Abstract
Background
The aim of the current study was to assess the HPV prevalence in unscreened and unvaccinated young women living in Norway, to provide important baseline data for early estimation of the impact of the HPV vaccination program.
Methods
A total of 13,129 self-sampled urine samples from two complete birth-cohorts of 17-year old women born in 1994 and 1996 and one third of a birth-cohort of 21-year old women born in 1990, were analysed for the presence of 37 HPV types using PCR and a DNA hybridization technique.
Results
In the two birth cohorts of 17-year old women, HPV was detected in 19.9% (95% CI 18.8–20.9) and 15.4% (95% CI 14.5–16.3), respectively. High-risk HPV types were detected in 11.2% (95% CI 10.3–12.0) and 7.6% (95% CI 6.9–8.2), respectively, while vaccine types were detected in 7.4% (95% CI 6.7–8.1) and 6.0% (95% CI 5.4–6.6), respectively. Among the 21-year old women HPV was detected in 45.4% (95% CI 42.9–47.8), whereas high-risk types were detected in 29.8% (95% CI 27.5–32.0). Vaccine types (HPV 6, 11, 16, 18) were detected in 16.2% (95% CI 14.4–18.1).
Conclusion
This large population based study confirms that HPV testing in urine samples is easy and highly feasible for epidemiological studies and vaccine surveillance in young women. HPV was very common and a broad spectrum of HPV types was identified. Differences in HPV prevalence was seen both between age groups and between the two birth cohorts of 17-year old women.
Keywords: Human papillomavirus, Immunization, Urine, Vaccine, HPV prevalence, HPV genotype
Highlights
-
•
Self-sampled urine proved suitable for large scale HPV testing.
-
•
HPV 16 and 18 was very common among young girls.
-
•
A wide variety of HPV types circulates in the population.
-
•
HPV was detected in nearly half of the 21-year old women.
-
•
HPV was detected in 15–20% of the 17-year old women.
1. Introduction
Infection with an oncogenic type of human papillomavirus (HPV) is a pre-requisite for developing cervical pre-cancerous lesions and carcinomas. More than 40 HPV types are known to infect the human anogenital tract. At least 12 types are considered carcinogenic and are commonly referred to as high-risk types [1], [2], [3].
Vaccination against HPV infection was introduced in the Norwegian childhood immunization program in the school year 2009/2010. All girls born in 1997 and later have been offered the vaccine in the 7th grade at age 11–12 years. No publically funded catch-up vaccination for the older age groups has been introduced. The 4-valent vaccine, Gardasil® is used in the program. The vaccine offers protection against HPV 16 and 18, which cause about 70% of invasive cervical carcinomas [4], as well as the low-risk types 6, 11, the main etiologic agent for external genital warts [5], [6].
Knowledge of the baseline HPV prevalence and type distribution in unscreened and unvaccinated birth cohorts is essential for estimating the impact of HPV vaccination. However, few population-based studies have been conducted to assess the prevalence of HPV and type distribution in pre-teens or young adults. Smaller studies of unvaccinated women from Scotland and the Netherlands show an HPV prevalence in urine of4.4–32.2% in the age groups of 14–16 and 20–21 years, respectively [7], [8]. A few studies have assessed the prevalence of circulating HPV types in Norway, generally focusing on HPV types present in cervical precancerous or cancerous lesions or in women visiting gynaecology clinics [9], [10], [11], [12], [13], [14], [15]. Less is known about the HPV prevalence and genotype distribution in women in their late teens or early twenties, who have not yet been invited to participate in the national screening program against cervical cancer. The aim of the current study was to describe the HPV type prevalence in young women in Norway who have not been offered the vaccine against HPV as part of the national childhood immunization program. We were also interested in comparing the HPV prevalence between 17-year olds and 21-year olds and between two birth cohorts of 17-year olds to document natural fluctuations of HPV prevalence.
2. Material and methods
2.1. Enrolment, sample collection, and study sample
Women eligible for the study were identified through the Norwegian Population Register.
