Abstract
The physiologic limitations of neural regeneration make peripheral nerve surgery challenging to both the surgeon and the patient. Presence of nerve gaps and local wound factors may all influence outcome, suggesting that barriers to reduce perineural scarring, minimize fibrosis, and avoid ischemia would be beneficial. To examine the evidence supporting their use, we reviewed the autologous and commercially-available options for barriers against scarring around a nerve. Numerous clinical case series demonstrated the effectiveness and safety of local/rotational flaps and autologous vein wrapping when used in the presence of recurrent compressive neuropathy. Translational research in animal models support the biocompatibility of commercially-available nerve wraps following nerve repair. To date, there are no reports of clinical use of commercially-available nerve wraps in acute nerve repair, but a growing number of case series demonstrate their effectiveness and safety in chronic compressive neuropathy. Limited clinical evidence exists to support the efficacy of flap coverage in acute nerve repairs, including the use of vein.
Keywords: Nerve wrap, nerve repair, compressive neuropathy, epineural scarring, cicatrix, recurrent carpal tunnel, recurrent cubital tunnel, neuropathic pain
Rationale for use of barriers to epineural scarring
When performing nerve repair, a favorable soft tissue envelope intuitively would seem to minimize the chances of ischemia and scar formation that can impede neural regeneration. In the case of revision surgery for chronic compressive neuropathy, surgery in an already-scarred tissue bed can create additional adhesions that lead to eventual symptom recurrence and traction-related pain. Ideally, a barrier could be used to promote nerve gliding and reduce scarring around the nerve, without proliferation of extraneural fibrosis and scarring. Scientists and surgeons have provided innovative solutions, ranging from synthetic and xenograft materials to autologous vein wrapping and pedicled/free tissue coverage. There is no clear guidance on which barriers provide the best results in either the acute or chronic situations, or the indications for their use.
We conducted a review of the published literature regarding perineural scarring and barriers. A search was conducted in Ovid Medline (1946-present), Embase (1946-present), Clinical Trials database, Cochrane Databases, Scopus (1823-present), Science Citation Index (1900-present). 8841 unique citations were filtered to 47 articles based on article titles and abstracts, with 20 articles included here to support our discussion and promote ongoing dialogue on this topic.
