Abstract
Introduction
Infection with Human papillomavirus (HPV) has been reported as one of the most prevalent agent sexually transmitted diseases, but its true prevalence in men is not precisely known, mainly due to the near absence of symptoms. Moreover, few studies evaluating the post-vaccination immune response have been performed to date in men, hence the hypotheses tested in this study can be important to enable a better understanding of both the immunopathogenesis and the response to vaccination in HIV-infected patients, and to help in the elaboration of strategies of vaccination against HPV in the HIV-infected population.
Objectives
To analyze the specific response to antigens of HPV vaccine in HIV-infected men.
Methods
A total of 25 HIV-infected male patients who met the inclusion criteria during the data collection period were vaccinated; however, six (30%) had anti-HPV at baseline, and were not considered further in the analysis. Therefore, 19 HIV-infected individuals were included in the study, along with five healthy, HPV-seronegative controls.
Results
Patients infected with HIV-1 were subdivided into two groups, A and B, according to their T CD4 cells count at the time of vaccination, namely: Group A: CD4>500; Group B: CD4<500. The proportion of seroconversion after immunization with three doses of a bivalent anti-HPV vaccine was 92%.
Conclusion
HIV-infected patients as well as HIV negative controls responded to anti-HPV vaccination, regardless of their T CD4 cells count and HIV plasma viral load. These results demonstrate that anti-HPV immunization in HIV-infected males is effective and should be encouraged, thus helping to decrease the risk of infection, mortality and morbidity of diseases associated with HPV in men.
Keywords: HIV-1, HPV, Vaccine, Immune response
Introduction
Human papillomavirus (HPV) infections rank among the most common virus sexually transmitted diseases worldwide [1]; it is an epitheliotropic virus, characterized by its icosahedral, non-encapsulated form, with a diameter of 50 nm, and includes as its genetic material a molecule of double-stranded circular DNA with approximately eight thousand base pairs [2]. To date, more than 200 types of human HPV have been described and classified into high and low risk subgroups; there are 45 potentially oncogenic types infecting the anogenital region [3].
HPV escapes recognition by the immune system by not causing cell lysis and not inducing a local inflammation [4]. In its infection cycle there is no viremia, and a very low amount of viral protein is expressed; also, the virus is not cytolytic. Thus, there is no signaling to the immune system to activate the innate response, as well as a delay in the adaptive response [1], leading to an immune dysfunction, with a decrease in the number of antigen-presenting cells (APCs) and T-cell lymphocytes. Immune surveillance is impaired, due both to a deficient presentation of antigens and to a reduced effecter phase of T-lymphocyte activation and antibody production [5].
Anti-HPV vaccine was the result of the mastering of the key technologies that are capable of producing virus-like (VLP) particles. Recombinant DNA was used to generate VLPs able to mimic the natural virus, causing high titers of neutralizing serum antibodies. Today there are two types of vaccines commercially available: Cervarix ® (GlaxoSmithKline Biologicals website) containing VLPs (Virus Like Particle) types 16 and 18, Gardasil® (Merck Dohme Shape, Whitehouse Station, New Jersey) containing VLPs of types 6, 11, 16 and 18. Both vaccines contain an adjuvant aluminum salt that precipitates VLP allowing a more slowly release of the antigen [6], [7]. More recently, a prophylactic nive-valent HPV (types 6/11/16/18/31/33/45/52/58) VLP (9vHPV) vaccine in young men 16–26 years of age in comparison to young women 16–26 years of age showed safety and immunogenetic [8].
In women with immunodeficiency, iatrogenic or acquired, the prevalence of HPV-associated lesions is higher than in immunocompetent individuals [9]. HIV-seropositive subjects experience an increased infection rate with a high number of relapse after treatment and an accelerated development of HPV-associated cancer [10], [11]. In HIV-infected men, the prevalence of HPV in the genital area is 18.2% while that in the anus is 59.3% [12], [13].
Besides the specific response to vaccine antigens, we analyzed the anti-HPV immune response from HIV-1-infected men in different strata of immune deficiency. The hypothesis was tested by stratification according to the number of T CD4+lymphocytes, in an attempt to assess the count range with the highest probability of vaccine response. The degree of vaccine response in HIV-infected patients may indicate which patients have a greater potential for in vivo vaccine response.
