Abstract
Background
This trial examined the efficacy of a clinic-based weight loss intervention in cancer survivors.
Methods
This single-center phase II trial randomized survivors of solid tumors and hematologic malignancies to a 15-week group-based weight loss intervention that included caloric restriction and physical activity (n=30) or a wait-list control intervention (n=30). The primary study outcome was body mass. Secondary study outcomes included body composition using dual-energy x-ray absorptiometry, physical fitness using the six-minute walk test (6MWT), and concentrations of serum biomarkers.
Results
Participants in the intervention group lost 5.6±4.4% of baseline weight (4.6±3.9 kg), whereas participants in the control group gained 0.2±2.4% of baseline weight (0.2±2.0 kg); intervention effect −5.8% (95% CI: −7.8, −3.8); −4.8 kg (95% CI: −6.6, −3.0); P=0.0001. A larger proportion of participants in the intervention group lost ≥5% of baseline weight compared to the control group (43% v 0%; P<0.0001). The intervention led to reductions in fat mass (−3.2±0.7 kg; P<0.0001), improvements in physical fitness (an increase of 22.6±10.8 m on 6MWT; P=0.03), and reductions in concentrations of insulin (−7.7±3.5 μU/mL; P=0.004) and leptin (−7.3±4.0 ng/mL; P=0.04).
Conclusion
A 15-week clinic-based weight loss intervention resulted in significant weight loss, and improvements in body composition, physical fitness, and concentrations of serum biomarkers in cancer survivors.
Implications for Cancer Survivors
Weight loss programs provide a number of benefits for cancer survivors; survivors should inquire about the availability of lifestyle programs offered at their cancer center and within their local communities.
Keywords: body composition, body mass index, obesity, insulin, adipokines, inflammation, prognosis
INTRODUCTION
One-in-three cancer survivors in the United States is obese [1]. Obesity is associated with disease recurrence and cancer-specific mortality among individuals with early-stage breast, gynecologic, genitourinary, gastrointestinal, and hematologic malignancies [2–5]. The biologic mechanisms that mediate the relationship between obesity and cancer prognosis may include dysregulation of insulin, adipokine, and inflammatory pathways [6–8]. Obesity is also associated with a higher risk of developing adverse treatment-related effects and cardiovascular disease in cancer survivors [9]. Effective weight management programs are therefore needed for cancer survivors.
Two recent large randomized trials demonstrated that telephone- and group-based weight loss interventions are feasible and produce statistically significant weight loss in cancer survivors [10, 11]. Smaller randomized trials have demonstrated that weight loss may lead to favorable changes in insulin, adipokine, and inflammatory measures in these patients [12–14]. However, most weight loss trials conducted to date have enrolled highly-selected homogeneous samples, required a high level of staff expertise for intervention delivery, have not been manualized or packaged for immediate dissemination, and have not been seamlessly embedded into referral pathways for oncology providers. The interaction among these factors may serve as barriers to the rapid implementation of weight loss programs in cancer centers across the United States [15].
Studies in primary care populations demonstrate that clinic-based weight loss programs are efficacious when led a range of trained intervention staff (e.g., medical assistants, exercise specialists, registered dietitians, health counselors, and laypersons) [16]. Consequently, we sought to develop a clinic-based weight loss intervention for cancer survivors—The Healthy Living and Eating Program—that utilized a manualized intervention guidebook, which could be delivered by a variety of health and wellness staff often employed within cancer centers, and therefore had the potential to be broadly disseminated and readily implemented at cancer centers across the United States.
The Healthy Living After Cancer Trial was a randomized trial designed to evaluate the efficacy of a 15-week clinic-based weight loss intervention in a diverse group of overweight and obese cancer survivors and to provide justification, feasibility data, and support for implementing this intervention in a clinic setting within our comprehensive cancer center. The primary aim of the study was to evaluate the efficacy of the intervention in lowering body mass. The secondary aims of the study were to evaluate the effect of the intervention on body composition, physical fitness, and concentrations of serum biomarkers linked to cancer risk and prognosis.
METHODS
Study Design
The Healthy Living After Cancer Trial was a single-center, phase II, randomized trial.
Participants
Patients were enrolled from Medical Oncology Clinics, the Leonard P. Zakim Center for Integrative Therapies, and the Adult Survivorship Clinic at the Dana-Farber Cancer Institute. Participants were recruited from March 2014 to August 2015 using electronic medical record review, flyers placed throughout the cancer center, and oncology provider referral. Eligibility criteria included prior diagnosis of malignancy, body mass index (BMI) >25.0 kg/m2, completion of all surgery, chemotherapy, and/or radiation at least one month prior to study enrollment (concurrent treatment with adjuvant hormonal or biologic therapies was acceptable); ECOG performance status of 0 (fully active without restriction) or 1 (restricted in physically strenuous activity, but ambulatory); and the ability to both walk two city blocks and to speak and read English. Patients were not eligible if they had other serious medical conditions such as unstable cardiovascular disease or digestive disorders that would preclude participation in a physical activity and dietary intervention. Inclusion and exclusion criteria were screened using the electronic medical record. Medical clearance was provided from all patients’ treating clinicians prior to enrollment. The study was approved by the Institutional Review Board at the Dana-Farber Cancer Institute, and all patients signed informed consent prior to study participation.