The ideal barrier
The ideal barrier to perineural scarring should have the following characteristics: (1) minimal or no chance of rejection or inflammatory reaction; (2) sufficient porosity to facilitate diffusion of nutrients without allowing axonal escape; (3) avoidance of scar induced ischemia; (4) promote nerve gliding; (5) minimal or no donor site morbidity; (6) minimal cost or supply restraints (Table 1).1
Table 1.
Comparison of nerve barrier options based on ideal characteristics for a barrier to epineural scarring.
| Ideal characteristics | Adipofascial or muscle flap |
Vein wrapping | Hyaluronic acid- carboxycellulose membrane (Seprafilm) |
Bovine collagen NeuraWrap; NeuraMend) |
Porcine small intestine submucosa (Axoguard) |
|---|---|---|---|---|---|
|
| |||||
| Biocompatible | Yes. | Yes | No reports of rejection. | No reports of rejection. | No reports of rejection. |
| Non-absorbable | Non-absorbable | Absorbs by 7 days | Absorbs by 4–8 months | Absorbs by 3 months. | |
|
| |||||
| Semipermeable | Yes | Yes | Yes | Yes | Yes |
|
| |||||
| Non-constricting | No reported cases of cicatrix formation after use. | No reported cases of cicatrix formation after use. | No reported cases of cicatrix formation after use. | No reported cases of cicatrix formation after use. | No reported cases of cicatrix formation after use. |
|
| |||||
| Promote nerve gliding | Yes | Yes – demonstrated in animal model | Unknown | Unknown | Unknown |
|
| |||||
| Minimal/no donor site morbidity | Depends on flap harvested (minimal for local fat pad flap) | Yes (typically edema) | No donor site morbidity | No donor site morbidity | No donor site morbidity |
|
| |||||
| Minimal cost or supply restraints | Increased surgery time | Increased surgery time | Subject to implant cost and availability | Subject to implant cost and availability | Subject to implant cost and availability |
Types of barriers available
Adipofascial or muscle flap (pedicle or free tissue)
The concept of using local tissues to provide a barrier around a nerve has been promoted extensively in the treatment of recurrent carpal tunnel syndrome (CTS) and cubital tunnel syndrome (CuTS). While incomplete release of the transverse carpal ligament during carpal tunnel release (CTR) or newly-created points of compression of the transposed ulnar nerve are common reasons for revision surgery, another frequent finding during revision