Material and methods
In total, 450 HIV-infected individuals have been in follow-up at our outpatient service for the last 25 years. Among them, 300 subjects are males, 56 of whom aged 18–45 years and were seen in the Clinic during the study. These patients underwent a screening to check for the presence of HPV in oral samples, previously published elsewhere [14]; eight tested positive for HPV and 23 had other exclusion criteria and were not vaccinated. Therefore, in this study, 25 patients satisfied the inclusion criteria, were invited and agreed to participate, all of them were men who make sex with men. Five HIV-negative control subjects were tested for HIV infection and submitted to an oral cleansing prior to vaccination. Patients and controls were recruited from October 2012 to February 2013. The Ethical Board of Hospital das Clínicas approved the clinical study; it is registered at the clinical trial identifier (NCT02236234).
Subject population
HIV-1-infected men aged 18–45 years old were invited to participate, regardless acquisition mode of transmission. We selected two groups according to the updated outpatient records: nine had T-CD4 cells counts above 500 cells/µL whereas 16 had T-CD4 cells counts below 500. Exclusion criteria included other types of immunedeficiencies, such as renal failure or hepatic malignancies, autoimmune diseases, immunosupressive medications, or other vaccination within the last 2–3 weeks. All patients gave informed consent, which contained information about the study. After agreeing to sign the term, they answered a questionnaire containing information on socio-behavioral, presence or history of sexually transmitted diseases, HIV viral load, CD4+T cells count and time of the first HIV testing.
Vaccine
Cervarix® (GlaxoSmith-Kline, Brentford, England, a bivalent human papillomavirus vaccine (types 16 and 18), is a recombinant protein virus-like particle. It was administered by intramuscular injection 0.5 mL at 0, 4 and 24 weeks. The primary immunogenicity endpoint was determined three weeks after the third vaccination dose.
Design study
Twenty-five HIV-infected patients who met the inclusion criteria during data collection period were analyzed and classified as follows: All were vaccinated; however, six (24%) had anti-HPV antibodies at baseline, and were not considered further in the analysis. Therefore, 19 HIV-infected individuals were described in the study (nine patients with T CD4 <500 cells/mm³ and 10 patients with CD4≥500 cells/mm³). Control group was made up of five healthy adults who were negative for HIV and HCV infections.
Laboratory methods
Samples were collected by venipuncture from the peripheral blood of research subjects and processed in tubes containing 5 mL of gel; the tubes were centrifuged at 2000xg at a room with a temperature of 15 °C for 15 min. Two aliquots of 500 µl of serum were stored at −80 °C in order to perform the analysis.
HPV serology
Presence of IgG antibodies to HPV 16 and 18 in the sera samples wasdetected using a type specific ELISA using L1VLPs from S. cerevisiae (kindly provided by Dr Ian Frazer, Univ of Queensland, Woolongaba, AU). Fifty microliters of 0.03 mg/ml of each VLP were used to coat the ELISA plates overnight at 4 °C. From every two wells coated with VLP, one was coated with PBS, pH 7.2. Plates were then washed once with PBST (0.02% Tween in PBS pH7.2) and blocked with 100 ml of BSA blocking solution (1.5% bovine serum Albumin in PBS) for 2 h. After the blocking step, plates were washed twice with PBST. Sera and control samples, diluted 1:50 with a solution of 5% fat free milk powder, 0.1% BSA in PBST, were tested in duplicate and incubated at 37 °C for one hour. Plates were washed five times with PBST and tested with horseradish peroxidase conjugated with anti-human IgG (TagoImmunobiologicals, USA) at appropriated dilutions. Following 45 min of incubation at 37 °C, the plates were washed 5 times with PBST and 50 microliters of ABTS solution (0.05% 2,2′-Azino Bis Ethylenebenzthiazoline-6-sulfonic acid, Sigma, USA) were added to each well. The reaction was stopped after 4 min by adding 50 ml of 1% Sodium Dodecyl Sulfate. Optical Density (OD) was read at 415 nm in a microplate reader (Biorad, USA). The OD value for each serum was calculated by subtracting the mean OD of wells coated with VLP from the OD of wells coated with PBS. The cut points for seropositivity for HPV 16 and 18 were determined from the positive controls provided by WHO [15].
