Abstract
The childhood high body mass index (BMI) is associated with cardiovascular risk, but the association between childhood BMI trajectory patterns and cardiovascular risk remains unclear. The purposes of this study are to identify subgroups of individuals with similar trajectories in BMI during childhood, and to determine the relationship of childhood BMI trajectories with subclinical cardiovascular disease in young adulthood, indexed by intima-media thickness (IMT) and left ventricular mass index (LVMI). The participants were from the Georgia Stress and Heart (GSH) study. A total of 626 participants with BMI measured 3–12 times during childhood (5–18 years old) were included. By using latent class models, three trajectory groups in BMI were identified, including high increasing (HI), moderate increasing (MI), and normal group. We found that childhood trajectory of BMI was significantly associated with IMT and LVMI in young adulthood even after adjustment for BMI in young adulthood. Our results suggested that different BMI trajectory patterns exist during childhood. We for the first time reported the association between childhood BMI trajectory patterns and subclinical cardiovascular risk in young adulthood, indicating that monitoring trajectories of BMI from childhood may help to identify a high cardiovascular risk population in early life.
Keywords: BMI trajectories, intima-media thickness, left ventricular mass index, longitudinal cohort study, youth
INTRODUCTION
Obesity has been well established as a major risk factor for cardiovascular disease (CVD),1 which are the leading causes of death and disability worldwide.2 Obesity have their beginnings in childhood and then track overtime, and childhood obesity has reached epidemic levels in developed as well as in developing countries.3, 4 Body mass index (BMI) is widely used to define obesity. Previous studies indicated that beside BMI levels, more rapid gains in BMI were also associated with an increased risk of CVD, and the BMI gain during childhood might be more critical.5, 6 Several studies have demonstrated that varied BMI trajectory patterns exist in population.7, 8 For example, Li et al. found that life-course BMI trajectories (between 7–43 years old) were associated with blood pressure at 43 years old.8 However, little is known about the BMI trajectory patterns during childhood and their effects on CVD risk in later life. Therefore, we aimed to identify subgroups of individuals with differential trajectories in BMI during childhood (5–18 years), and to determine the associations of childhood BMI trajectory patterns with the risk of subclinical CVD in young adulthood (mean age: 24 years), indexed by intima-media thickness (IMT) and left ventricular mass index (LVMI). The results of our study could shed some light on the prevention strategy for early identification of high CVD risk population.
METHODS
The participants were from the Georgia Stress and Heart (GSH) study, an ongoing longitudinal study designed to evaluate the development of CV risk factors in youth and young adults. Recruitment and evaluation of participants have been described in detail elsewhere.9 The participants who had at least 3 BMI measurements prior to 18 years old, regardless of whether they had CVD subclinical measurements in young adults, were used to identify subgroups with similar underlying BMI trajectory patterns. The Institutional Review Board of the Medical College of Georgia have approved the study and informed consent of each participant was obtained. Participants’ height and weight were measured with a Healthometer medical scale that was calibrated daily. Body surface area (BSA) was calculated as SQRT ([height (cm) * weight (kg)]/ 3600).10 IMT and LVM were measured up to 3 times in 501 participants (a total of 1115 measurements were eligible for analysis) and 496 participants (a total of 1118 measurements were eligible for analysis) respectively at visit 12, 14 and 15. Hewlett-Packard Sonos 5500 (Andover, MA) equipped with a 7.5 MHz linear array probe was used to measure the common carotid artery IMT. Sector-guided M-mode echocardiograms were performed with a Hewlett Packard Sonos 1500 echocardiograph to measure the LVM. LVM index was calculated using the necropsy-validated formula of Devereux et al and normalized to BSA to obtain LVMI. 11, 12
Latent class modeling was used to identify subgroups that share a similar underlying trajectory in BMI.13 A Stata plugin program (Traj) was used for estimating group-based trajectory model using maximum likelihood method.14 Briefly, this method is designed to identify clusters of individuals following a similar developmental trajectory on an outcome of interest, based on a semipara metric, group-based approach.13, 15 Selection of the best-fitting trajectory model was assessed using the Bayesian Information Criterion. Linear and quadratic terms of age were considered and evaluated based on their significance level. Once the optimal latent class model was estimated, posterior probabilities would be calculated from the recruitment probabilities (which are the conditional probabilities of class membership for a given response pattern), then individuals were assigned to latent class with the highest posterior probability.13 The baseline characteristics of participants, including age, race, sex, father’s education level, systolic and diastolic BP, among BMI trajectories were compared using ANOVA test for continuous variables or Chi-squared test for categorical variables. The associations of the trajectory groups with IMT and LVMI were examined by using mixed linear regression model with unstructured covariance, meanwhile trend test was performed by treating BMI trajectories as a continuous variable. A two-sided P < 0.05 was considered significant. All data analyses were performed using Stata software version 12.1 (STATA Corp., TX, US).