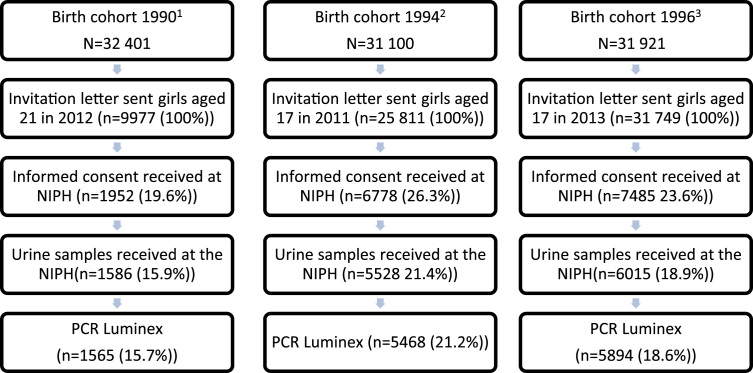
In 2011, an invitation letter was sent to all women born in 1994 residing in Norway as of January 1st 2011, except some born at the end of 1994, in total 83.0% of the birth cohort (Fig. 1). In 2013, a total of 99.5% of the women born in 1996 were invited to participate in the study. The invitation was sent the same month the women became 17 years (in 2011 and 2013, respectively). Women born between August and December in 1990 were invited in the period January to May 2012. The HPV prevalence in this age-group was expected to be at least twice the HPV prevalence in the 17-year olds. Therefore, only women born between August and December were invited, in total 30.8% of the birth cohort. From the initial birth cohort list obtained at the beginning of the year of sample collection, some were not invited due to missing address, invalid social security number, death, or emigration.
Fig. 1.
Flow-chart study population. 1,2,3Total female birth cohort alive the 1st of January the year of sample collection [29].
The invitation was sent by mail and included information about the study, an informed consent form, and a pre-franked envelope for returning the signed informed consent. Women who consented to participate received a sample kit and instructions for obtaining a first void urine sample together with a pre-franked return envelope. The sample device contained a preservative (boric acid), to prevent bacterial growth. The urine samples were shipped by mail to the Norwegian Institute of Public Health (NIPH) where the samples were marked, processed and stored at −80 °C until further analysis. An aliquot was sent to the Norwegian HPV Reference Laboratory (Akershus University Hospital) for isolation of nucleic acids and HPV genotyping. HPV results were not routinely communicated to the participants, but were provided upon request. Participants could withdraw from the study at any time. All participants were rewarded with two cinema tickets for their contribution.
A total of 13,129 women contributed with a urine sample. The participation rates were similar for the northern, middle, western, southern and eastern region of Norway, and ranged from 14.2 to 17.8% for the 21-year olds and 16.9–22.3% for the 17-year olds.
Linkage to the immunization register for individual vaccination status was not performed since the current study population is largely unvaccinated. These young women were not offered vaccine against HPV as part of the national immunization program. According to distribution numbers, very few vaccine doses have been distributed for sale to this group.
The study was approved by the Regional Committee for Medical and Health Research Ethics and the Norwegian Data Protection Authority.
2.1.1. Isolation of nucleic acids
Nucleic acids were isolated using Boom's isolation method [16] and the automatic NucliSENS easyMAG extraction device (bioMérieux Corporate, Marcy l’Etoile, France). Total nucleic acids were kept cold and analysed within four hours or stored at −80 °C until analysis.
Validation of sample adequacy and HPV genotyping
Human β-globin quantitative real-time PCR for validation of sample adequacy and HPV genotyping using PCR and DNA hybridization and Luminex based technology was performed as previously described [17], [18]. The HPV genotyping method detects 37 HPV types; 12 high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59), six probable high-risk types (26, 53, 66, 68, 73, 82), and 19 undetermined or low-risk types (6, 11, 30, 40, 42, 43, 54, 61, 67, 69, 70, 74, 81, 83, 86, 87, 89, 90, 91)[1], [2].
In order not to compete with the HPV PCR, the β-globin PCR was run in a separate reaction. The PCR products were kept frozen at −20 °C until further analysis.
Upon Luminex detection of values in the range from the cut-off value up to two times the cut-off value for any HPV-type, a re-analysis was performed in duplicate. Individual cut-off values for each HPV-type were calculated for each run based on the level of background noise. Cut-off modification values and factors for cross-hybridization correction used to calculate the cut-off were adapted from the WHO HPV reference laboratory in Sweden. A total of 202 samples (1.5%) did not give a valid result for β-globin or for HPV, and were excluded from the analyses.
2.1.2. Statistical analyses
The prevalence of HPV types was defined as the number of positive specimens divided by the total number of specimens with valid PCR result (β-globin and Luminex). We calculated the corresponding 95% Wald confidence intervals (CIs).
