Abstract
Individuals with schizophrenia (SZ) exhibit multiple premature age-related phenotypes and die ~20 years prematurely. The accelerated aging hypothesis of SZ has been advanced to explain these observations, it posits that SZ-associated factors accelerate the progressive biological changes associated with normal aging. Testing the hypothesis has been limited by the absence of robust, meaningful, and multi-tissue measures of biological age. Recently, a method was described in which DNA methylation (DNAm) levels at 353 genomic sites are used to produce “DNAm age”, an estimate of biological age with advantages over existing measures. We used this method and 3 publicly-available DNAm datasets, 1 from brain and 2 from blood, to test the hypothesis. The brain dataset was composed of data from the dorsolateral prefrontal cortex of 232 non-psychiatric control (NPC) and 195 SZ subjects. Blood dataset #1 was composed of data from whole blood of 304 NPC and 332 SZ subjects, and blood dataset #2 was composed of data from whole blood of 405 NPC and 260 SZ subjects. DNAm age and chronological age correlated strongly (r=0.92–0.95, p<0.0001) in both NPC and SZ subjects in all 3 datasets. DNAm age acceleration did not differ between NPC and SZ subjects in the brain dataset (t=0.52, p=0.60), blood dataset #1 (t=1.51, p=0.13), or blood dataset #2 (t=0.93, p=0.35). Consistent with our previous findings from a smaller study of postmortem brains, our findings suggest there is no acceleration of brain or blood aging in SZ and, thus, do not support the accelerated aging hypothesis of SZ.
Keywords: schizophrenia, accelerated aging, DNA methylation, epigenetic clock, aging, biomarkers
1. Introduction
Schizophrenia (SZ) is associated with premature age-related phenotypes throughout the body. For example, the brains of individuals with SZ exhibit alterations that are frequently associated with old age including dendritic spine loss (Glausier and Lewis, 2013; Moyer et al., 2015), cerebral cortical atrophy (van Haren et al., 2011), and cognitive dysfunction (Jeste et al., 2011). Premature age-related phenotypes including telomere shortening (Darrow et al., 2016; Wolkowitz et al., 2016), increased inflammatory markers (Lee et al., 2017), and elevated levels of oxidative stress (Lee et al., 2016; Okusaga, 2014) have been measured from blood of SZ subjects and suggest the involvement of multiple organ systems.
Individuals with SZ also die ~20 years prematurely (Laursen et al., 2014). Rates of suicide, homicide, and accidental death are increased among individuals with SZ but most of the excess mortality has been attributed to natural causes such a cardiovascular and respiratory disease (Saha et al., 2007). Both endogenous (e.g., polygenic risk) and environmental factors (e.g., health behaviors and health care access) are thought to contribute to premature mortality in SZ. Tobacco smoking (Brown et al., 1999), sedentary lifestyle (Osborn et al., 2007), obesity (Allison et al., 1999), insulin resistance (Greenhalgh et al., 2017; Pillinger et al., 2017), and hyperlipidemia (Henderson et al., 2015) are all more common in SZ than in the general population.
The accelerated aging hypothesis of SZ has been advanced to explain these observations, it posits that SZ-associated factors, either endogenous or environmental, accelerate the progressive biological changes of normal aging (Kirkpatrick et al., 2008). Testing this hypothesis has been challenging due the absence of robust and meaningful measures of biological age across multiple cell and tissue types. However, a recently described DNA methylation (DNAm)-based method of measuring biological age offers promise for addressing this challenge (Horvath, 2013). By combining DNAm levels at 353 genomic sites, the method produces “DNAm age”, a measure of biological age. This method estimates chronological age for healthy individuals with unprecedented accuracy, and this estimate is consistent across most cell and tissue types (Horvath, 2013). Importantly, DNAm age appears to capture an aspect of biological age as demonstrated by the fact that the difference between an individual’s DNAm age and chronological age is associated with clinically meaningful outcomes. For example, individuals exhibiting age acceleration (i.e., DNAm age > chronological age) are at greater risk for all-cause mortality (Chen et al., 2016; Marioni et al., 2015).
Given the advantages of the above DNAm-based method of measuring biological age, we previously used it to test the accelerated aging hypothesis of SZ in the superior temporal gyrus (STG), a region affected by cortical atrophy and dendritic spine loss in SZ (Shelton et al., 2015; Sun et al., 2009; Sweet et al., 2009). We found age acceleration did not differ between non-psychiatric control (NPC) and SZ subjects. That study, however, was limited in that we studied only a single brain region in a small cohort (N=44). Here, we follow-up on that study by using the same approach in 3 large, publicly-available DNAm datasets—one from brain and two from blood.
2. Experimental Methods
2.1 Dataset Selection and Description
The datasets were chosen such that one measured DNAm a brain region distinct from that which we studied previously and the other two measured DNAm in a peripheral tissue. Additional criteria for selecting the datasets for analysis included that they i) were publicly available, ii) measured DNAm using Human Methylation 450K array platform (HM450K Array; Illumina, San Diego, CA, USA), iii) included an age range of at least 30 years for mixed-age samples, and iv) made up of a relatively large number of samples compared to other similar datasets.
2.1.1 Brain Dataset
These data were generated by Jaffe and colleagues (Jaffe et al., 2016) using DLPFC, defined as the middle one-third of the middle frontal gyrus immediately anterior to the genu of the corpus callosum, DNA from postmortem brains. They extracted DNA from DLPFC gray matter with the phenol-chloroform method, and bisulfite conversion of DNA was performed with the EZ DNA methylation kit (Zymo Research, Irvine, CA, USA) for analysis of DNAm using the HM450K array.