Randomization, Stratification, and Blinding
Participants were randomly allocated in a 1:1 ratio using computer allocation to either a weight loss intervention or wait-list control group. Randomization was stratified by sex. After completing baseline measures, the study coordinator informed the participant of the randomized group assignment. Outcome measures were obtained by assessors blinded to treatment assignment.
Study Intervention
Participants randomized to receive the weight loss intervention participated in a 15-week, in-person, group-based program that was led by a health coach with a background in nutrition and an exercise physiologist. The behavioral content of the program described herein was modeled after the Lifestyle Intervention in Adjuvant Treatment of Early Breast Cancer (LISA) study [10]. The goal of the intervention was to induce a 7% weight loss (0.5–1.0 kg per week) to a BMI not less than 21 kg/m2, as this amount of weight loss is associated with cardio-metabolic benefits, while still maintaining a healthy body mass [17]. This was achieved using individualized caloric restriction goals to attain a 500–1,000 kcal per day deficit, with an initial caloric consumption recommendation of 1,250–1,750 kcal per day, based on baseline BMI. Dietary recommendations were tailored throughout the intervention as necessary, based on current weight and weight loss target. Moderate-intensity aerobic physical activity was individualized using baseline physical activity volume, and prescribed towards the goal of achieving 150–200 minutes per week. Behavioral change techniques using the Social Cognitive Theory were focused on motivation, relapse prevention, emotional distress, time management, and overcoming barriers. Behavioral change techniques were integrated into study materials and in-person group meetings [18]. Weekly, in-person, 50-minute intervention group meetings included up to eight participants. Planned weekly topics of the group meetings are described in detail elsewhere [19], but broadly included lessons relating to behavioral self-management of healthy eating, portion control, and physical activity. At the in-person meetings, participants were weighed by study staff. Participants received a workbook that included worksheets to complement the topics discussed at the in-person meetings. At the completion of the didactic portion of the group session, participants were provided with individualized dietary and exercise goals for the subsequent week. At the start of the intervention, participants were provided with an activity monitor (Fitbit Flex™) and a journal to record the food they consumed and recreational physical activity they performed each day. The Fitbit was used as a motivational device; using the Fitbit online portal, participants could review their activity and could friend other group members to promote accountability in attaining weekly physical activity goals. Fitbit data were available to the study staff for weekly goal setting, but were not used as efficacy endpoints. Participants reviewed their journals each week with study staff. The Healthy Living intervention was manualized pragmatically, such that health coaches, personal trainers, or other allied health professionals would be able to successfully deliver the intervention to a diverse population of cancer survivors.
Participants randomized to receive the wait-list control intervention were not given support to make diet and exercise changes during the 15-week control period. Following this control period, control group participants were provided with the same weight loss program delivered to the intervention group.
Measurements
Demographic and Clinical Characteristics
Demographic characteristics (age, sex, race, and education) were self-reported using paper surveys. Clinical characteristics (type of cancer and cancer therapies) were obtained from the electronic medical record.
Body Mass and Body Composition
The following measures were obtained at baseline and 15-weeks. Body mass (kg) and height (m) were measured in duplicate and used to calculate BMI (kg/m2). Body composition was measured using whole-body dual-energy x-ray absorptiometry (DXA) imaging (Hologic, Bedford MA). The DXA scanner was calibrated daily using an anthropomorphic spine phantom and thrice weekly using a whole-body phantom. DXA was used to quantify fat mass (kg), lean mass (kg), and bone mineral density (g/m2) using Hologic APEX software.
Physical Fitness
Physical fitness was quantified using the six-minute walk test (m) [20]. Participants were asked to walk for six-minutes at a sustainable pace, on a 30-m (100’) course. The total distance walked in six-minutes was recorded.
Physical Activity
Moderate-to-vigorous intensity physical activity (min/wk) was quantified using an accelerometer (ActiGraph GT3X+) applying the Trioano cut points [21]. Participants were provided with the accelerometer at an in-person visit and asked to wear for seven-days; four-days of valid wear with ≥600 minutes each day were required for analysis. Accelerometers were returned using a self-addressed stamped envelope. Self-reported physical activity was quantified using an interviewer-administered seven-day recall [22].
Dietary Intake
Dietary intake was quantified using the Block 2005 food frequency questionnaire in a paper format [23]. A three-month recall period was used for dietary intake.
Serum Biomarkers
A fasting (≥12 hour) blood draw was conducted and serum samples were stored at −80°C until assayed. Insulin (μU/mL), leptin (ng/mL), and adiponectin (ng/mL) concentrations were quantified with a radioimmunoassay; C-Reactive Protein (CRP) concentration (ng/L) was quantified with an automated chemistry analyzer; and interleukin (IL)-6 concentration (pg/mL) was quantified with an enzyme-linked immunosorbent assay. Baseline and 15-week serum samples were assayed simultaneously and in duplicate at the end of the study (the coefficients of variation for all assays were ≤10%).