surgeries for both CTS and CuTS is adherence of the nerve to the surrounding tissues. Soltani, et al performed a systematic review for surgical treatment of recurrent carpal tunnel syndrome.2 Of the 14 articles describing rotational or free flap coverage options, 7 discussed hypothenar fat pad or ulnar artery-based perforator flaps. Additional options include other rotational flaps (synovium, pronator quadratus, palmaris brevis, abductor digiti minimi, radial artery perforator) and free flaps (omentum and anterolateral thigh flaps). Of all options for flap coverage in revision carpal tunnel release, we prefer the hypothenar fat pad flap because of minimal morbidity and reliable blood supply (Figures 1A–1C). In the meta-analysis of 14 studies (n=294) using flap coverage during revision CTR, there was an 86% success rate. This was substantially higher than the 74% success rate seen in patients treated with decompression alone (7 studies; n=364). More recently, Pace, et al performed an unmatched, retrospective cohort study of patients who underwent flap interposition or decompression only during revision CTR.3 The authors did not detect a difference in outcomes, but they did not report a power analysis.3 With regard to recurrent CuTS, there is little evidence-based guidance in the literature about the type of procedure to use during revision cases. We have found that perineural fibrosis may form after any of the procedures used for primary treatment. Submuscular transposition provides a reliable gliding surface for the ulnar nerve, provided that new points of compression are not created. The decision about whether to add an additional barrier to scarring around the nerve is described in more detail below. With regard to nerve repairs, we are unaware of any published series dedicated to examining outcomes of flap coverage of repaired nerves alone.
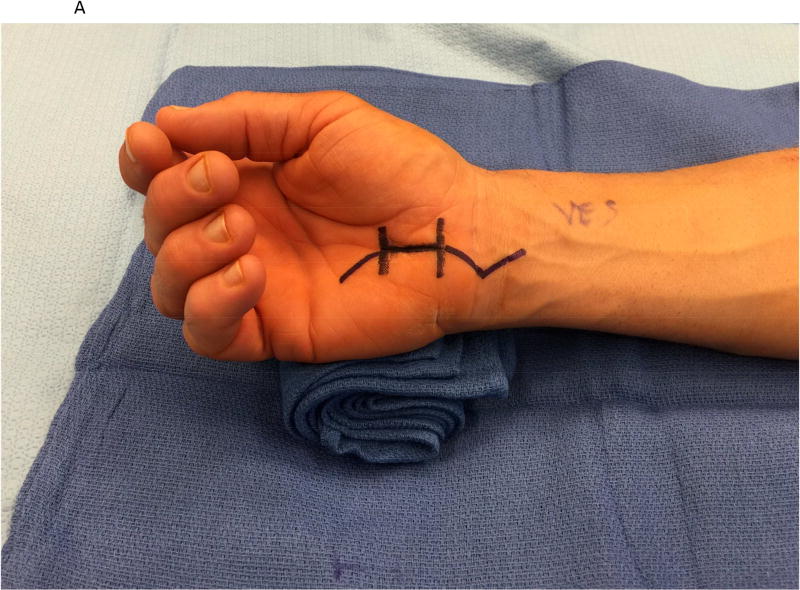
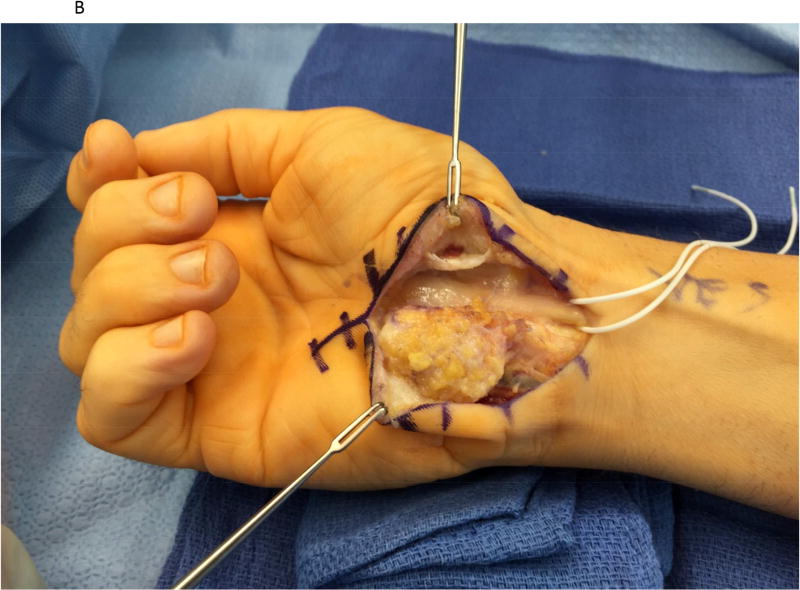
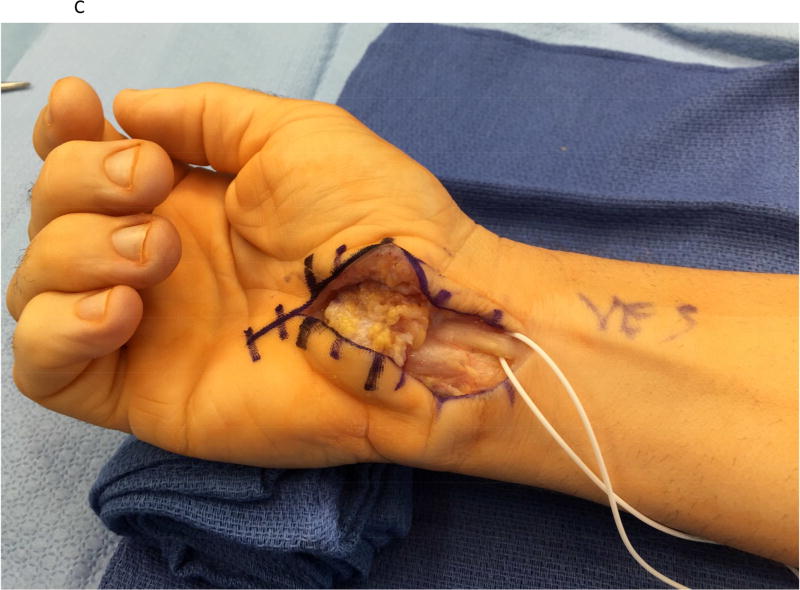
Figure 1.
A hypothenar fat pad flap was used as a protective barrier in a case of recurrent carpal tunnel syndrome. (1A) The prior skin incision is marked with the transverse lines and is extended proximally and distally. (1B) The hypothenar fat pad is dissected from the overlying skin of the palm, preserving its vascular supply from the ulnar artery. The transverse carpal ligament has been re-released. (1C) The hypothenar fat pad has been placed over the median nerve and loosely secured to the radial leaflet of the transverse carpal ligament.