Postmortem brains from which they generated these data were recovered during routine autopsies at the Offices of the Chief Medical Examiners of the District of Columbia and of the Commonwealth of Virginia, Northern District following informed consent from legal next-of-kin for donation to the National Institute of Mental Health Brain Tissue Collection at the National Institutes of Health in Bethesda, Maryland. Details of the postmortem brain donation process have been previously described (Deep-Soboslay et al., 2005; Jaffe et al., 2016; Lipska et al., 2006). Briefly, consensus Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) diagnoses were made by psychiatrists based on clinical information obtained via structured interviews with the next-of-kin, including the Structured Clinical Interview for DSM-IV–clinician version (First, 1997) and the NIMH psychological autopsy interview, and psychiatric record reviews with the Diagnostic Evaluation After Death (Zalcman, 1983). All brains underwent neuropathological examination and those with evidence of neurological disorder were excluded. SZ group subjects met DSM-IV criteria for schizophrenia or schizoaffective disorder, and NPC group subjects did not meet DSM-IV criteria for any psychiatric or substance-related disorders and toxicology screening at time of death excluded acute drug and/or alcohol use.
For the present study, data from 232 NPC subjects (160 males, 72 females) and 195 SZ subjects (119 males, 76 females), all with ages greater than 17 years of age, were downloaded from Gene Expression Omnibus (GEO) (GSE74193). Ages ranged from 17–85 years of age for NPC subjects and 17–96 years of age for SZ subjects. Additional phenotypic information for the subjects in this dataset, including antipsychotic use at time of death, illness duration, and manner of death, was obtained from the authors of the original study.
2.1.2 Blood Datasets
2.1.2.1 Blood Dataset #1
These data were generated by Hannon and colleagues (Hannon et al., 2016) using whole blood DNA of subjects from The University College London (UCL) case–control sample (Datta et al., 2010). They extracted DNA from frozen whole blood samples with the phenol-chloroform method, and bisulfite conversion of DNA was performed with the EZ-96 DNA methylation kit (Zymo Research, Irvine, CA, USA) for analysis of DNAm using the HM450K array.
Subjects in the UCL case-control sample were recruited from UK National Health Service (NHS) clinics in London and South England. Subjects were included only if both parents were of English, Irish, Welsh, or Scottish descent and if three out of four grandparents were of the same descent. SZ group subjects were recruited based on having a clinical diagnosis of schizophrenia and then interviewed using the Schedule for Affective Disorders and Schizophrenia-Lifetime Version (SADS-L) (Spitzer, 1977), those that received a probable diagnosis of schizophrenia per Research Diagnostic Criteria (RDC) were included. Individuals with bipolar disorder, schizoaffective disorder bipolar type, and schizophrenia associated with brain damage were excluded. NPC group subjects interviewed with the SADS-L screening questions, those with neither a personal history of an RDC-defined psychiatric disorder nor a family history of schizophrenia, bipolar disorder, or alcohol use disorder were included (Datta et al., 2010).
For the present study, data from 322 NPC and 353 SZ subjects were downloaded from GEO (GEO identifier GSE80417). Chronological age information was missing for 17 NPC and 20 SZ subjects, and there was an evident error in the entry of chronological age for one subject from each of the NPC and SZ groups (recorded as 891 and 883 years of age, respectively). Analysis was performed on the 304 NPC subjects (135 males, 169 females) and 332 SZ subjects (242 males, 90 females) with correct chronological age information. Ages ranged from 18–87 years of age for NPC subjects, and 19–90 years of age for SZ subjects.
2.1.2.2 Blood Dataset #2
These data were generated by Hannon and colleagues (Hannon et al., 2016) using whole blood DNA of subjects from the Aberdeen case–control sample (International Schizophrenia, 2008). They extracted DNA from frozen whole blood samples with the phenol-chloroform method, and bisulfite conversion of DNA was performed with the EZ-96 DNA methylation kit (Zymo Research, Irvine, CA, USA) for analysis of DNAm using the HM450K array.
Subjects in the Aberdeen case–control sample self-identified as being born in the British Isles (95% in Scotland). SZ subjects were initially recruited through Scottish psychiatric hospitals based on meeting DSM-IV (1994) and ICD-10 criteria for schizophrenia using the Operational Criteria Checklist (Azevedo et al., 1999; McGuffin et al., 1991) and then interviewed using the Structured Clinical Interview for DSM-IV (SCID), those for which a consensus (2 psychiatrists) diagnosis of schizophrenia based on the SCID and inspection of psychiatric case notes were included. NPC subjects were recruited through general practices in the same region of Scotland and were ethnically matched. NPC subjects were recruited based on age, sex, and the absence of psychiatric disorder and then interviewed using a questionnaire about personal and family psychiatric history, those without personal or family history of a major psychiatric disorder were included (International Schizophrenia, 2008).
For the present study, data from 433 NPC subjects and 414 SZ subjects were downloaded from GEO (GEO identifier GSE80427). Chronological age information was missing from 28 NPC and 155 SZ subjects. Analysis was performed on the 405 NPC subjects (303 males, 102 females) and 260 SZ subjects (187 males, 83 females) with correct chronological age information. Ages ranged from 18–66 years of age for NPC subjects, and 18–80 years of age for SZ subjects.
