of Neurological Disorders and Stroke (NINDS), and she received funding from Society of Critical Care Medicine (SCCM) (travel grant). Dr. Bell’s institution received funding from NINDS, and he received support for article research from the National Institutes of Health (NIH). Dr. Buttram’s institution received funding from the National Institute of Child Health and Human Development; she received support for article research from the NIH; and she disclosed she is a DSMB member for study evaluating Early Rehabilitation after Acute Brain Injury at the University of Pittsburgh funded by PICORI. Dr. Kochanek received funding from SCCM via an editor stipend for his services as Editor-in-Chief for Pediatric Critical Care Medicine. Dr. Wisniewski’s institution received funding from the NIH, and he received support for article research from the NIH. Dr. Adelson’s institution received funding from the NIH, Codman Neuro, and the National Center for Advancing Translational Sciences; he received funding from Phoenix Children's Hospital, Adelson Medical Consulting, and Thieme Publishing; and he received support for article research from the NIH.
Footnotes
Copyright form disclosure: Dr. Balasubramani disclosed that the does not have any potential conflicts of interest.
References
- 1.Coronado VG, Xu L, Basavaraju SV, et al. Surveillance for traumatic brain injury-related deaths--United States, 1997–2007. MMWR Surveill Summ. 2011;60(5):1–32. [PubMed] [Google Scholar]
- 2.Zaloshnja E, Miller T, Langlois JA, et al. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J Head Trauma Rehabil. 2008;23(6):394–400. doi: 10.1097/01.HTR.0000341435.52004.ac. [DOI] [PubMed] [Google Scholar]
- 3.Bullock R, Chesnut RM, Clifton G, et al. Guidelines for the management of severe head injury. Brain Trauma Foundation. Eur J Emerg Med. 1996;3(2):109–127. doi: 10.1097/00063110-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Carney NA, Chesnut R, Kochanek PM, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Pediatr Crit Care Med. 2003;4(3 Suppl):S1. doi: 10.1097/01.CCM.0000067635.95882.24. [DOI] [PubMed] [Google Scholar]
- 5.Young B, Ott L, Norton J, et al. Metabolic and nutritional sequelae in the non-steroid treated head injury patient. Neurosurgery. 1985;17(5):784–791. doi: 10.1227/00006123-198511000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Clifton GL, Robertson CS, Choi SC. Assessment of nutritional requirements of head-injured patients. J Neurosurg. 1986;64(6):895–901. doi: 10.3171/jns.1986.64.6.0895. [DOI] [PubMed] [Google Scholar]
- 7.Deutschman CS, Konstantinides FN, Raup S, et al. Physiological and metabolic response to isolated closed-head injury. Part 1: Basal metabolic state: correlations of metabolic and physiological parameters with fasting and stressed controls. J Neurosurg. 1986;64(1):89–98. doi: 10.3171/jns.1986.64.1.0089. [DOI] [PubMed] [Google Scholar]
- 8.Phillips R, Ott L, Young B, et al. Nutritional support and measured energy expenditure of the child and adolescent with head injury. J Neurosurg. 1987;67(6):846–851. doi: 10.3171/jns.1987.67.6.0846. [DOI] [PubMed] [Google Scholar]
- 9.Moore R, Najarian MP, Konvolinka CW. Measured energy expenditure in severe head trauma. J Trauma. 1989;29(12):1633–1636. doi: 10.1097/00005373-198912000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Young B, Ott L, Dempsey R, et al. Relationship between admission hyperglycemia and neurologic outcome of severely brain-injured patients. Ann Surg. 1989;210(4):466–472. doi: 10.1097/00000658-198910000-00007. discussion 472-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam AM, Winn HR, Cullen BF, et al. Hyperglycemia and neurological outcome in patients with head injury. J Neurosurg. 1991;75(4):545–551. doi: 10.3171/jns.1991.75.4.0545. [DOI] [PubMed] [Google Scholar]
- 12.Michaud LJ, Rivara FP, Longstreth WT, Jr, et al. Elevated initial blood glucose levels and poor outcome following severe brain injuries in children. J Trauma. 1991;31(10):1356–1362. doi: 10.1097/00005373-199110000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Carney N, Totten AM, O'Reilly C, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2016 doi: 10.1227/NEU.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 14.Briassoulis G, Filippou O, Kanariou M, et al. Temporal nutritional and inflammatory changes in children with severe head injury fed a regular or an immune-enhancing diet: A randomized, controlled trial. Pediatr Crit Care Med. 2006;7(1):56–62. doi: 10.1097/01.pcc.0000192339.44871.26. [DOI] [PubMed] [Google Scholar]
- 15.Kochanek PK, Carney NA, Adelson PD, et al. Guidelines for the Acute Medical Management of Severe Traumatic Brain Injury in Infants, Children and Adolescents: Second Edition. Pediatr Crit Care Med. 2012;13(1 (Suppl)):S1–S82. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- 16.Malakouti A, Sookplung P, Siriussawakul A, et al. Nutrition support and deficiencies in children with severe traumatic brain injury. Pediatr Crit Care Med. 2012;13(1):e18–24. doi: 10.1097/PCC.0b013e31820aba1f. [DOI] [PubMed] [Google Scholar]
- 17.Taha AA, Badr L, Westlake C, et al. Effect of early nutritional support on intensive care unit length of stay and neurological status at discharge in children with severe traumatic brain injury. The Journal of neuroscience nursing : journal of the American Association of Neuroscience Nurses. 2011;43(6):291–297. doi: 10.1097/JNN.0b013e318234e9b2. [DOI] [PubMed] [Google Scholar]
- 18.Vavilala MS, Kernic MA, Wang J, et al. Acute care clinical indicators associated with discharge outcomes in children with severe traumatic brain injury. Crit Care Med. 2014;42(10):2258–2266. doi: 10.1097/CCM.0000000000000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lingsma HF, Roozenbeek B, Li B, et al. Large between-center differences in outcome after moderate and severe traumatic brain injury in the international mission on prognosis and clinical trial design in traumatic brain injury (IMPACT) study. Neurosurgery. 2011;68(3):601–607. doi: 10.1227/NEU.0b013e318209333b. discussion 607–608. [DOI] [PubMed] [Google Scholar]
- 20.Clifton GL, Choi SC, Miller ER, et al. Intercenter variance in clinical trials of head trauma--experience of the National Acute Brain Injury Study: Hypothermia. J Neurosurg. 2001;95(5):751–755. doi: 10.3171/jns.2001.95.5.0751. [DOI] [PubMed] [Google Scholar]
- 21.Adelson PD, Wisniewski SR, Beca J, et al. Comparison of hypothermia and normothermia after severe traumatic brain injury in children (Cool Kids): a phase 3, randomised controlled trial. Lancet Neurol. 2013;12(6):546–553. doi: 10.1016/S1474-4422(13)70077-2. [DOI] [PubMed] [Google Scholar]
- 22.Fivez T, Kerklaan D, Mesotten D, et al. Early versus Late Parenteral Nutrition in Critically Ill Children. N Engl J Med. 2016;374(12):1111–1122. doi: 10.1056/NEJMoa1514762. [DOI] [PubMed] [Google Scholar]