Autologous vein wraps
Veins provide an ideal surface to place around a nerve after it has been repaired or dissected from scar, as it is biologically compatible and vein intima provides a gliding surface (Figure 2A–2E). In a rat sciatic nerve compressive neuropathy model, Xu, et al demonstrated gliding of the vein wrap along the nerve trunk, improvement in electrophysiologic testing, and less scarring on histologic examination in nerves wrapped with autologous vein compared to controls.4 Murakami, et al also noted decreased perineural scarring in a rat compressive neuropathy model after vein wrapping.5 Since the concept was introduced by Masear in 1989 for the treatment of recurrent tarsal tunnel syndrome, there have been numerous case series describing the effectiveness of autologous vein wraps in treatment of recurrent CTS6 and CuTS7. The potential disadvantages of using autologous vein wraps include donor site morbidity (including swelling), as well as increased operative time and technical difficulty if adequate assistance is not available. Promising results with vein allografts have been reported, but availability in the United States is limited. There are no clinical studies comparing patients with compressive neuropathy treated with vein wraps to either control patients or other types of nerve barriers. Regarding nerve repair, there is a single case series describing vein wrapping after nerve repair – Sadek, et al reported that patients with saphenous vein wrapping of ulnar nerve repairs had improved motor, sensory, and electrophysiologic recovery compared to unmatched historical controls.8
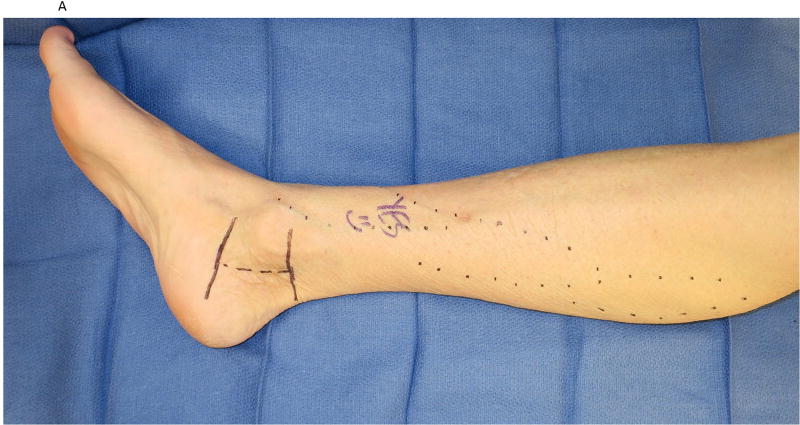
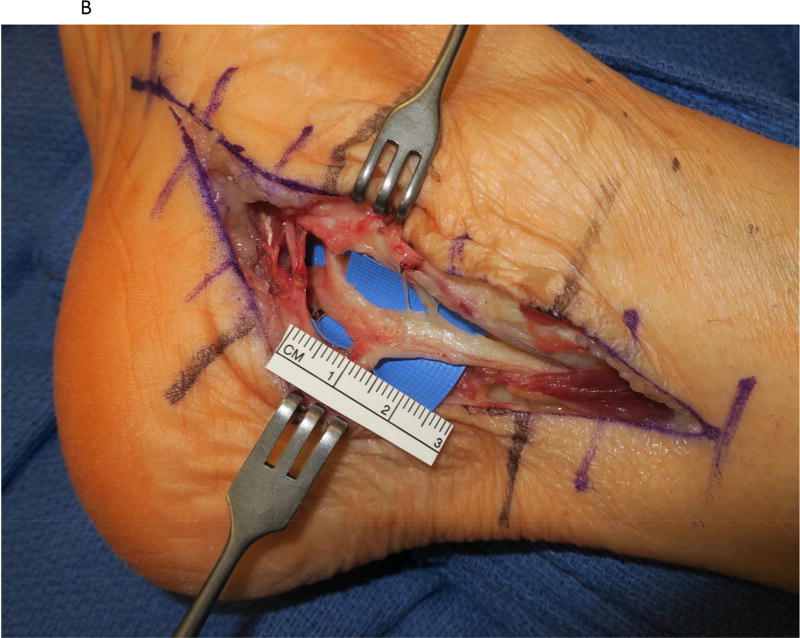
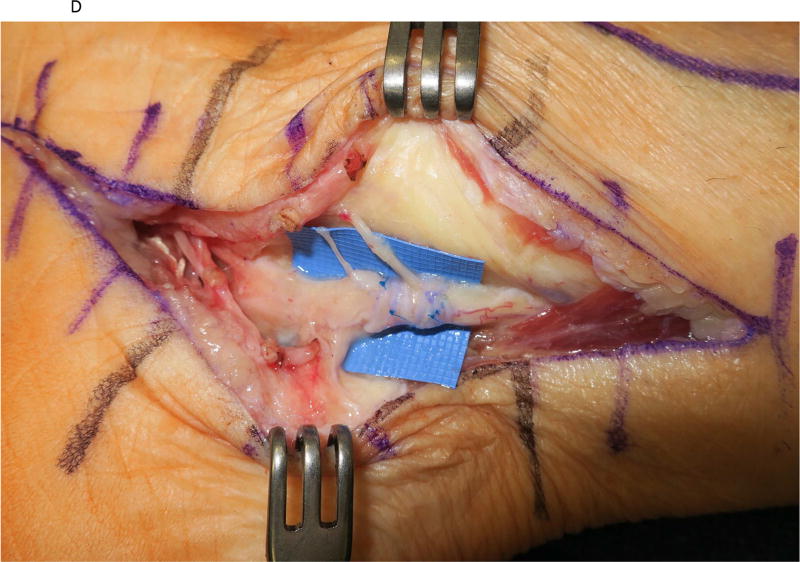
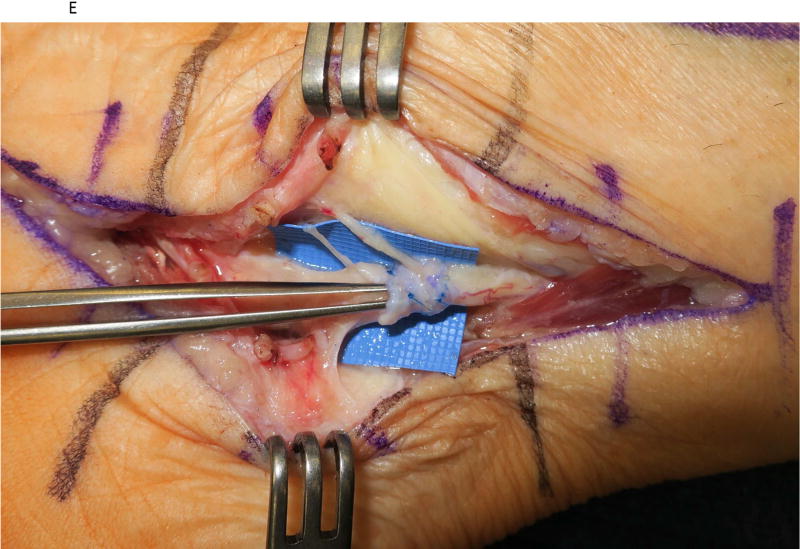
Figure 2.