RESULTS
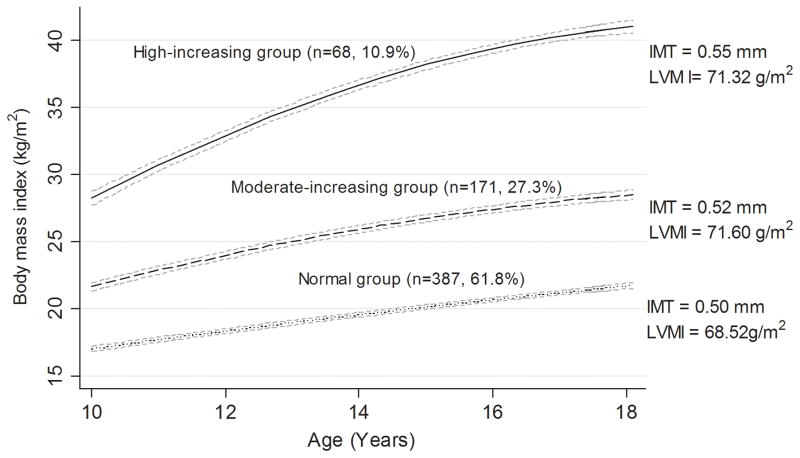
The trajectory patterns in BMI were examined among 626 participants aged 5–18 (a total of 4146 measurements, up to 12 visits). Three trajectory groups in BMI were identified. As shown in the Figure, 387 (61.8%) participants started with a low level and maintained a low increase in BMI (considered as normal group); 171 (27.3%) participants started with a moderate level and experienced a moderate increase in BMI (moderate-increasing [MI] group); and 68 (10.9%) participants started with a high level and had relatively fast increase in BMI level during childhood (high-increasing [HI] group). The participants in the HI group tended to be male, black, higher systolic blood pressure level at the baseline, and more likely to have a father with a lower educational level (P < 0.05).
Figure.
Trajectory groups identified for body mass index in childhood
Their patterns by age, the number and percentage were shown for each group. The mean levels of IMT and LVMI in adulthood were also shown for each group. Dash lines are 95% confidence interval lines.
IMT = intima-media thickness, LVMI = left ventricular mass index
The mean IMT and LVMI in the HI and MI group were higher than in the normal group (0.55, 0.52 and 0.50 mm for IMT; 71.32, 71.60 and 68.52g/m2 for LVMI). Increased rate of growth in BMI during childhood was significantly associated with increased LVMI in young adulthood (P for trend <0.05). Elevated BMI trajectory groups were independently associated with LVMI after adjustment for age, race, sex, blood pressure, father’s education. Compared to the normal group, individuals in the MI and HI groups showed higher IMT (β=0.014, P=0.043 for the MI group; β=0.034, P =0.001 for the HI group) and LVMI (β=4.148, P<0.001 for the MI group; β=3.079, P =0.100 for the HI group), respectively. The associations for LVMI were not virtually changed after adjustment for BMI at baseline or in young adulthood, but the associations for IMT were not significant after adjustment for BMI at baseline (Table).
Table.
The associations between childhood BMI trajectories and left ventricular mass index
| Intima-media thickness
|
Left ventricular mass index
|
|||||
|---|---|---|---|---|---|---|
| N | β (SE) | P value | N | β (SE) | P value | |
| Model 1 | N=501 | N=496 | ||||
| normal | 302 | Reference | 310 | Reference | ||
| Moderate-increasing | 143 | 0.017 (0.007) | 0.013 | 138 | 3.287 (1.355) | 0.015 |
| High-increasing | 56 | 0.044 (0.010) | <0.001 | 48 | 1.917 (2.103) | 0.362 |
| P for trend <0.001 | P for trend 0.052 | |||||
| Model 2* | N=496 | N=490 | ||||
| normal | 301 | Reference | 308 | Reference | ||
| Moderate-increasing | 141 | 0.014 (0.007) | 0.043 | 136 | 4.148 (1.153) | <0.001 |
| High-increasing | 54 | 0.034 (0.010) | 0.001 | 46 | 3.079 (1.914) | 0.100 |
| P for trend <0.001 | P for trend 0.002 | |||||
| Model 3* | N=496 | N=490 | ||||
| normal | 301 | Reference | 308 | Reference | ||
| Moderate-increasing | 141 | 0.012 (0.008) | 0.152 | 136 | 4.763 (1.353) | <0.001 |
| High-increasing | 54 | 0.030 (0.015) | 0.043 | 46 | 4.492 (2.515) | 0.074 |
| P for trend =0.044 | P for trend 0.003 | |||||
| Model 4* | N=496 | N=490 | ||||
| normal | 301 | Reference | 308 | Reference | ||
| Moderate-increasing | 141 | 0.007 (0.009) | 0.354 | 136 | 4.547 (1.328) | 0.008 |
| High-increasing | 54 | 0.017 (0.015) | 0.250 | 46 | 1.427 (2.631) | 0.588 |
| P for trend =0.228 | P for trend 0.065 | |||||
BMI = body mass index; SE = standard error
Model 1= Unadjusted model; Model 2= age, race, sex, father’s education level, systolic BP and diastolic BP; Model 3= Model 2+BMI in young adulthood; Model 4= Model 2+BMI at baseline
Five participants were excluded due to missing values of father’s education in Model 2–4 for intima-media thickness, and six participants were excluded due to missing values of father’s education in Model 2–4 for left ventricular mass index
DISCUSSIONS
Three trajectory groups in BMI during childhood were identified. We for the first time found that childhood trajectories of BMI were significantly associated with mean IMT and LVMI in young adulthood, which are two accepted subclinical markers for cardiovascular disease.16, 17 The associations with LVMI were independent of the BMI at baseline or in young adulthood, while the associations with IMT were not significant after adjustment for baseline BMI.