Adverse Events
Adverse events were assessed prospectively using the Common Terminology Criteria for Adverse Events (CTCAE v4.0) at each intervention group session and measurement time collection.
Study Outcomes
The primary study outcome was change in body mass. Secondary study outcomes included changes in body composition (fat mass, lean mass, and bone mineral density), physical fitness, and concentrations of serum biomarkers (insulin, leptin, adiponectin, CRP, and IL-6).
Statistical Analysis
Descriptive statistics presented for baseline variables include counts and proportions for categorical variables and means ± standard deviations for continuous variables. Categorical baseline characteristics were compared between the two randomized groups using Fisher’s exact test, and continuous baseline characteristics were compared between the two randomized groups using the Wilcoxon rank-sum test. A sample size of 60 participants provided 80% statistical power to detect a between-group difference of 2.4 kg, assuming a 3.1 kg standard deviation of change, 5% type-I error rate, and 5% attrition rate. Study outcomes were analyzed using a non-parametric Wilcoxon rank-sum test in per-protocol (completers) analysis set. A non-parametric test statistic was selected for the primary efficacy analysis to minimize concern regarding non-normally distributed outcome variables that may be observed due to the modest sample size. Sensitivity analyses were conducted on all outcomes using baseline observation carried forward imputation and linear mixed-effect regression modeling [24]. Post hoc regression models were used to correlate adherence to the in-person sessions (divided evenly at the median) with changes in weight loss, physical activity, and dietary consumption. Post hoc regression models were also used to correlate change in body mass with change in biomarker concentrations from baseline to 15-weeks. Participants were divided into thirds to align with meaningful weight changes: tertile 1, no weight loss or weight gain [median Δ: 0.0%]; tertile 2, intermediate weight loss [median Δ: −1.3%]; and tertile 3, ≥5% weight loss [median Δ: −7.5%]. Percent change in biomarker concentrations was quantified using geometric mean ratios derived from the logarithmically transformed biomarker concentrations in the linear regression models. All statistical testing was two-sided.
RESULTS
Participant Characteristics & Disposition
Baseline characteristics of study participants are presented in Table 1. In the study sample, the average age was 52±9 years and average BMI was 31.8±5.4 kg/m2. All but two of the participants were women. Most patients were breast cancer survivors (77%); other malignancies represented included gynecologic, hematologic, genitourinary, gastrointestinal, and sarcomas. Figure 1 depicts the screening, randomization, and follow-up of participants in the study. Twelve participants were lost to follow-up (80% follow-up rate). Follow-up rates did not differ between groups (P=0.75). Participants who did not complete the 15-week intervention were more likely to be treated with hormonal therapy (100% vs 62.5%; P=0.01). No other reported study variables differed between participants who did, versus, did not, complete the study. Participants attended an average of 10.5±3.9 of the 15 in-person meetings [median=11].
Table 1.
Baseline characteristics of the participants
| Characteristic | Intervention(n = 30) | Control(n = 30) | P |
|---|---|---|---|
| Age, years | 52±9 | 52±10 | 0.80 |
| Sex, % | |||
| Female | 29 (97%) | 29 (97%) | 0.99 |
| Male | 1 (3%) | 1 (3%) | |
| Race, % | |||
| White | 26 (87%) | 26 (87%) | 0.99 |
| Black | 2 (7%) | 1 (3%) | |
| Other | 2 (7%) | 3 (10%) | |
| Education, % | |||
| High School or Less | 1 (3%) | 4 (13%) | 0.54 |
| Some College | 5 (17%) | 5 (17%) | |
| College Degree or More | 24 (80%) | 21 (70%) | |
| Body Mass Index, kg/m2 | 32.1±5.6 | 31.5±5.3 | 0.67 |
| Cancer Type, % | |||
| Breast | 24 (80%) | 22 (73%) | 0.25 |
| Gynecologic | 0 (0%) | 4 (13%) | |
| Hematologic | 2 (7%) | 2 (7%) | |
| Genitourinary | 1 (3%) | 1 (3%) | |
| Gastrointestinal | 1 (3%) | 1 (3%) | |
| Sarcoma | 2 (7%) | 0 (0%) | |
| Time Since Diagnosis, mo. | 39.1±31.0 | 41.1±38.9 | 0.95 |
| Cancer Treatment, % | |||
| Chemotherapy | 19 (63%) | 21 (70%) | 0.58 |
| Radiation | 22 (73%) | 18 (60%) | 0.41 |
| Hormone | 20 (67%) | 22 (73%) | 0.57 |
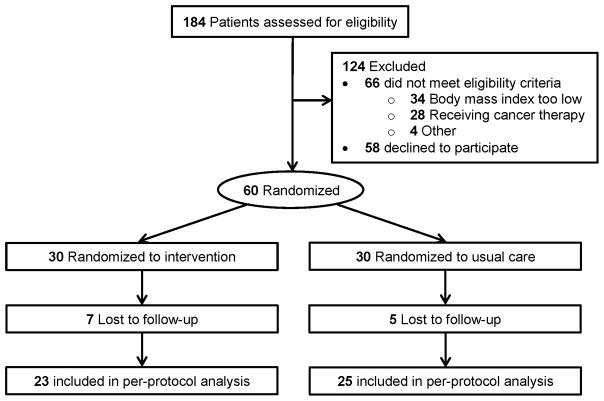
Figure 1.