A saphenous vein wrap was used as a protective barrier in a case of recurrent tarsal tunnel syndrome. (2A) The prior skin incision is marked with the transverse lines and the subcutaneous veins are marked prior to limb exsanguination. (2B) Dissection of the tibial nerve within the tarsal tunnel demonstrates epineural scarring deep to the reformed lancinate ligament. (2C) The saphenous vein is harvested and split longitudinally. (2D) The longitudinally-split saphenous vein is wrapped around the scarred segment of the tibial nerve. (2E) Care is taken to avoid wrapping the nerve too tightly, as demonstrated by the ability to place the forceps deep to the vein wrap.
Commercially-available nerve wraps
Many of the commercially-available nerve wraps are comprised largely of collagen, the main component of the extracellular matrix, and are similar in material composition to nerve conduit products. Type 1 collagen is an appealing material to use as a nerve wrap, as it has a long track record of biocompatibility and has selective permeability (Table 2). The biocompatibility of these wraps has been demonstrated in laboratory testing of nerve repair conduits composed of the same material. The most commonly-used source for the Type 1 collagen wraps is bovine tendon, with a degradation time ranging from 4–8 months. There is limited laboratory evidence demonstrating the efficacy or safety of these wraps following nerve repair. In a rat sciatic nerve model, Lee, et al9 demonstrated decreased perineural scarring compared to controls, but no difference in axon counts or density. To our knowledge, there are no clinical reports of Type 1 collagen wrap use after nerve repair. While there are no laboratory models of Type 1 collagen wrap use in compressive neuropathy, recent clinical series have described use of Type 1 collagen wraps after revision CTS cases. Kokkalis, et al described two cases with clinical improvement and no signs of intolerance or complications.10 Additionally, Soltani, et al used a Type 1 collagen wrap in 9 recurrent CTS and 6 recurrent CuTS cases; all but 2 patients demonstrated improvement in symptoms. There were no reoperations or signs of collagen wrap intolerance in the series.11
Table 2.