2.1.3 Calculation of DNAm age and Age Acceleration
For each of the 3 data sets, DNAm was measured at 485,577 sites using HM450K arrays. Details regarding preprocessing of HM450K array data including color adjustment, background correction, and normalization can be found in the articles in which the data were originally published (Hannon et al., 2016; Jaffe et al., 2016). The normalized β-values, the ratio of signal from a methylated probe relative to the sum of both methylated and unmethylated probes, were downloaded from GEO directly for each data set. The β-values for the 353 DNAm sites necessary for calculating DNAm age were extracted from the downloaded data, and DNAm age was calculated as described in Horvath et al (Horvath, 2013). Briefly, for each dataset, a linear model was built by regressing DNAm age on chronological age in NPC subjects (black line in Figures 1–3). Then, DNAm age acceleration for each subject (NPC or SZ) was calculated as the residual value resulting from the regression model. Finally, a two-sided t-test was performed to test whether DNAm age acceleration (i.e., residual value) in SZ subjects is the same as in NPC subjects.
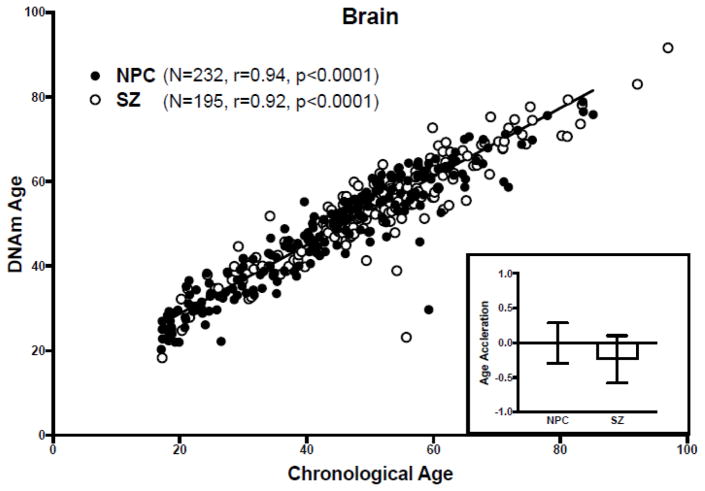
Figure 1. DNAm age analysis of the brain dataset.
(Main) Scatter plot of DNAm age versus chronological age. Filled circles correspond to NPC subjects, unfilled circles to SZ subjects. The regression line of DNAm age on chronological age in NPC subjects is shown in black. (Inset) Bar graph of age acceleration in NPC and SZ subjects. The bars represent mean ± standard error of the mean. A negative value for age acceleration means the subject’s observed DNAm age is younger than the predicted DNAm age for an NPC subject of the same chronologic age. The average age acceleration in NPC subjects is 0 by definition.
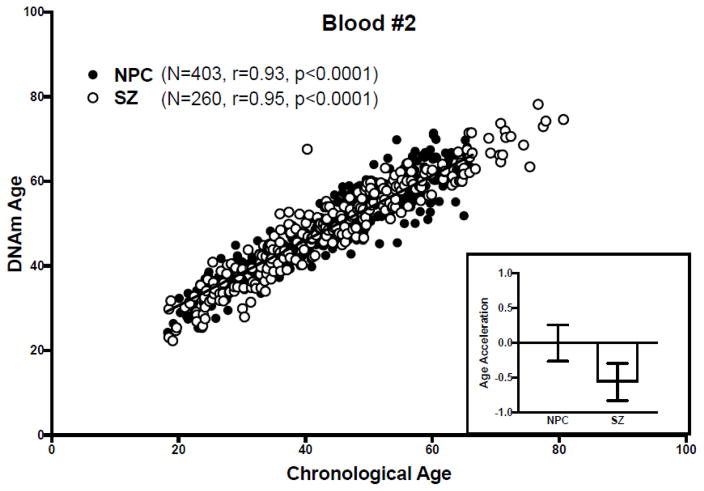
Figure 3. DNAm age analysis of blood dataset #2.
(Main) Scatter plot of DNAm age versus chronological age. Filled circles correspond to NPC subjects, unfilled circles to SZ subjects. The regression line of DNAm age on chronological age in NPC subjects is shown in black. (Inset) Bar graph of age acceleration in NPC and SZ subjects. The bars represent mean ± standard error of the mean. A negative value for age acceleration means the subject’s observed DNAm age is younger than the predicted DNAm age for an NPC subject of the same chronologic age. The average age acceleration in NPC subjects is 0 by definition.
For blood dataset #1, DNAm age acceleration was corrected for differences in blood cell counts using the “Horvath” approach (Horvath and Levine, 2015). Specifically, DNAm age was regressed on chronological age + naive CD8+ T cells + exhausted CD8+ T cells + plasmablasts + CD4+ T cells + natural killer cells + monocytes + granulocytes in NPC subjects, the resulting model applied to both NPC and SZ subjects separately, the residuals calculated, and a 2-sample t-test performed. The data necessary for IEAA was not available for blood dataset #2.