CONSORT diagram that depicts the flow of participants through the study. Among the 184 participants assessed for eligibility, 60 were eligible and successfully randomized: 30 to the intervention group and 30 to the usual care group. After 15-weeks, 48 participants completed the study: 23 in the intervention group and 25 in the usual care group.
Body Mass & Body Composition Outcomes
Body mass and body composition outcomes are presented in Table 2. After 15-weeks, participants in the intervention group lost 5.6±4.4% of baseline weight (4.6±3.9 kg), whereas participants in the control group gained 0.2±2.4% of baseline weight (0.2±2.0 kg); intervention effect −5.8% (95% CI: −7.8, −3.8); −4.8 kg (95% CI: −6.6, −3.0); P=0.0001. A larger proportion of participants in the intervention group lost ≥5% of baseline weight compared to the control group (43% v 0%; P<0.001). The intervention reduced fat mass (−3.2±0.7 kg; P<0.0001) and lean mass (−1.7±0.4 kg; P=0.0005) compared to the control group. Bone mineral density did not change (0.002±0.006 g/cm2; P=0.98) compared to the control group. Results using baseline observation carried forward imputation and linear mixed-effect regression modeling were consistent with the per-protocol analysis. Post hoc analysis demonstrated that participants who attended ≥12 in-person meetings lost significantly more weight than those who attended ≤11 in-person meetings (−3.1 kg [95% CI: −5.4 to −0.8]; P=0.005).
Table 2.
Body composition outcomes at baseline and change during 15 weeks
| Outcome | Baseline(Mean ± SD) | Δ Baseline to Week 15(Mean ± SD) | Δ from Control(Mean ± SE) | P |
|---|---|---|---|---|
| Body Mass, kg | ||||
| Intervention | 86.3±13.9 | −4.6±3.9 | −4.8±0.9 | 0.0001 |
| Control | 86.5±15.3 | 0.2±2.0 | ||
| Fat Mass, kg | ||||
| Intervention | 39.1±9.3 | −3.3±2.8 | −3.2±0.7 | <0.0001 |
| Control | 38.3±9.5 | −0.1±1.4 | ||
| Lean Mass, kg | ||||
| Intervention | 42.4±6.0 | −1.0±1.3 | −1.7±0.4 | 0.0005 |
| Control | 43.4±8.0 | 0.7±1.5 | ||
| Bone Mineral Density, g/cm2 | ||||
| Intervention | 1.2±0.1 | 0.001±0.021 | 0.002±0.006 | 0.98 |
| Control | 1.2±0.1 | −0.001±0.019 | ||
SD, standard deviation; SE, standard error. This analysis was conducted using the per-protocol (completers) analysis set, including n=23 for the intervention group and n=25 for the control group.
Physical Fitness & Health Behavior Outcomes
Physical fitness and health behavior outcomes are presented in Table 3. At baseline, participants were moderately active, engaging in approximately 94±36 minutes of moderate or vigorous-intensity physical activity per week as measured by the accelerometer. They consumed an average of 1.4±1.0 servings of fruit and 3.3±2.4 servings of vegetables per day, and 36–39% of calories were consumed as fat.
Table 3.
Physical fitness and health behavior outcomes at baseline and change during 15 weeks
| Outcome | Baseline(Mean ± SD) | Δ Baseline to Week 15(Mean ± SD) | Δ from Control(Mean ± SE) | P |
|---|---|---|---|---|
| Six-minute walk test, m | ||||
| Intervention | 479.0±59.8 | 18.8±27.0 | 22.6±10.8 | 0.03 |
| Control | 499.2±79.3 | −3.7±44.6 | ||
| Accelerometer moderate-vigorous activity, minutes/week | ||||
| Intervention | 96.6±41.3 | 8.6±52.1 | 18.4±14.6 | 0.20 |
| Control | 91.0±29.2 | −9.9±38.3 | ||
| Self-reported physical activity, minutes/week | ||||
| Intervention | 142.0±143.2 | 76.4±158.3 | 12.1±50.3 | 0.66 |
| Control | 126.8±120.9 | 64.3±185.1 | ||
| Caloric Intake, kcals/day | ||||
| Intervention | 1405±546 | −191±461 | −43±134 | 0.80 |
| Control | 1661±540 | −148±427 | ||
| Fat, % kcals/day | ||||
| Intervention | 36.8±5.6 | 1.3±6.2 | 2.1±1.7 | 0.32 |
| Control | 39.2±7.5 | −0.8±5.2 | ||
| Carbohydrate, % kcals/day | ||||
| Intervention | 41.1±6.4 | −2.0±8.3 | −1.6±2.2 | 0.47 |
| Control | 45.8±10.8 | −0.4±6.4 | ||
| Fruits, servings/day | ||||
| Intervention | 1.4±1.1 | 0.3±1.0 | 0.2±0.3 | 0.46 |
| Control | 1.5±1.0 | 0.1±0.7 | ||
| Vegetables, servings/day | ||||
| Intervention | 3.3±2.4 | 0.8±1.5 | 1.2±0.4 | 0.03 |
| Control | 3.2±2.5 | −0.4±1.5 | ||
| Fiber, grams/day | ||||
| Intervention | 16.5±7.4 | 0.5±5.0 | 0.7±1.6 | 0.10 |
| Control | 18.0±7.4 | −2.0±5.6 | ||
SD, standard deviation; SE, standard error. This analysis was conducted using the per-protocol (completers) analysis set, including n=23 for the intervention group and n=25 for the control group.