Chi squared tests were used to test for differences between proportions. All tests were two-sided, and p<0.05 was considered statistically significant. The data were analysed with STATA/SE version 13.0 (StataCorp, College Station, Texas, USA).
3. Results
HPV prevalence by birth cohort is shown in Table 1. The overall HPV prevalence in urine specimens from 21-year old women was 45.4% (95% CI 42.9, 47.8). High-risk types were detected in 29.8% (95% CI 27.5, 32.0), corresponding to 65.5% of all HPV positive samples. The vaccine types 6, 11, 16, and 18, were detected in 16.2% (95% CI 14.4, 18.1), corresponding to 35.7% of all HPV positive samples. Multiple infections were observed in 26.1% (95% CI 23.9, 28.2).
Table 1.
HPV prevalence in urine samples from Norwegian women by birth cohort.
| 21 yr 1990 (N=1565) |
17 yr 1994 (N=5468) |
17 yr 1996 (N=5894) |
||||
|---|---|---|---|---|---|---|
| HPV | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) |
| Totala | 710 | 45.4 (42.9–47.8) | 1087 | 19.9 (18.8–20.9) | 907 | 15.4 (14.5–16.3) |
| High-risk (HR)b | 466 | 29.8 (27.5–32.0) | 611 | 11.2 (10.3–12.0) | 445 | 7.6 (6.9–8.2) |
| Probably HRc | 129 | 8.2 (6.9–9.6) | 174 | 3.2 (2.7–3.6) | 173 | 2.9 (2.5–3.4) |
| Low-riskd | 455 | 29.1 (26.8–31.3) | 640 | 11.7 (10.9–12.6) | 500 | 8.5 (7.8–9.2) |
| Vaccine typese | 254 | 16.2 (14.4–18.1) | 403 | 7.4 (6.7–8.1) | 283 | 4.8 (4.3–5.3) |
| Multiple infectionf | 408 | 26.1 (23.9–28.2) | 504 | 9.2 (8.5–10.0) | 352 | 6.0 (5.4–6.6) |
Wald's method was used to calculate 95% CIs.
HPV total includes those who are positive for at least one of the 37 HPV types tested for and those who are HPV type negative, but positive for HPV using generic primers.
HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59.
HPV types 26, 53, 66, 68, 73, 82.
HPV types 6, 11, 30, 40, 42, 43, 54, 61, 67, 69, 70, 74, 81, 83, 86, 87, 89, 90, 91.
HPV types 6, 11, 16, 18.
Infection positive for two or more HPV types.
For 17-year old women born in 1994, the overall HPV prevalence was 19.9% (95% CI 18.8, 20.9). High-risk types were detected in 11.2% (95% CI 10.3, 12.0). Vaccine types 6, 11, 16, and 18, were detected in 7.4% (95% CI 6.7, 8.1). Multiple infections were observed in 9.2% (95% CI 8.5, 10.0). Among all positive HPV samples, 56.2% were high-risk types and 37.1% were positive for any vaccine type.
For 17-year old women born in 1996, the overall HPV prevalence was 15.4% (95% CI 14.5, 16.3). High-risk types were detected in 7.6% (95% CI 6.9, 8.2). Vaccine types 6, 11, 16, and 18, were detected in 4.8% (95% CI 4.3, 5.3). Multiple infections were observed in 6.0% (95% CI 5.4, 6.6). Among all HPV positive samples, 49.1% were positive for high-risk types, and 31.2% were positive for any vaccine types.
The HPV prevalence was significantly higher (p<0.001) in 21-year old women as compared to the 17-year old women combined for HPV overall (45.4% vs. 17.5%), high-risk HPV types (29.8% vs. 9.3%), probable high-risk HPV types (8.2% vs. 3.1%), low-risk HPV types (29.1% vs. 10.0%), vaccine HPV types (16.2% vs. 6.0%), and multiple infections (26.1% vs. 7.5%). Moreover, the prevalence was significantly higher (p<0.001) among 17-year olds born in 1994 than 17-year olds born in 1996 for HPV total, high-risk HPV types, low-risk HPV types, vaccine HPV types, and multiple infections, whereas the prevalence of probable high-risk types was not significantly different (p=0.45).