Nerve wraps that are commercially available in the United States
| Product Name Manufacturer) |
Description | Lab evidence | Clinical evidence |
|---|---|---|---|
|
| |||
| Autologous vein wrapping | Intimal surface of vein placed along epineurium and wrapped circumferentially | Nerve repair: Rat sciatic nerve – decreased perineural scar compared to controls13 | Nerve repair: 1 case series8 |
| Compressive neuropathy: Rat sciatic nerve – decreased perineural scar, improved electrophysiologic testing, larger axons compared to controls4 | Compressive neuropathy: 4 case series6,7 21 22 | ||
|
| |||
| NeuraWrap Integra Life Sciences) | Bovine-derived type 1 collagen | Nerve repair: Rat sciatic nerve – decreased perineural scar, no difference in axon counts compared to controls9 | Nerve repair: None |
| Compressive neuropathy: None | Compressive neuropathy: 2 case series10,11 | ||
|
| |||
| AxoGuard Nerve Wrap (Axogen) | Porcine small intestine submucosa | Nerve repair: Rat sciatic nerve – more effectively reduced intraneural fibrosis than vein wrapping13 | Nerve repair: None |
| Compressive neuropathy: None | Compressive neuropathy: 1 case series14 | ||
|
| |||
| Seprafilm (Genzyme) Non FDA-indicated | Hyaluronic acid-carboxymethylcellulose film | Nerve repair: Rat sciatic nerve – decreased adhesions compared to control group17 | Nerve repair: None |
| Compressive neuropathy: None | Compressive neuropathy: None | ||
|
| |||
| XWrap Dry (Applied Biologics) Non FDA-indicated | Human amniotic membrane | Nerve repair: Rabbit ulnar nerve and rat sciatic nerve repair models: less perineural fibrosis and adhesion compared to controls18 | Nerve repair: None |
| Compressive neuropathy: None | Compressive neuropathy: 1 case series19 | ||
Porcine small intestine submucosa, composed of both Types 1 and 3 collagen, has recently been developed for use as both a nerve conduit and nerve wrap (Table 2). The advantage of this material is that it retains its ability to serve as an extracellular matrix scaffold for the regenerating nerve. Like all xenografts, concerns arise from potential immune response or transmission of infectious disease. Processing techniques have been successful in allowing porcine xenograft implantation without clinically obvious rejection. Pertici, et al circumferentially sutured a porcine-derived nerve wrap around an acutely-repaired rat peroneal nerve.12 There were no differences in functional or histologic measures among the repair-only, repair+wrap, and repair+fibrin glue cohorts at final follow-up. Although there was an increased histologic grade of inflammatory reaction in the repair+wrap group compared to repair+fibrin glue at 1 month post-repair, no difference was observed at 3 months post-repair. The authors attributed the initial difference to degradation of the wrap, which was complete by 3 months. In the only study that compared two different nerve barriers, Mathieu, et al demonstrated that a porcine-derived nerve wrap more effectively reduced intraneural fibrosis than vein wrapping in a rat sciatic nerve repair model13. Although the nerve wrap used by Pertici12 and Mathieu13 is (1) different from the porcine wrap available in the United States and (2) applied in rats, the lessons regarding porcine xenograft biocompatibility may hold value. To our knowledge, there are no clinical reports of porcine-derived nerve wrap use in association with acute nerve repair. Papatheodorou, et al recently described the use of the porcine wrap in a case series of 12 patients with recurrent CuTS.14 There were no adverse reactions or complications, and all patients had clinical improvement in patient-reported outcomes and grip strength.
While not specifically designed, marketed, or FDA-indicated for use as a perineural barrier to scarring, hyaluronic acid-carboxymethylcellulose film (HA-CMC) has been examined in both clinical and laboratory studies. Hyaluronic acid is a component of the extracellular membrane and contributes to wound healing and nerve regeneration. Mixing the hyaluronic acid with CMC slows the resorption of the HA, allowing it to exert its effect over a longer period of time (commercially-available HA-CMC films typically absorb within 7 days). The benefits of HA-CMC membranes have also been demonstrated in preventing restrictive adhesions in a chicken flexor tendon model15 and in reducing the incidence, extent, and severity of postoperative abdominal adhesions.16 In a rat sciatic nerve model, Magill, et al noted less perineural scarring on histomorphometric and stereological analyses after a commercially-available HA-CMC film (Seprafilm; Genzyme, Cambridge, MA, USA) was wrapped around a rat sciatic nerve after injury and repair,17 relative to a control group. This study also included a non-injury phase in which the HA-CMC film was placed onto, or wrapped around, an intact nerve; no significant differences were found compared to a control group, suggesting that it is biocompatible.17 To our knowledge, there are no clinical data in the peer-reviewed literature demonstrating the efficacy of HA-CMC films used for acute nerve repair or chronic compressive neuropathy.