Several studies have incorporated intercepts and slopes, or latent trajectory methods to identify heterogeneity in the development of body fatness and obesity across the life course, and found that BMI trajectories associated with cardio-metabolic risk factors. In the National Longitudinal Study of Adolescent Health study, Attard et al. found that for adults at equivalent BMI, odds of diabetes, hypertension and inflammation differed according to BMI trajectories from adolescence to adulthood.5 Thompson et al. found that those with higher, steeper weight gains were more likely to have elevated high-sensitivity C-reactive protein regardless of initial weight across sex and age strata. The authors also found that steeper weight gains at younger ages (aged 18 to 30) may be riskier for the development of inflammation than weight gain at older ages (aged 31 to 66) in men.6 Four BMI trajectory patterns from ages 18 to 49 were identified in the National Longitudinal Survey of Youth 1979 Cohort, in which higher BMI trajectories were associated with elevated risk of adverse health outcomes, measured through a self-rated health survey.18 Consistent to our results, previous studies also found that participants being blacks, with lower socio-economic status and higher blood pressure had higher odds of being in the groups with faster increase of BMI, 18, 19 suggesting that the identified BMI trajectories to some extent do capture the population with varied cardiovascular risk exposures. The Amsterdam Growth and Health Longitudinal Study demonstrated that the “progressively overweight” BMI trajectory, measured from adolescence into adulthood, was associated with higher arterial pressure and lower HDL cholesterol at age 42.20
In this study, we confirmed that the differences of the BMI trajectories could start in early life, and these differences were associated with the increased risk of subclinical CVD, partially independent of the baseline level of BMI. More interestingly, individuals in the moderate increasing group had significantly increased risk of higher LVMI, indicating that regular screening and monitoring trajectories of BMI in children with relatively normal weight may assist in a more accurate identification of individuals with higher cardiovascular risk in early life. A major strength of the present study is that it involves up to 12 BMI measurements (median: 6, range: 3–12) in childhood, gives us a unique opportunity to examine the childhood trajectory patters of BMI. However, several limitations need to be noted. First, our cohort only includes European and African Americans. The trajectory groups identified may not be generalizable to other populations. In addition, not all participants who were used to identify the BMI trajectories have IMT and LVMI information available. However, we repeated the analysis using the participants with available IMT or LVMI data only. We identified similar childhood BMI trajectories, and the same associations of BMI trajectories with IMT/LVMI. More studies with large sample sizes should be performed to confirm our results.
In conclusion, our study confirmed that subgroups with different BMI trajectories exist in youth population, and found that trajectories of BMI in childhood were significant predictors of subclinical CVD. Our data suggested that regular screening and monitoring trajectories of BMI from childhood may assist in a more accurate identification of individuals with higher cardiovascular risk in early life.
Acknowledgments
We would like to thank all the participants in the study and the staff at the Georgia Prevention Institute.
FUNDING SOURCES
The present study was supported in part by NIH/NHLBI HL69999 and HL125577.
Footnotes
CONFLICT OF INTEREST
None.