Results
Mean age of group A was 30.44 years, and most patients reported having a college degree (66.7%) and being single (77.8%). The average age of group B was 34, 50% reported having completed high school and 80% were single (these characteristics were not different).
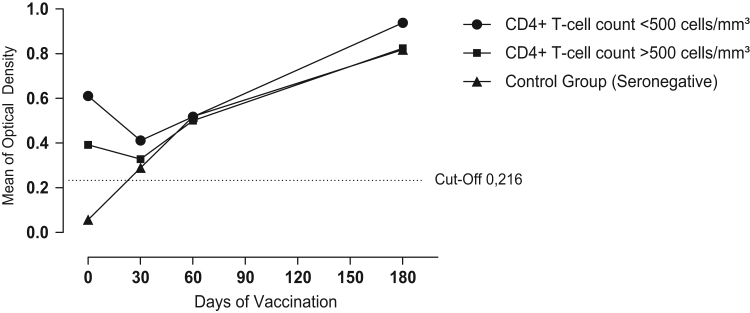
Six patients had anti-HPV antibodies before baseline and therefore were excluded from further analysis. The remaining 19 HIV-infected patients and five controls had titers of anti-HPV antibodies below the cut-off of 0.216. Four weeks after the administration of the first dose of vaccine an increase in titers of circulating antibodies was detected. Three control subjects seroconverted (60%), as well as five patients in group A (55.6%) and three patients in group B (30%) as judged by increased antibody titers measured by ELISA using VLP 16 HPV ranked by standard titles (Fig. 1).
Fig. 1.
: Anti-HPV serum titers from HIV-1-infected subjects and negative controls after vaccination with bivalent human papillomavirus vaccine (types 16 and 18).
After the 2nd dose (at 30 days), four weeks after immunization, there was an increase in titers of circulating antibodies in the serum of patients. Four patients in the control group seroconverted (80%), eight patients in group A (88.8%) and eight patients in group B (80.0%) had increased titers of anti-HPV, ranked by titles׳ standard. Finally, after the 3rd dose (at 180 days), four weeks after immunization, there was an increase in circulating antibodies in the serum of patients: five volunteers from the control group (100%), eight HIV-infected patients from group A (88.8%) and nine HIV-infected patients from group B (90%) seroconverted (Fig. 1) after vaccination.
Discussion
This study demonstrated that the bivalent HPV vaccine (Cervarix) was efficacious when administered to HIV-infected-oral- HPV- negative men, with more than 90 percent of patients producing antibodies to HPV in high titers, after a full vaccination scheme. It was also safe, with no major adverse effect reported. The satisfactory response of HIV patients to the vaccine was independent of their CD4 count, since both groups studied (CD4+ T-cells count <500 and CD4+ T-cells count >=500 cells/mm3) responded similarly to the vaccine, with approximately 90% of seroconversion. Our results are in agreement with findings from other authors [9], who also worked with HIV-infected men, obtaining a 95% proportion of seroconversionwith a tetravalent vaccine (2010) administered to sevento 12 years-old HIV-positive children [13]. Our data strongly suggest that HPV vaccination induces protective immunity against vaccine-specific HPV infection in persons with HIV [16]. Similarly, this efficacy was found in HIV-1-positive women from Africa [17].
The VLP-based bivalent vaccine administered to our patients did not change their CD4+ counts or HIV viral loads. These findings were also observed in two other studies involving immune depressed patients [13], [18]. Several factors may contribute to the nearly absence of deleterious effects of the vaccine on the immune system, such as the intrinsic immunogenicity of the VLPs, keeping an orderly and repetitive arrangement of their epitopes, enabling a specific recognition by B cells. Another likely factor may be the characteristics of the epitopes of the L1 region, which induce a broad neutralizing antibody response, enabling the recognition of a certain type of HPV and its variants through its amino acids sequences.