Human amniotic membrane wraps have also been described as a barrier to perineural fibrosis. Laboratory studies using human amniotic membrane wraps in a rabbit model demonstrate less perineural fibrosis and adhesion compared to controls.18 To our knowledge, there are no clinical reports of amnionic membrane wrap use in the nerve injury setting. Gaspar, et al reported the use of a commercially-available amniotic membrane wrap (XWrap Dry; Applied Biologics, Scottsdale, AZ) during 8 revision cubital tunnel cases.19 There were no signs of adverse reactions or complications reported, and all patients had clinical improvement in patient-reported outcomes and grip strength. However, preliminary results using amniotic wraps placed around repaired flexor tendons demonstrated unfavorable results with inflammatory responses and local fibrosis.20 FDA indications for XWrap do not include use as a barrier to nerve adhesion.
Author preferences
For recurrent CTS, our preference is to use the hypothenar fat pad flap if intraoperative inspection demonstrates scarring of the median nerve to the surrounding tissue. If the transverse carpal ligament has reconstituted and there is no perineural fibrosis, we will perform a decompression only. For CuTS, our preference is to perform a submuscular ulnar nerve transposition (with a very loose approximation of the flexor-pronator fascia) to provide a favorable environment for the ulnar nerve. We will apply an autologous vein wrap if a submuscular transposition has already been performed and there is epineural fibrosis on intraoperative inspection. For nerve repairs, we do not routinely use any of the commercially-available scar barriers due to the absence of laboratory or clinical evidence of commercially-available barriers for primary nerve repair. If the nerve coaptation will be vulnerable to traction from surrounding structures, we will use an autologous vein wrap or local/rotational soft tissue flap for coverage.
Supplementary Material
Acknowledgments
This publication was made possible by Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448, Sub award KL2 TR000450 (CJD) from the NIH-National Center for Advancing Translational Sciences (NCATS), components of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official view of NCATS or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Isaacs J, McMurtry J. Different nerve grafting and wrapping options in upper extremity surgery. Current Orthopaedic Practice. 2014;25(5):456–461. doi: 10.1097/BCO.0000000000000146. [published Online First: Epub Date] [DOI] [Google Scholar]
- 2.Soltani AM, Allan BJ, Best MJ, Mir HS, Panthaki ZJ. A systematic review of the literature on the outcomes of treatment for recurrent and persistent carpal tunnel syndrome. Plast Reconstr Surg. 2013;132(1):114–121. doi: 10.1097/PRS.0b013e318290faba. [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- 3.Pace GI, Zale CL, Gendelberg D, Taylor KF. Self-Reported Outcomes for Patients Undergoing Revision Carpal Tunnel Surgery With or Without Hypothenar Fat Pad Transposition. Hand (N Y) 2017:1558944717701243. doi: 10.1177/1558944717701243. [published Online First: Epub Date] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J, Varitimidis SE, Fisher KJ, Tomaino MM, Sotereanos DG. The effect of wrapping scarred nerves with autogenous vein graft to treat recurrent chronic nerve compression. J Hand Surg Am. 2000;25(1):93–103. doi: 10.1053/jhsu.2000.jhsu025a0093. [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- 5.Murakami K, Kuniyoshi K, Iwakura N, et al. Vein wrapping for chronic nerve constriction injury in a rat model: study showing increases in VEGF and HGF production and prevention of pain-associated behaviors and nerve damage. J Bone Joint Surg Am. 2014;96(10):859–867. doi: 10.2106/JBJS.L.01790. [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- 6.Sotereanos DG, Giannakopoulos PN, Mitsionis GI, Xu J, Herndon JH. Vein-graft wrapping for the treatment of recurrent compression of the median nerve. Microsurgery. 1995;16(11):752–756. doi: 10.1002/micr.1920161110. [DOI] [PubMed] [Google Scholar]