3. Results
3.1.1 Brain Dataset
DNAm age correlated with chronological age in both NPC (r=0.94, p<0.0001) and SZ subjects (r=0.92, p<0.0001) (Figure 1). We found that age acceleration did not differ between NPC and SZ groups (t=0.52, p=0.60) (Figure 1, inset), and this did not change when males and females were analyzed separately (p=0.22 and p=0.07, respectively). Further, age acceleration in the NPC group did not differ between either the subgroup of SZ subjects taking antipsychotics at the time of death (p=0.28) or the subgroup of SZ subjects not taking antipsychotics at the time of death (p=0.57), and the regression of DNAm age acceleration on illness duration for SZ samples is not significant (p = 0.26).
3.1.2 Blood Datasets
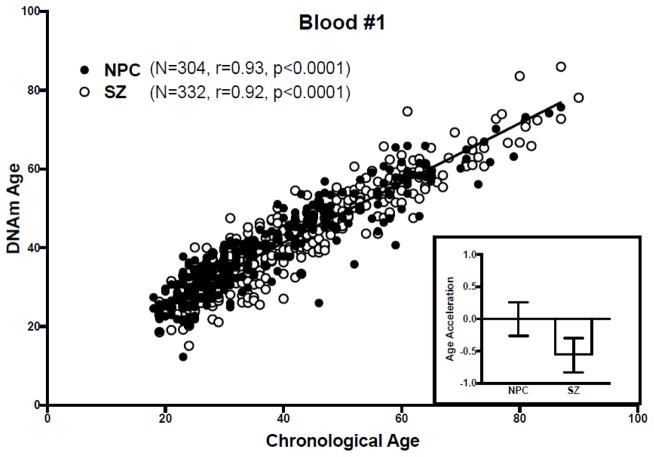
In blood dataset #1, as in the brain dataset, DNAm age correlated with chronological age in both NPC (r=0.93, p<0.0001) and SZ subjects (r=0.92, p<0.0001) (Figure 2), and age acceleration did not differ between NPC and SZ groups (t=1.51, p=0.13) (Figure 2, inset), and this did not change upon correcting for blood cell counts. Similarly, in blood dataset #2, DNAm age correlated with chronological age in both NPC (r=0.93, p<0.0001) and SZ subjects (r=0.95, p<0.0001) (Figure 3), and age acceleration did not differ between NPC and SZ groups (t=0.93, p=0.35) (Figure 3, inset). Of note, the authors of the study in which the original blood DNAm datasets were described also found DNAm age correlated strongly with chronological age (r=0.93).
Figure 2. DNAm age analysis of blood dataset #1.
(Main) Scatter plot of DNAm age versus chronological age. Filled circles correspond to NPC subjects, unfilled circles to SZ subjects. The regression line of DNAm age on chronological age in NPC subjects is shown in black. (Inset) Bar graph of age acceleration in NPC and SZ subjects. The bars represent mean ± standard error of the mean. A negative value for age acceleration means the subject’s observed DNAm age is younger than the predicted DNAm age for an NPC subject of the same chronologic age. The average age acceleration in NPC subjects is 0 by definition.
DNAm age in male SZ subjects appears to be decelerated compared to male NPC subjects when data from males and females are analyzed separately, the effect reaches statistical significance in blood dataset #1 (p=0.03) and approaches being statistically significant in blood dataset #2 (p=0.06). No differences in DNAm age acceleration are observed between female SZ and NPC subjects in either blood dataset #1 (p=0.75) or blood dataset #2 (p=0.20).
4. Discussion
In this study, the largest DNAm-based test of the accelerated aging hypothesis of SZ to date, and the only one to study multiple tissue types, we did not find DNAm age to be accelerated in the DLPFC or blood of SZ subjects. These findings are consistent with earlier small study of DNAm age in postmortem STG. Here, we overcome the major limitations of that study by leveraging large publicly-available DNAm datasets.
Our findings that DNAm age is not accelerated in SZ subjects, now from 2 distinct brain regions with key roles in SZ pathophysiology, argue against accelerated brain aging in SZ. The fact that we did not find acceleration of DNAm age in blood from SZ subjects argues against accelerated aging in the periphery in SZ. In fact, our findings in the blood datasets suggest that age may be decelerated in males with SZ. However, it may also be that SZ subjects with older DNAm age (i.e., biologically ‘older‘) selectively died earlier thus leading to an underrepresentation of SZ subjects with older DNAm age in the study. Of note, the differences in DNAm age between NPC and SZ subjects that we observed were subtle and limited to one subgroup, but the primary analyses of all three datasets found many significant differences in DNAm levels between NPC and SZ subjects (Hannon et al., 2016; Jaffe et al., 2016).
Aging in SZ may be accelerated in other brain regions and/or other peripheral tissues. Using the same DNAm-based approach we used, brains from Huntington‘s disease subjects were shown to undergo brain-region-specific age acceleration (Horvath et al., 2016). Similarly, DNAm age was shown to be greater than chronological age in the brain and blood, but not buccal epithelium, of Down‘s syndrome subjects (Horvath et al., 2015a). However, a recent study found that DNAm age did not differ between SZ and NPC subjects in the prefrontal cortex, striatum, hippocampus and cerebellum (Viana et al., 2017). Age acceleration may occur in some cell types and not others, which will require cell type-specific DNAm quantification to evaluate. STG and DLPFC layer 3 pyramidal neurons will be of particular interest given that these neurons exhibit dendritic spine alterations which could reflect premature aging (Glausier and Lewis, 2013; Moyer et al., 2015). The effect of treatment with antipsychotics on DNAm age should be considered. Evidence suggests that antipsychotics do alter DNAm (Castellani et al., 2015) and, in some cases, SZ-associated DNAm alterations are normalized by antipsychotics (Abdolmaleky et al., 2015). Though we did not observe an effect of antipsychotics at the time of death on DNAm age in the brain dataset, we were unable to assess the effect of antipsychotic medications on DNAm age in the blood datasets because information about antipsychotic treatment was not available. Notably, some studies suggest that SZ-associated effects on telomere length, another measure of biological age, are reversed by antipsychotics (Lindqvist et al., 2015; Wolkowitz et al., 2016).