The high titers of antibodies induced by VLP may inhibit the establishment of an infection through the occlusion of its binding sites on the cell. VLP may also avoid the immunologic escape of HPV, decreasing the expression of the capsid proteins, delaying the production of virions until the cell differentiation is complete [19]. Patients did not report any serious adverse effect that could be attributed to the vaccine, only minor complaints such as pain at the injection site and headaches, considered mild or moderate and similar to what has been observed for other vaccines when administered to HIV-infected patients [9], [18], [20].
The efficacy of the bivalent (16 and 18) HPV vaccine has been demonstrated in several other studies, with consistent, comparable results for the two currently used vaccines. The long term efficacy of the vaccine is unknown, and follow-up studies are currently been undertaken to examine whether a booster dose of the vaccine will be needed in the future [21]. On a previous study developed by our research team, Veiga et al. demonstrated that the viral load from HIV carriers was the determinant factor for the unsuccessful vaccination against hepatitis B [22]. Our results from the current study are in agreement with two other studies which found that the viral loads of HIV-1-infected patients, immunized with Merck׳s tetravalent anti-HPV vaccine, were not associated with their response to the vaccine.
Our study has some limitations such as the small number of patients and controls, short follow-up time, precluding a better comparison with the HIV negative population. We also did not investigate the possibility of a cross-reaction of the vaccinethat could protect the vaccinated against other HPV types not present on the vaccine; another aspect not contemplated on our study was the long term behavior of the antibodies’ titers in the patients sera. We hope that our results help stimulate the HPV immunization of the HIV-infected men, in order to decrease their risks of infection, morbidity and mortality from HPV diseases, as well as averting the risks of transmission.
Author contributions
A.S, M.A.A. and K.G collected the data and wrote the manuscript. L.A.M.F.; LLV and T.A.S. made the statistical analysis and revised the final version of the MS. A.J.S.D. and J.C. designed the study and read the final version of the MS.
Additional information
LLV was involved in clinical trials of the Quadrivalent HPV vaccine and is member of the board of Merck, Sharp and Dohme for HPV vaccines.
Competing financial interests
The authors declare no competing financial interests.
Acknowledgments
We thank the financial support from FAPESP, Grant 2010/07076-4. supported by grants to LLV from FAPESP 2008/05889-1 and CNPq 573799/2008-3. Our thanks to Dr. Roberto Carvalho from the Referral Center and Training in STD/AIDS – Sao Paulo for assistance in patient recruitment. Our thanks to all the patients who participated in this research.
Contributor Information
Adriele Fontes, Email: adrielefontes@gmail.com.
Jorge Casseb, Email: jcasseb10@gmail.com.
References
- 1.Stanley M. HPV-immune response to infection and vaccination. Infect. Agents Cancer. 2010;5:19. doi: 10.1186/1750-9378-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.zur Hausen H., de Villiers E.M., Gissmann L. Papillomavirus infections and human genital cancer. Gynecol. Oncol. 1981;12:S124–S128. doi: 10.1016/0090-8258(81)90067-6. [DOI] [PubMed] [Google Scholar]
- 3.Hoots B.E., Palefsky J.M., Pimenta J.M., Smith J.S. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int. J. Cancer J. Int. Du. Cancer. 2009;124:2375–2383. doi: 10.1002/ijc.24215. [DOI] [PubMed] [Google Scholar]
- 4.Betiol J., Villa L.L., Sichero L. Impact of HPV infection on the development of head and neck cancer. Braz. J. Med. Biol. Res. 2013;46:217–226. doi: 10.1590/1414-431X20132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiller J.T., Lowy D.R. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat. Rev. Microbiol. 2012;10:681–692. doi: 10.1038/nrmicro2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palefsky J.M., Giuliano A.R., Goldstone S., Moreira E.D., Jr., Aranda C., Jessen H. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. New Engl. J. Med. 2011;365:1576–1585. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 7.Munoz N., Kjaer S.K., Sigurdsson K., Iversen O.E., Hernandez-Avila M., Wheeler C.M. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J. Natl. Cancer Inst. 2010;102:325–339. doi: 10.1093/jnci/djp534. [DOI] [PubMed] [Google Scholar]