Participants randomized to the weight loss intervention significantly increased the distance that they could walk in six minutes over the intervention period as compared to the control group (22.6±10.8 m; P=0.03). Accelerometer-quantified moderate-to-vigorous intensity physical activity was non-statistically significantly but numerically higher in the intervention compared to the control group (18.5±14.6 min/week; P=0.20); a similar pattern was observed for self-reported physical activity. The intervention significantly increased vegetable consumption compared to the control group (1.2±0.4 servings/day; P=0.03). The weight loss group also reported changes in caloric intake and other dietary measures, but none were statistically significantly different compared to the control group. Post hoc analysis demonstrated that participants who attended ≥12 in-person meetings consumed numerically fewer calories than those who attended ≤11 in-person meetings (−157 kcal/day [−517 to 203]; P=0.70) and consumed statistically significantly more vegetables (+1.8 [95% CI: 0.6–3.0]; P=0.03).
Serum Biomarker Concentration Outcomes
Concentrations of biomarker outcomes are presented in Table 4. After 15-weeks, the intervention reduced concentrations of insulin (−7.7±3.5 μU/mL; P=0.004) and leptin (−7.3±4.0 ng/mL; P=0.04) compared to the control group. The intervention did not change concentrations of adiponectin (26.2±15.4 ng/mL; P=0.09), CRP (−10.0±12.7 ng/mL; P=0.40), or IL-6 (0.6±0.4; P=0.24) compared to the control group. Post hoc correlation analysis of change in body mass with change in biomarker concentrations from baseline to 15-weeks is presented in Table 5. Changes in insulin (R2=0.41; P<0.001), leptin (R2=0.34; P=0.003), and adiponectin (R2=0.21; P=0.007) were all correlated with degree of weight loss. No dose-response relationship was observed for CRP (P=0.13) or IL-6 (P=0.08).
Table 4.
Concentrations of biomarker outcomes at baseline and change during 15 weeks
| Outcome | Baseline(Mean±SD) | Δ Baseline to Week 15(Mean ± SD) | Δ from Control(Mean ± SE) | P |
|---|---|---|---|---|
| Insulin, μU/mL | ||||
| Intervention | 8.5±15.0 | −5.4±16.8 | −7.7±3.5 | 0.004 |
| Control | 8.0±5.6 | 2.3±5.0 | ||
| Leptin, ng/mL | ||||
| Intervention | 43.1±26.4 | −12.8±26.9 | −7.3±4.0 | 0.04 |
| Control | 38.5±22.5 | −2.3±28.6 | ||
| Adiponectin, ng/mL | ||||
| Intervention | 99.2±43.5 | 14.7±51.6 | 26.2±15.4 | 0.09 |
| Control | 92.7±41.3 | −11.5±54.8 | ||
| C-Reactive Protein, ng/mL | ||||
| Intervention | 28.4±26.6 | −8.5±44.1 | −10.0±12.7 | 0.40 |
| Control | 41.0±33.0 | 1.5±44.2 | ||
| Interleukin-6, pg/mL | ||||
| Intervention | 2.2±1.2 | 0.3±1.6 | 0.6±0.4 | 0.24 |
| Control | 2.4±1.5 | −0.3±1.3 | ||
SD, standard deviation; SE, standard error. This analysis was conducted using the per-protocol (completers) analysis set, including n=23 for the intervention group and n=25 for the control group.
Table 5.
Post hoc analysis of biomarker concentration outcomes stratified by tertile of percentage change in body mass during 15 weeks
| Outcome | Δ Biomarker Concentration(Log10 LS Mean ± SE) | Ratio of Geometric Means | Model R2 | P |
|---|---|---|---|---|
| Insulin, μU/mL | 0.41 | <0.001 | ||
| Tertile 2 vs 1 | −0.145±0.09 | 0.72 | ||
| Tertile 3 vs 1 | −0.434±0.08 | 0.37 | ||
| Leptin, ng/mL | 0.34 | 0.003 | ||
| Tertile 2 vs 1 | −0.288±0.09 | 0.52 | ||
| Tertile 3 vs 1 | −0.429±0.10 | 0.37 | ||
| Adiponectin, ng/mL | 0.21 | 0.007 | ||
| Tertile 2 vs 1 | −0.068±0.07 | 0.86 | ||
| Tertile 3 vs 1 | 0.149±0.08 | 1.41 | ||
| C-Reactive Protein, ng/L | 0.09 | 0.13 | ||
| Tertile 2 vs 1 | −0.315±0.16 | 0.48 | ||
| Tertile 3 vs 1 | −0.262±0.17 | 0.55 | ||
| Interleukin-6, pg/mL | 0.24 | 0.08 | ||
| Tertile 2 vs 1 | −0.179±0.09 | 0.66 | ||
| Tertile 3 vs 1 | −0.050±0.08 | 0.89 |
Participants were divided into thirds to align with meaningful weight changes: tertile 1, no weight loss or weight gain [median Δ: 0.0%]; tertile 2, intermediate weight loss [median Δ: −1.3%]; and tertile 3, ≥5% weight loss [median Δ: −7.5%]. This analysis was conducted using the per-protocol (completers) analysis set, including n=48 overall with n=16 per tertile.