References
- 1.Litwin SE. Childhood obesity and adulthood cardiovascular disease: quantifying the lifetime cumulative burden of cardiovascular risk factors. Journal of the American College of Cardiology. 2014;64(15):1588–90. doi: 10.1016/j.jacc.2014.07.962. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Praveen PA, Roy A, Prabhakaran D. Cardiovascular disease risk factors: a childhood perspective. Indian journal of pediatrics. 2013;80(Suppl 1):S3–12. doi: 10.1007/s12098-012-0767-z. [DOI] [PubMed] [Google Scholar]
- 4.Sahoo K, Sahoo B, Choudhury AK, Sofi NY, Kumar R, Bhadoria AS. Childhood obesity: causes and consequences. Journal of family medicine and primary care. 2015;4(2):187–92. doi: 10.4103/2249-4863.154628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attard SM, Herring AH, Howard AG, Gordon-Larsen P. Longitudinal trajectories of BMI and cardiovascular disease risk: the national longitudinal study of adolescent health. Obesity. 2013;21(11):2180–8. doi: 10.1002/oby.20569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson AL, Koehler E, Herring AH, Paynter L, Du S, Zhang B, et al. Weight Gain Trajectories Associated With Elevated C-Reactive Protein Levels in Chinese Adults. Journal of the American Heart Association. 2016;5(9) doi: 10.1161/JAHA.116.003262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berenson GS. Childhood risk factors predict adult risk associated with subclinical cardiovascular disease. The Bogalusa Heart Study The American journal of cardiology. 2002;90(10C):3L–7L. doi: 10.1016/s0002-9149(02)02953-3. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Hardy R, Kuh D, Power C. Life-course body mass index trajectories and blood pressure in mid life in two British birth cohorts: stronger associations in the later-born generation. International journal of epidemiology. 2015;44(3):1018–26. doi: 10.1093/ije/dyv106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su S, Wang X, Pollock JS, Treiber FA, Xu X, Snieder H, et al. Adverse childhood experiences and blood pressure trajectories from childhood to young adulthood: the Georgia stress and Heart study. Circulation. 2015;131(19):1674–81. doi: 10.1161/CIRCULATIONAHA.114.013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosteller RD. Simplified calculation of body-surface area. The New England journal of medicine. 1987;317(17):1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 11.Devereux RB, Lutas EM, Casale PN, Kligfield P, Eisenberg RR, Hammond IW, et al. Standardization of M-mode echocardiographic left ventricular anatomic measurements. Journal of the American College of Cardiology. 1984;4(6):1222–30. doi: 10.1016/s0735-1097(84)80141-2. [DOI] [PubMed] [Google Scholar]
- 12.Noble EE, Billington CJ, Kotz CM, Wang C. The lighter side of BDNF. American journal of physiology Regulatory, integrative and comparative physiology. 2011;300(5):R1053–69. doi: 10.1152/ajpregu.00776.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCutcheon AL. Latent Class Analysis. SAGE Publications; 1987. [Google Scholar]
- 14.Jones BL, Nagin DS. A Note on a Stata Plugin for Estimating Group-based Trajectory Models. Sociological Methods & Research. 2013;42(4):608–613. [Google Scholar]
- 15.JONES BL, NAGIN DS, ROEDER K. A SAS Procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research. 2001;29:374–393. [Google Scholar]
- 16.Baldassarre D, Hamsten A, Veglia F, de Faire U, Humphries SE, Smit AJ, et al. Measurements of carotid intima-media thickness and of interadventitia common carotid diameter improve prediction of cardiovascular events: results of the IMPROVE (Carotid Intima Media Thickness [IMT] and IMT-Progression as Predictors of Vascular Events in a High Risk European Population) study. Journal of the American College of Cardiology. 2012;60(16):1489–99. doi: 10.1016/j.jacc.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 17.Liao J, Karnik R, Gu H, Ziller MJ, Clement K, Tsankov AM, et al. Targeted disruption of DNMT1, DNMT3A and DNMT3B in human embryonic stem cells. Nature genetics. 2015;47(5):469–78. doi: 10.1038/ng.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostbye T, Malhotra R, Landerman LR. Body mass trajectories through adulthood: results from the National Longitudinal Survey of Youth 1979 Cohort (1981–2006) International journal of epidemiology. 2011;40(1):240–50. doi: 10.1093/ije/dyq142. [DOI] [PubMed] [Google Scholar]
- 19.Koning M, Hoekstra T, de Jong E, Visscher TL, Seidell JC, Renders CM. Identifying developmental trajectories of body mass index in childhood using latent class growth (mixture) modelling: associations with dietary, sedentary and physical activity behaviors: a longitudinal study. BMC public health. 2016;16(1):1128. doi: 10.1186/s12889-016-3757-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilmoth JM, London AS, Himes CL. Inter-Cohort Variation in the Consequences of U.S. Military Service for Men’s Mid- to Late-Life Body Mass Index Trajectories. In: Burton-Jeangros C, Cullati S, Sacker A, Blane D, editors. A Life Course Perspective on Health Trajectories and Transitions. Cham (CH): 2015. [PubMed] [Google Scholar]