Individuals younger than 17 years of age were not represented in the current study so it is possible that DNAm age is accelerated relative to chronological age among individuals with SZ, or at risk for SZ, in this age group. Notably, a recent study found that telomere length, another measure of biological age, was shorter in whole blood samples from 14 and 26 year old subjects at ultra-high risk for psychosis (Maurya et al., 2017). Such observations raise the question of whether they represent accelerated aging or, alternatively, precocious development. Nonetheless, the observation that biological age is greater than chronological age during development among subjects that go on to have SZ is consistent with SZ being a disorder of aberrant neurodevelopment (Lewis and Levitt, 2002).
Evidence from the study of aging and diseases of accelerated aging support the concept that many paths can lead to the aging phenotype (Prolla, 2005). Thus, it is possible that accelerated aging does occur in SZ, but is not captured by the measure used in the present study. That is, DNAm age may measure a path towards the aging phenotype that is not accelerated in SZ. Other indices of biological aging (e.g., age-dependent gene expression (Glorioso et al., 2011; Peters et al., 2015), telomere length (Darrow et al., 2016), and basic blood biochemistry and cell counts (Putin et al., 2016)) may be better suited for detecting accelerated aging in SZ subjects. Thus, our use of only one measure of biological aging limits the interpretation of the current study. As the many paths to the aging phenotype become better understood, it may be that different measures will prove to be better at quantifying acceleration down each of the different paths. Such an idea may explain the observation that 2 validated measures of biological age, DNAm age and telomere length, are independently associated with chronological age and mortality (Marioni et al., 2016).
Table 1.
Cohort characteristics. Data for continuous variables are presented as group average ± SEM. NPC, non-psychiatric control; SZ, schizophrenia; M, male; F, female; W, white; B, black; ATOD, at time of death; AP+, antipsychotic medication positive; AP-, antipsychotic medication negative; NA, not available; Sm, smoker; NS, non-smoker; N, natural death; A, accidental death; H, homicide; Su, suicide; PMI, postmortem interval.
| Cohort | Brain | Blood #1 | Blood #2 | |||
|---|---|---|---|---|---|---|
|
|
||||||
| Group | NPC | SZ | NPC | SZ | NPC | SZ |
| Number | 232 | 195 | 304 | 332 | 405 | 260 |
| Sex | 160 M, 72 F | 119 M, 76 F | 135 M, 169 F | 242 M, 90 F | 303 M, 102 F | 187 M, 83 F |
| Race | 100 W, 132 B | 108 W, 87 B | 304 W | 332 W | 405 | 260 |
| Anipsychotics ATOD | 0 AP+, 150 AP-, 82 NA | 125 AP+, 67 AP-, 3 NA | -- | -- | -- | -- |
| Smoking Cigarettes ATOD | 54 Sm, 171 NS, 7 NA | 131 Sm, 53 NS, 11 NA | -- | -- | -- | -- |
| Manner of Death | 179 N, 20 A, 31 H, 2 NA | 128 N, 24 A, 36 Su, 7 NA | -- | -- | -- | -- |
| PMI (Hours) | 30.4 ± 0.97 | 39.1 ± 1.73 | -- | -- | -- | -- |
Acknowledgments
Funding: This work was supported by NIH Grants RO1 MH071533 (RAS), RO3 MH108849 (YD), T32 MH016804 (BCM), KL2 TR001856 (BCM), and K23 MH112798 (BCM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs, or the United States Government.
We thank Andrew E. Jaffe, Eilis Hannon, and Jonathan Mill for their assistance with the data associated with their publications.
This work was supported by NIH Grants RO1 MH071533 (RAS), RO3 MH108849 (YD), KL2 TR001856 (BCM), and K23 MH112798 (BCM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs, or the United States Government.
Footnotes
5. Contributions.
Study was conceived of and designed by BCM and RAS. Data was acquired by BCM; analyzed by YD and HL; and interpreted by BCM, DAL, and RAS. Manuscript was drafted by BCM. All authors critically reviewed manuscript drafts and approved the final manuscript. BCM is the corresponding author. RAS is the manuscript’s guarantor.
6. Conflict of Interest
David A. Lewis currently receives investigator-initiated research support from Pfizer and in 2017 served as a consultant to Merck.