Adverse Events
No serious or unexpected adverse events occurred that were related to the intervention.
DISCUSSION
The 15-week lifestyle intervention utilized in this clinical trial led to a statistically significant and clinically meaningful improvement in body weight among overweight and obese cancer survivors. The intervention produced an average −5.8% (−4.8 kg) reduction in body weight, and 43% of participants in the intervention lost ≥5% of baseline weight. Substantiating the observed reductions in body weight, the intervention significantly reduced fat mass. The intervention improved physical fitness and did not cause any severe or unexpected adverse events. The intervention reduced serum measures that are hypothesized to mediate the relationship between obesity and cancer prognosis.
Results of this study can be compared to the short-term (six-month) outcomes of two recent large randomized trials of breast cancer patients, LISA and ENERGY [10, 11]. The LISA trial randomized 338 overweight and obese breast cancer survivors to a 24-month telephone-based lifestyle intervention or a general health information control group [10]. At six-months, the intervention group lost 5.3% of their baseline weight and the control group lost 0.7%, producing an intervention effect of 4.6% (P<0.001). The ENERGY trial randomized 692 overweight and obese breast cancer survivors to a 24-month group-based behavioral intervention or an attention control group [11]. At six-months, the intervention group lost 5.9% of their baseline weight and the control group lost 1.3%, producing an intervention effect of 4.6% (P<0.001). Although our intervention was shorter in duration (≈4 months), the average intervention effect of 5.8% weight loss is comparable to that of LISA and ENERGY.
Results of this trial can also be compared to weight loss interventions in other cancer populations, such as endometrial cancer [25, 26]. Haggerty and colleagues examined the feasibility of two technology-based weight loss interventions, including telemedicine or text messaging, in 20 endometrial cancer survivors. At 24-weeks, participants in the telemedicine group lost 7.6% of their baseline body weight, and participants in the text message group lost 4.1% of their baseline body weight (P=0.04) [25]. The SUCCEED trial examined the feasibility of a lifestyle intervention compared to usual care in 75 endometrial cancer survivors. At 12-months, participants in the intervention group lost 1.3 kg/m2 whereas the usual care group gained 0.3 kg/m2 (between group difference of ≈4.2 kg) [26]. The findings from the current study provided the scientific foundation that enabled us to translate the Healthy Living intervention into a routine clinical service offered through the Leonard P. Zakim Center for Integrative Therapies at the Dana-Farber Cancer Institute. This clinical service has been available for four months, and to date has enrolled 39 cancer patients, and 45 staff members.
The etiology of the relationship between obesity and cancer prognosis is not completely understood, but believed to be mediated through interconnected metabolic and inflammatory pathways [27]. Metabolically active adipose tissue is associated with insulin, adipokine, and cytokine dysregulation [6–8]. These cell signaling proteins may act directly on cancer cells, such as through the PI3K-Akt-mTOR pathway or indirectly by fostering a tumor microenvironment that promotes cancer recurrence and progression [27]. In our study, the weight loss intervention significantly reduced concentrations of serum insulin and leptin. In exploratory post hoc analysis that consolidated both groups, magnitude of weight loss was correlated with favorable changes in insulin, leptin, and adiponectin in a dose-response fashion; compared to participants with no weight loss or weight gain, those who lost ≥5% of baseline weight had 63% lower insulin (P<0.001), 63% lower leptin (P=0.003), and 41% higher adiponectin (P=0.007). This observation parallels several prior trials that demonstrate a weight loss of ≥5% is associated with improvements in concentrations of insulin and adipokines [12–14]. Contrary to our hypothesis and the findings of prior studies [12, 13], we did not observe significant reductions in inflammation biomarkers CRP and IL-6, possibly due to the small sample size or the relatively short duration of our intervention. These data demonstrate that weight loss may induce a multitude of physiologic changes that are hypothesized to relate to cancer prognosis.