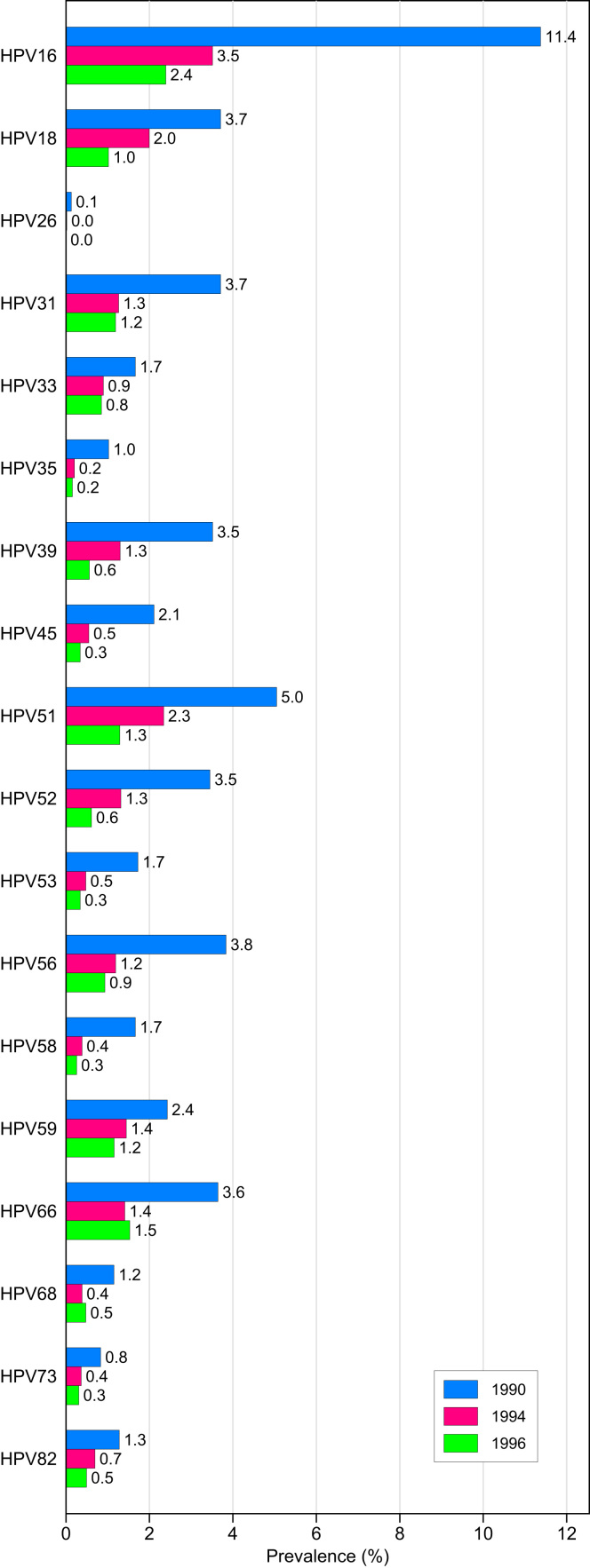
The prevalence of HPV types defined as high-risk or probable high-risk are presented in Fig. 2. HPV 16 was the most common HPV genotype detected in 21-year old women, with a prevalence of 11.4%. Following HPV 16, the four most common high-risk or probable high-risk HPV types were in decreasing order; HPV 51, 56, 18, and 31.
Fig. 2.
Prevalence of high-risk and probably high-risk HPV types in urine samples from unvaccinated Norwegian women by birth cohort.
Among 17-year old women born in 1994, HPV 16, the most common HPV type, was detected in 3.5% of the samples. After HPV 16, the four most common high-risk or probable high-risk HPV types were in decreasing order; HPV 51, 18, 59, and 66.
Among 17-year old girls born in 1996, HPV 16, the most common HPV type, was detected in 2.4% of the samples. After HPV 16, the four most common high-risk or probable high-risk HPV types were in decreasing order; HPV 66, 51, 31 and 59.