All other authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Diagnostic and Statistical Manual for Mental Disorders. 4. American Psychiatric Press; Washington DC: 1994. [Google Scholar]
- Abdolmaleky HM, Pajouhanfar S, Faghankhani M, Joghataei MT, Mostafavi A, Thiagalingam S. Antipsychotic drugs attenuate aberrant DNA methylation of DTNBP1 (dysbindin) promoter in saliva and post-mortem brain of patients with schizophrenia and Psychotic bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2015;168(8):687–696. doi: 10.1002/ajmg.b.32361. [DOI] [PubMed] [Google Scholar]
- Allison DB, Fontaine KR, Heo M, Mentore JL, Cappelleri JC, Chandler LP, Weiden PJ, Cheskin LJ. The distribution of body mass index among individuals with and without schizophrenia. J Clin Psychiatry. 1999;60(4):215–220. doi: 10.4088/jcp.v60n0402. [DOI] [PubMed] [Google Scholar]
- Azevedo MH, Soares MJ, Coelho I, Dourado A, Valente J, Macedo A, Pato M, Pato C. Using consensus OPCRIT diagnoses. An efficient procedure for best-estimate lifetime diagnoses. Br J Psychiatry. 1999;175:154–157. doi: 10.1192/bjp.175.2.154. [DOI] [PubMed] [Google Scholar]
- Brown S, Birtwistle J, Roe L, Thompson C. The unhealthy lifestyle of people with schizophrenia. Psychol Med. 1999;29(3):697–701. doi: 10.1017/s0033291798008186. [DOI] [PubMed] [Google Scholar]
- Castellani CA, Melka MG, Diehl EJ, Laufer BI, O’Reilly RL, Singh SM. DNA methylation in psychosis: insights into etiology and treatment. Epigenomics. 2015;7(1):67–74. doi: 10.2217/epi.14.66. [DOI] [PubMed] [Google Scholar]
- Chen BH, Marioni RE, Colicino E, Peters MJ, Ward-Caviness CK, Tsai PC, Roetker NS, Just AC, Demerath EW, Guan W, Bressler J, Fornage M, Studenski S, Vandiver AR, Moore AZ, Tanaka T, Kiel DP, Liang L, Vokonas P, Schwartz J, Lunetta KL, Murabito JM, Bandinelli S, Hernandez DG, Melzer D, Nalls M, Pilling LC, Price TR, Singleton AB, Gieger C, Holle R, Kretschmer A, Kronenberg F, Kunze S, Linseisen J, Meisinger C, Rathmann W, Waldenberger M, Visscher PM, Shah S, Wray NR, McRae AF, Franco OH, Hofman A, Uitterlinden AG, Absher D, Assimes T, Levine ME, Lu AT, Tsao PS, Hou L, Manson JE, Carty CL, LaCroix AZ, Reiner AP, Spector TD, Feinberg AP, Levy D, Baccarelli A, van Meurs J, Bell JT, Peters A, Deary IJ, Pankow JS, Ferrucci L, Horvath S. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY) 2016;8(9):1844–1865. doi: 10.18632/aging.101020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow SM, Verhoeven JE, Revesz D, Lindqvist D, Penninx BW, Delucchi KL, Wolkowitz OM, Mathews CA. The Association Between Psychiatric Disorders and Telomere Length: A Meta-Analysis Involving 14,827 Persons. Psychosom Med. 2016;78(7):776–787. doi: 10.1097/PSY.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, McQuillin A, Rizig M, Blaveri E, Thirumalai S, Kalsi G, Lawrence J, Bass NJ, Puri V, Choudhury K, Pimm J, Crombie C, Fraser G, Walker N, Curtis D, Zvelebil M, Pereira A, Kandaswamy R, St Clair D, Gurling HM. A threonine to isoleucine missense mutation in the pericentriolar material 1 gene is strongly associated with schizophrenia. Mol Psychiatry. 2010;15(6):615–628. doi: 10.1038/mp.2008.128. [DOI] [PubMed] [Google Scholar]
- Deep-Soboslay A, Akil M, Martin CE, Bigelow LB, Herman MM, Hyde TM, Kleinman JE. Reliability of psychiatric diagnosis in postmortem research. Biol Psychiatry. 2005;57(1):96–101. doi: 10.1016/j.biopsych.2004.10.016. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders--Clinician Version (SCID-CV) American Psychiatric Press; Washington DC: 1997. [Google Scholar]
- Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107. doi: 10.1016/j.neuroscience.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorioso C, Oh S, Douillard GG, Sibille E. Brain molecular aging, promotion of neurological disease and modulation by sirtuin 5 longevity gene polymorphism. Neurobiol Dis. 2011;41(2):279–290. doi: 10.1016/j.nbd.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh AM, Gonzalez-Blanco L, Garcia-Rizo C, Fernandez-Egea E, Miller B, Arroyo MB, Kirkpatrick B. Meta-analysis of glucose tolerance, insulin, and insulin resistance in antipsychotic-naive patients with nonaffective psychosis. Schizophr Res. 2017;179:57–63. doi: 10.1016/j.schres.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon E, Dempster E, Viana J, Burrage J, Smith AR, Macdonald R, St Clair D, Mustard C, Breen G, Therman S, Kaprio J, Toulopoulou T, Hulshoff Pol HE, Bohlken MM, Kahn RS, Nenadic I, Hultman CM, Murray RM, Collier DA, Bass N, Gurling H, McQuillin A, Schalkwyk L, Mill J. An integrated genetic-epigenetic analysis of schizophrenia: evidence for co-localization of genetic associations and differential DNA methylation. Genome Biol. 2016;17(1):176. doi: 10.1186/s13059-016-1041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson DC, Vincenzi B, Andrea NV, Ulloa M, Copeland PM. Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses. Lancet Psychiatry. 2015;2(5):452–464. doi: 10.1016/S2215-0366(15)00115-7. [DOI] [PubMed] [Google Scholar]
- Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Garagnani P, Bacalini MG, Pirazzini C, Salvioli S, Gentilini D, Di Blasio AM, Giuliani C, Tung S, Vinters HV, Franceschi C. Accelerated epigenetic aging in Down syndrome. Aging Cell. 2015a;14(3):491–495. doi: 10.1111/acel.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Langfelder P, Kwak S, Aaronson J, Rosinski J, Vogt TF, Eszes M, Faull RL, Curtis MA, Waldvogel HJ, Choi OW, Tung S, Vinters HV, Coppola G, Yang XW. Huntington’s disease accelerates epigenetic aging of human brain and disrupts DNA methylation levels. Aging (Albany NY) 2016;8(7):1485–1512. doi: 10.18632/aging.101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Levine AJ. HIV-1 Infection Accelerates Age According to the Epigenetic Clock. J Infect Dis. 2015;212(10):1563–1573. doi: 10.1093/infdis/jiv277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Pirazzini C, Bacalini MG, Gentilini D, Di Blasio AM, Delledonne M, Mari D, Arosio B, Monti D, Passarino G, De Rango F, D’Aquila P, Giuliani C, Marasco E, Collino S, Descombes P, Garagnani P, Franceschi C. Decreased epigenetic age of PBMCs from Italian semi-supercentenarians and their offspring. Aging (Albany NY) 2015b;7(12):1159–1170. doi: 10.18632/aging.100861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Schizophrenia C. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455(7210):237–241. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AE, Gao Y, Deep-Soboslay A, Tao R, Hyde TM, Weinberger DR, Kleinman JE. Mapping DNA methylation across development, genotype and schizophrenia in the human frontal cortex. Nat Neurosci. 2016;19(1):40–47. doi: 10.1038/nn.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV, Wolkowitz OM, Palmer BW. Divergent trajectories of physical, cognitive, and psychosocial aging in schizophrenia. Schizophr Bull. 2011;37(3):451–455. doi: 10.1093/schbul/sbr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Messias E, Harvey PD, Fernandez-Egea E, Bowie CR. Is schizophrenia a syndrome of accelerated aging? Schizophr Bull. 2008;34(6):1024–1032. doi: 10.1093/schbul/sbm140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen TM, Nordentoft M, Mortensen PB. Excess early mortality in schizophrenia. Annu Rev Clin Psychol. 2014;10:425–448. doi: 10.1146/annurev-clinpsy-032813-153657. [DOI] [PubMed] [Google Scholar]
- Lee EE, Eyler LT, Wolkowitz OM, Martin AS, Reuter C, Kraemer H, Jeste DV. Elevated plasma F2-isoprostane levels in schizophrenia. Schizophr Res. 2016;176(2–3):320–326. doi: 10.1016/j.schres.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EE, Hong S, Martin AS, Eyler LT, Jeste DV. Inflammation in Schizophrenia: Cytokine Levels and Their Relationships to Demographic and Clinical Variables. Am J Geriatr Psychiatry. 2017;25(1):50–61. doi: 10.1016/j.jagp.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Epel ES, Mellon SH, Penninx BW, Revesz D, Verhoeven JE, Reus VI, Lin J, Mahan L, Hough CM, Rosser R, Bersani FS, Blackburn EH, Wolkowitz OM. Psychiatric disorders and leukocyte telomere length: Underlying mechanisms linking mental illness with cellular aging. Neurosci Biobehav Rev. 2015;55:333–364. doi: 10.1016/j.neubiorev.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipska BK, Deep-Soboslay A, Weickert CS, Hyde TM, Martin CE, Herman MM, Kleinman JE. Critical factors in gene expression in postmortem human brain: Focus on studies in schizophrenia. Biol Psychiatry. 2006;60(6):650–658. doi: 10.1016/j.biopsych.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Marioni RE, Harris SE, Shah S, McRae AF, von Zglinicki T, Martin-Ruiz C, Wray NR, Visscher PM, Deary IJ. The epigenetic clock and telomere length are independently associated with chronological age and mortality. Int J Epidemiol. 2016 doi: 10.1093/ije/dyw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni RE, Shah S, McRae AF, Chen BH, Colicino E, Harris SE, Gibson J, Henders AK, Redmond P, Cox SR, Pattie A, Corley J, Murphy L, Martin NG, Montgomery GW, Feinberg AP, Fallin MD, Multhaup ML, Jaffe AE, Joehanes R, Schwartz J, Just AC, Lunetta KL, Murabito JM, Starr JM, Horvath S, Baccarelli AA, Levy D, Visscher PM, Wray NR, Deary IJ. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015;16:25. doi: 10.1186/s13059-015-0584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya PK, Rizzo LB, Xavier G, Tempaku PF, Zeni-Graiff M, Santoro ML, Mazzotti DR, Zugman A, Pan P, Noto C, Maes M, Asevedo E, Mansur RB, Cunha GR, Gadelha A, Bressan RA, Belangero SI, Brietzke E. Shorter leukocyte telomere length in patients at ultra high risk for psychosis. Eur Neuropsychopharmacol. 2017 doi: 10.1016/j.euroneuro.2017.02.008. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Farmer A, Harvey I. A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatry. 1991;48(8):764–770. doi: 10.1001/archpsyc.1991.01810320088015. [DOI] [PubMed] [Google Scholar]