There are several limitations to this trial. The main limitation is the small sample size, which may constrain the generalizability of our findings. The intervention was 15-weeks, which limits our ability to comment on the sustainability of the observed weight loss over a longer time horizon. Though we intended for the study sample to represent an array of cancer types, only a minority of participants (23%) were diagnosed with cancers other than breast cancer. This limitation precludes our ability to conduct informative subgroup analyses to compare the efficacy of the intervention among survivors of breast versus non-breast cancers. Throughout the recruitment period, focused efforts were made to attract cancer survivors of non-breast cancer. However, the cancer survivorship and integrative medicine programs at our comprehensive cancer center include a concentrated volume of highly-motivated breast cancer survivors. Our experience is similar to that of the Livestrong at the YMCA [28]. Most participants (97%) were female, which does not allow us to comment on the efficacy of this intervention among male cancer survivors. In addition, although our intervention led to a significant reduction in fat mass, participants randomized to the weight loss intervention also experienced a significant reduction in lean (muscle) mass. The rate of decline in lean mass during caloric restriction can potentially be attenuated by engaging in physical activity, such as walking [29]. Given that our intervention only modestly increased physical activity (18.5±14.6 min/week; P=0.20), participants may have lost more lean mass than they might have with an intervention that led to greater increases in physical activity, underscoring the dual importance of caloric restriction and physical activity for weight loss and weight management programs. Finally, we observed only modest changes in dietary intake, possibly due to our small sample size, which led to a limited ability to see differences between groups, and to the use of self-reported assessments that typically have limited ability to detect changes in dietary intake, compared to objectively-measured dietary phenotyping methods such as urinary metabolites [30].
There are several strengths to this trial. The randomized design provides a high degree of internal validity. The evidence-based weight loss intervention, which was modeled after the LISA study [10], was successful at inducing a statistically significant and clinically meaningful weight loss. The study integrated objective measures of body composition using DXA to provide insight as to how fat and muscle are mobilized in cancer survivors during periods of weight loss using caloric restriction. The study also integrated biomarker measures to quantify how concentrations of metabolic, adipokine and inflammatory measures change with weight loss, providing additional insight into hypothesized biologic mediators of the relationship between obesity and cancer prognosis. Lessons that we learned included the importance of being able to connect patients to the study and understanding how to prompt oncology providers to refer patients onto the trial. Several of the major successes of this study included inducing a statistically significant weight loss in a 15-week period, utilizing a manualized intervention that could be delivered by a variety of health and wellness professionals, and adding an efficacious evidence-based intervention to our toolbox of supportive care services offered at our comprehensive cancer center. The interventional manual described in this report is available by contacting the corresponding author.
In conclusion, a 15-week clinic-based weight loss intervention among overweight and obese cancer survivors resulted in significant weight loss, and improvements in body composition, physical fitness, and concentrations of serum biomarkers. The findings from this randomized trial provide additional evidence regarding the feasibility of weight loss and potential physiologic mediators of the relationship between obesity and cancer prognosis and demonstrates the feasibility of translating an evidence-based intervention into the clinical care continuum at a comprehensive cancer center.
Acknowledgments
Funding: This study was supported by a grant from the Friends of Dana-Farber Cancer Institute.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
Clinicaltrials.gov Registry: NCT01978899
Compliance with Ethical Standards
Conflict of interest: The authors declare no conflicts of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the Dana-Farber Cancer Institute and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Greenlee H, Shi Z, Sardo Molmenti CL, Rundle A, Tsai WY. Trends in Obesity Prevalence in Adults With a History of Cancer: Results From the US National Health Interview Survey, 1997 to 2014. J Clin Oncol. 2016;34(26):3133–40. doi: 10.1200/JCO.2016.66.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown JC, Meyerhardt JA. Obesity and Energy Balance in GI Cancer. J Clin Oncol. 2016;34(35):4217–24. doi: 10.1200/JCO.2016.66.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiralerspong S, Goodwin PJ. Obesity and Breast Cancer Prognosis: Evidence, Challenges, and Opportunities. J Clin Oncol. 2016;34(35):4203–16. doi: 10.1200/JCO.2016.68.4480. [DOI] [PubMed] [Google Scholar]