- 8.Castellsague X., Giuliano A.R., Goldstone S., Guevara A., Mogensen O., Palefsky J.M. Immunogenicity and safety of the 9-valent HPV vaccine in men. Vaccine. 2015 doi: 10.1016/j.vaccine.2015.06.088. [DOI] [PubMed] [Google Scholar]
- 9.Wilkin T., Lee J.Y., Lensing S.Y., Stier E.A., Goldstone S.E., Berry J.M. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J. Infect. Dis. 2010;202:1246–1253. doi: 10.1086/656320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciavarra G., Ho A.T., Cobrinik D., Zacksenhaus E. Critical role of the Rb family in myoblast survival and fusion. Plos One. 2011;6:e17682. doi: 10.1371/journal.pone.0017682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Figliuolo G., Maia J., Jalkh A.P., Miranda A.E., Ferreira L.C. Clinical and laboratorial study of HPV infection in men infected with HIV. Int. Braz. J. Urol.: Off. J. Braz. Soc. Urol. 2012;38:411–418. doi: 10.1590/s1677-55382012000300015. [DOI] [PubMed] [Google Scholar]
- 12.Kreuter A., Brockmeyer N.H., Weissenborn S.J., Gambichler T., Stucker M., Altmeyer P. Penile intraepithelial neoplasia is frequent in HIV-positive men with anal dysplasia. J. Investig. Dermatol. 2008;128:2316–2324. doi: 10.1038/jid.2008.72. [DOI] [PubMed] [Google Scholar]
- 13.Levin M.J., Moscicki A.B., Song L.Y., Fenton T., Meyer W.A., 3rd, Read J.S. Safety and immunogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. J. Acquir. Immune Defic. Syndr. 2010;55:197–204. doi: 10.1097/QAI.0b013e3181de8d26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaester K., Fonseca L.A., Luiz O., Assone T., Fontes A.S., Costa F. Human papillomavirus infection in oral fluids of HIV-1-positive men: prevalence and risk factors. Sci. Rep. 2014;4:6592. doi: 10.1038/srep06592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson M., Heath A., Johnes S., Pagliusi S., Dillner J. Collaborative Study P. Results of the first WHO international collaborative study on the standardization of the detection of antibodies to human papillomaviruses. Int. J. Cancer J. Int. Du. Cancer. 2006;118:1508–1514. doi: 10.1002/ijc.21515. [DOI] [PubMed] [Google Scholar]
- 16.Toft L., Tolstrup M., Storgaard M., Ostergaard L., Sogaard O.S. Vaccination against oncogenic human papillomavirus infection in HIV-infected populations: review of current status and future perspectives. Sex Health. 2014;11:511–523. doi: 10.1071/SH14015. [DOI] [PubMed] [Google Scholar]
- 17.Denny L., Hendricks B., Gordon C., Thomas F., Hezareh M., Dobbelaere K. Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in HIV-positive women in South Africa: a partially-blind randomised placebo-controlled study. Vaccine. 2013;31:5745–5753. doi: 10.1016/j.vaccine.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 18.Villa L.L., Costa R.L., Petta C.A., Andrade R.P., Ault K.A., Giuliano A.R. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 19.Bernard H.U., Calleja-Macias I.E., Dunn S.T. Genome variation of human papillomavirus types: phylogenetic and medical implications. Int. J. Cancer J. Int. Du. Cancer. 2006;118:1071–1076. doi: 10.1002/ijc.21655. [DOI] [PubMed] [Google Scholar]
- 20.Giuliano A.R., Palefsky J.M., Goldstone S., Moreira E.D., Jr., Penny M.E., Aranda C. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N. Engl. J. Med. 2011;364:401–411. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michels K.B., zur Hausen H. HPV vaccine for all. Lancet. 2009;374:268–270. doi: 10.1016/S0140-6736(09)61247-2. [DOI] [PubMed] [Google Scholar]
- 22.Veiga A.P., Casseb J., Duarte A.J. Humoral response to hepatitis B vaccination and its relationship with T CD45RA+ (naive) and CD45RO+ (memory) subsets in HIV-1-infected subjects. Vaccine. 2006;24:7124–7128. doi: 10.1016/j.vaccine.2006.06.079. [DOI] [PubMed] [Google Scholar]