- 7.Kokkalis ZT, Jain S, Sotereanos DG. Vein wrapping at cubital tunnel for ulnar nerve problems. J Shoulder Elbow Surg. 2010;19(2 Suppl):91–97. doi: 10.1016/j.jse.2009.12.019. [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- 8.Sadek AF, Fouly EH, Hamdy M. Functional and electrophysiological outcome after autogenous vein wrapping of primary repaired ulnar nerves. Microsurgery. 2014;34(5):361–356. doi: 10.1002/micr.22207. [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- 9.Lee JY, Parisi TJ, Friedrich PF, Bishop AT, Shin AY. Does the addition of a nerve wrap to a motor nerve repair affect motor outcomes? Microsurgery. 2014;34(7):562–567. doi: 10.1002/micr.22274. [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- 10.Kokkalis ZT, Mavrogenis AF, Ballas EG, Papagelopoulos PJ, Soucacos PN. Collagen nerve wrap for median nerve scarring. Orthopedics. 2015;38(2):117–121. doi: 10.3928/01477447-20150204-04. [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- 11.Soltani AM, Allan BJ, Best MJ, Mir HS, Panthaki ZJ. Revision decompression and collagen nerve wrap for recurrent and persistent compression neuropathies of the upper extremity. Ann Plast Surg. 2014;72(5):572–578. doi: 10.1097/SAP.0b013e3182956475. [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- 12.Pertici V, Laurin J, Marqueste T, Decherchi P. Comparison of a collagen membrane versus a fibrin sealant after a peroneal nerve section and repair: a functional and histological study. Acta Neurochir (Wien) 2014;156(8):1577–1590. doi: 10.1007/s00701-014-2117-6. [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- 13.Mathieu L, Adam C, Legagneux J, Bruneval P, Masmejean E. Reduction of neural scarring after peripheral nerve suture: an experimental study about collagen membrane and autologous vein wrapping. Chir Main. 2012;31(6):311–317. doi: 10.1016/j.main.2012.10.167. [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- 14.Papatheodorou LK, Williams BG, Sotereanos DG. Preliminary results of recurrent cubital tunnel syndrome treated with neurolysis and porcine extracellular matrix nerve wrap. J Hand Surg Am. 2015;40(5):987–992. doi: 10.1016/j.jhsa.2015.02.031. [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- 15.Isik S, Ozturk S, Gurses S, et al. Prevention of restrictive adhesions in primary tendon repair by HA-membrane: experimental research in chickens. Br J Plast Surg. 1999;52(5):373–379. doi: 10.1054/bjps.1999.3128. [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- 16.Kumar S, Wong PF, Leaper DJ. Intra-peritoneal prophylactic agents for preventing adhesions and adhesive intestinal obstruction after non-gynaecological abdominal surgery. Cochrane Database Syst Rev. 2009;(1):CD005080. doi: 10.1002/14651858.CD005080.pub2. [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- 17.Magill CK, Tuffaha SH, Yee A, et al. The short- and long-term effects of Seprafilm on peripheral nerves: a histological and functional study. J Reconstr Microsurg. 2009;25(6):345–354. doi: 10.1055/s-0029-1215526. [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- 18.Ozgenel GY, Filiz G. Effects of human amniotic fluid on peripheral nerve scarring and regeneration in rats. J Neurosurg. 2003;98(2):371–377. doi: 10.3171/jns.2003.98.2.0371. [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- 19.Gaspar MP, Abdelfattah HM, Welch IW, Vosbikian MM, Kane PM, Rekant MS. Recurrent cubital tunnel syndrome treated with revision neurolysis and amniotic membrane nerve wrapping. J Shoulder Elbow Surg. 2016;25(12):2057–2065. doi: 10.1016/j.jse.2016.09.013. [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- 20.Leppanen OV, Karjalainen T, Goransson H, et al. Outcomes After Flexor Tendon Repair Combined With the Application of Human Amniotic Membrane Allograft. J Hand Surg Am. 2017;42(6):474 e1–474 e8. doi: 10.1016/j.jhsa.2017.03.006. [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.