- 4.Onstad MA, Schmandt RE, Lu KH. Addressing the Role of Obesity in Endometrial Cancer Risk, Prevention, and Treatment. J Clin Oncol. 2016;34(35):4225–30. doi: 10.1200/JCO.2016.69.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L, Drake BF, Colditz GA. Obesity and Other Cancers. J Clin Oncol. 2016;34(35):4231–7. doi: 10.1200/JCO.2016.68.4837. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins BD, Goncalves MD, Cantley LC. Obesity and Cancer Mechanisms: Cancer Metabolism. J Clin Oncol. 2016;34(35):4277–83. doi: 10.1200/JCO.2016.67.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J Clin Oncol. 2016;34(35):4270–6. doi: 10.1200/JCO.2016.67.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lohmann AE, Goodwin PJ, Chlebowski RT, Pan K, Stambolic V, Dowling RJ. Association of Obesity-Related Metabolic Disruptions With Cancer Risk and Outcome. J Clin Oncol. 2016;34(35):4249–55. doi: 10.1200/JCO.2016.69.6187. [DOI] [PubMed] [Google Scholar]
- 9.Schmitz KH, Neuhouser ML, Agurs-Collins T, Zanetti KA, Cadmus-Bertram L, Dean LT, et al. Impact of obesity on cancer survivorship and the potential relevance of race and ethnicity. J Natl Cancer Inst. 2013;105(18):1344–54. doi: 10.1093/jnci/djt223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodwin PJ, Segal RJ, Vallis M, Ligibel JA, Pond GR, Robidoux A, et al. Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: the LISA trial. J Clin Oncol. 2014;32(21):2231–9. doi: 10.1200/JCO.2013.53.1517. [DOI] [PubMed] [Google Scholar]
- 11.Rock CL, Flatt SW, Byers TE, Colditz GA, Demark-Wahnefried W, Ganz PA, et al. Results of the Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) Trial: A Behavioral Weight Loss Intervention in Overweight or Obese Breast Cancer Survivors. J Clin Oncol. 2015;33(28):3169–76. doi: 10.1200/JCO.2015.61.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrigan M, Cartmel B, Loftfield E, Sanft T, Chagpar AB, Zhou Y, et al. Randomized Trial Comparing Telephone Versus In-Person Weight Loss Counseling on Body Composition and Circulating Biomarkers in Women Treated for Breast Cancer: The Lifestyle, Exercise, and Nutrition (LEAN) Study. J Clin Oncol. 2016;34(7):669–76. doi: 10.1200/JCO.2015.61.6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pakiz B, Flatt SW, Bardwell WA, Rock CL, Mills PJ. Effects of a weight loss intervention on body mass, fitness, and inflammatory biomarkers in overweight or obese breast cancer survivors. Int J Behav Med. 2011;18(4):333–41. doi: 10.1007/s12529-010-9079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rock CL, Pande C, Flatt SW, Ying C, Pakiz B, Parker BA, et al. Favorable changes in serum estrogens and other biologic factors after weight loss in breast cancer survivors who are overweight or obese. Clin Breast Cancer. 2013;13(3):188–95. doi: 10.1016/j.clbc.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips SM, Alfano CM, Perna FM, Glasgow RE. Accelerating translation of physical activity and cancer survivorship research into practice: recommendations for a more integrated and collaborative approach. Cancer Epidemiology and Prevention Biomarkers. 2014 doi: 10.1158/1055-9965.EPI-13-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wadden TA, Butryn ML, Hong PS, Tsai AG. Behavioral treatment of obesity in patients encountered in primary care settings: a systematic review. JAMA. 2014;312(17):1779–91. doi: 10.1001/jama.2014.14173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. Circulation. 2013;2013 01. cir. 0000437739.71477. ee. [Google Scholar]
- 18.Glanz K, Rimer BK, Viswanath K. Health Behavior and Health Education: Theory, Research, and Practice. John Wiley & Sons; 2008. [Google Scholar]
- 19.Group DPPR. The diabetes prevention program (DPP) Diabetes care. 2002;25(12):2165–71. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enright PL. The six-minute walk test. Respir Care. 2003;48(8):783–5. [PubMed] [Google Scholar]
- 21.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 22.Hayden-Wade HA, Coleman KJ, Sallis JF, Armstrong C. Validation of the telephone and in-person interview versions of the 7-day PAR. Med Sci Sports Exerc. 2003;35(5):801–9. doi: 10.1249/01.MSS.0000064941.43869.4E. [DOI] [PubMed] [Google Scholar]
- 23.Gibson RS. Principles of nutritional assessment. Oxford university press; USA: 2005. [Google Scholar]
- 24.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. Springer Science & Business Media; 2009. [Google Scholar]
- 25.Haggerty AF, Huepenbecker S, Sarwer DB, Spitzer J, Raggio G, Chu CS, et al. The use of novel technology-based weight loss interventions for obese women with endometrial hyperplasia and cancer. Gynecologic oncology. 2016;140(2):239–44. doi: 10.1016/j.ygyno.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCarroll ML, Armbruster S, Frasure HE, Gothard MD, Gil KM, Kavanagh MB, et al. Self-efficacy, quality of life, and weight loss in overweight/obese endometrial cancer survivors (SUCCEED): a randomized controlled trial. Gynecol Oncol. 2014;132(2):397–402. doi: 10.1016/j.ygyno.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 27.Goodwin PJ, Stambolic V. Impact of the obesity epidemic on cancer. Annu Rev Med. 2015;66:281–96. doi: 10.1146/annurev-med-051613-012328. [DOI] [PubMed] [Google Scholar]
- 28.Irwin ML, Cartmel B, Harrigan M, Li F, Sanft T, Shockro L, et al. Effect of the LIVESTRONG at the YMCA exercise program on physical activity, fitness, quality of life, and fatigue in cancer survivors. Cancer. 2017;123(7):1249–58. doi: 10.1002/cncr.30456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller CT, Fraser SF, Levinger I, Straznicky NE, Dixon JB, Reynolds J, et al. The effects of exercise training in addition to energy restriction on functional capacities and body composition in obese adults during weight loss: a systematic review. PLoS One. 2013;8(11):e81692. doi: 10.1371/journal.pone.0081692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Perez I, Posma JM, Gibson R, Chambers ES, Hansen TH, Vestergaard H, et al. Objective assessment of dietary patterns by use of metabolic phenotyping: a randomised, controlled, crossover trial. The Lancet Diabetes & Endocrinology. 2017;5(3):184–95. doi: 10.1016/S2213-8587(16)30419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]