- Moyer CE, Shelton MA, Sweet RA. Dendritic spine alterations in schizophrenia. Neurosci Lett. 2015;601:46–53. doi: 10.1016/j.neulet.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okusaga OO. Accelerated aging in schizophrenia patients: the potential role of oxidative stress. Aging Dis. 2014;5(4):256–262. doi: 10.14336/AD.2014.0500256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn DP, Nazareth I, King MB. Physical activity, dietary habits and Coronary Heart Disease risk factor knowledge amongst people with severe mental illness: a cross sectional comparative study in primary care. Soc Psychiatry Psychiatr Epidemiol. 2007;42(10):787–793. doi: 10.1007/s00127-007-0247-3. [DOI] [PubMed] [Google Scholar]
- Peters MJ, Joehanes R, Pilling LC, Schurmann C, Conneely KN, Powell J, Reinmaa E, Sutphin GL, Zhernakova A, Schramm K, Wilson YA, Kobes S, Tukiainen T, Ramos YF, Goring HH, Fornage M, Liu Y, Gharib SA, Stranger BE, De Jager PL, Aviv A, Levy D, Murabito JM, Munson PJ, Huan T, Hofman A, Uitterlinden AG, Rivadeneira F, van Rooij J, Stolk L, Broer L, Verbiest MM, Jhamai M, Arp P, Metspalu A, Tserel L, Milani L, Samani NJ, Peterson P, Kasela S, Codd V, Peters A, Ward-Caviness CK, Herder C, Waldenberger M, Roden M, Singmann P, Zeilinger S, Illig T, Homuth G, Grabe HJ, Volzke H, Steil L, Kocher T, Murray A, Melzer D, Yaghootkar H, Bandinelli S, Moses EK, Kent JW, Curran JE, Johnson MP, Williams-Blangero S, Westra HJ, McRae AF, Smith JA, Kardia SL, Hovatta I, Perola M, Ripatti S, Salomaa V, Henders AK, Martin NG, Smith AK, Mehta D, Binder EB, Nylocks KM, Kennedy EM, Klengel T, Ding J, Suchy-Dicey AM, Enquobahrie DA, Brody J, Rotter JI, Chen YD, Houwing-Duistermaat J, Kloppenburg M, Slagboom PE, Helmer Q, den Hollander W, Bean S, Raj T, Bakhshi N, Wang QP, Oyston LJ, Psaty BM, Tracy RP, Montgomery GW, Turner ST, Blangero J, Meulenbelt I, Ressler KJ, Yang J, Franke L, Kettunen J, Visscher PM, Neely GG, Korstanje R, Hanson RL, Prokisch H, Ferrucci L, Esko T, Teumer A, van Meurs JB, Johnson AD Consortium NU. The transcriptional landscape of age in human peripheral blood. Nat Commun. 2015;6:8570. doi: 10.1038/ncomms9570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillinger T, Beck K, Gobjila C, Donocik JG, Jauhar S, Howes OD. Impaired Glucose Homeostasis in First-Episode Schizophrenia: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2017;74(3):261–269. doi: 10.1001/jamapsychiatry.2016.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prolla TA. Multiple roads to the aging phenotype: insights from the molecular dissection of progerias through DNA microarray analysis. Mech Ageing Dev. 2005;126(4):461–465. doi: 10.1016/j.mad.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Putin E, Mamoshina P, Aliper A, Korzinkin M, Moskalev A, Kolosov A, Ostrovskiy A, Cantor C, Vijg J, Zhavoronkov A. Deep biomarkers of human aging: Application of deep neural networks to biomarker development. Aging (Albany NY) 2016;8(5):1021–1033. doi: 10.18632/aging.100968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- Shelton MA, Newman JT, Gu H, Sampson AR, Fish KN, MacDonald ML, Moyer CE, DiBitetto JV, Dorph-Petersen KA, Penzes P, Lewis DA, Sweet RA. Loss of Microtubule-Associated Protein 2 Immunoreactivity Linked to Dendritic Spine Loss in Schizophrenia. Biol Psychiatry. 2015;78(6):374–385. doi: 10.1016/j.biopsych.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer R, Endicott J. The schedule for affective disorders and schizophrenia, lifetime version. 3. New York State Psychiatric Institute; New York: 1977. [Google Scholar]
- Sun J, Maller JJ, Guo L, Fitzgerald PB. Superior temporal gyrus volume change in schizophrenia: a review on region of interest volumetric studies. Brain Res Rev. 2009;61(1):14–32. doi: 10.1016/j.brainresrev.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34(2):374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haren NE, Schnack HG, Cahn W, van den Heuvel MP, Lepage C, Collins L, Evans AC, Hulshoff Pol HE, Kahn RS. Changes in cortical thickness during the course of illness in schizophrenia. Arch Gen Psychiatry. 2011;68(9):871–880. doi: 10.1001/archgenpsychiatry.2011.88. [DOI] [PubMed] [Google Scholar]
- Viana J, Hannon E, Dempster E, Pidsley R, Macdonald R, Knox O, Spiers H, Troakes C, Al-Saraj S, Turecki G, Schalkwyk LC, Mill J. Schizophrenia-associated methylomic variation: molecular signatures of disease and polygenic risk burden across multiple brain regions. Hum Mol Genet. 2017;26(1):210–225. doi: 10.1093/hmg/ddw373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Jeste DV, Martin AS, Lin J, Daly RE, Reuter C, Kraemer H. Leukocyte telomere length: Effects of schizophrenia, age, and gender. J Psychiatr Res. 2016;85:42–48. doi: 10.1016/j.jpsychires.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Zalcman S, Endicott J. Diagnostic Evaluation After Death. New York State Psychiatric Institute; New York, NY: 1983. [Google Scholar]