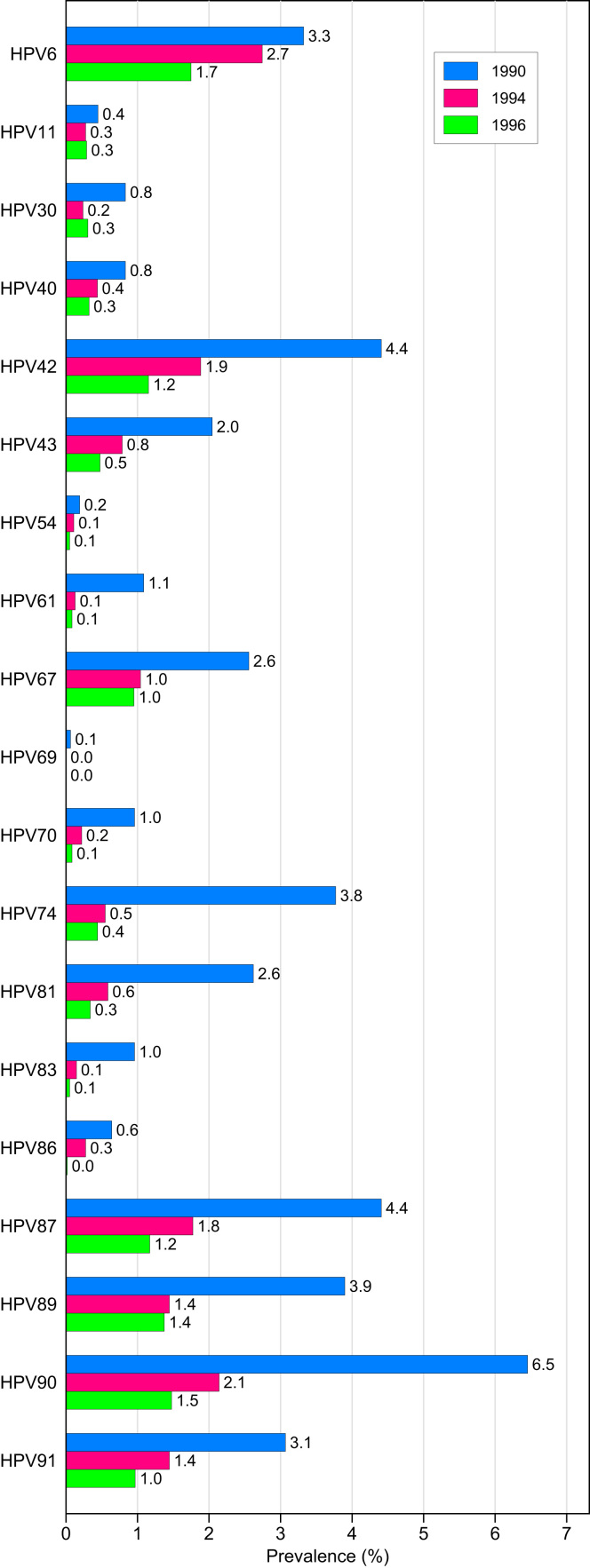
The prevalence of HPV types defined as undetermined or low-risk is presented in Fig. 3. For 21-year old women, HPV 90 was the most common low-risk HPV genotype with a prevalence of 6.4%. Other common low-risk HPV types were in decreasing order; HPV 42, 87, and 89. The vaccine types HPV 6 and 11 were detected in 3.3% and 0.04% of the 21-year old women, respectively.
Fig. 3.
Prevalence of undetermined or low-risk HPV types in urine samples from unvaccinated Norwegian women by birth cohort.
Among 17-year old girls born in 1994, the most common low-risk HPV type was HPV 6 (2.7%), followed by HPV 90, 42, and 87. The vaccine type HPV 11 was detected in 0.3%.
Among 17-year old girls born in 1996, the most common low-risk HPV type was HPV 6 (1.8%), followed by HPV90, 89, and 87. The vaccine type HPV 11 was detected in 0.3%.
4. Discussion
This study is the first of a series of nationwide, population-based, cross-sectional studies with the aim to estimate the early impact of the HPV vaccination program in Norway. We assessed HPV prevalence in self-sampled urine specimens in 17- and 21-year old women who have not been offered the vaccine against HPV as part of the national childhood immunization program.
All the 37 HPV types included in the HPV Luminex assay were detected in our study sample. Knowledge of the prevalence of high-risk HPV types 16 and 18 as well as the low-risk HPV types 6 and 11 in the population prior to vaccination is of primary interest for future studies of the impact of the 4-valent vaccine. The vaccine types HPV16 and 18 were detected in nearly half of the HPV high-risk positive samples across all age groups, which correspond well with previous results from unvaccinated 20–21 year old women in Scotland [8]. Of the other vaccine types, HPV 6 was common, whereas HPV 11 was quite rare. These results are in accordance with a Swedish study [19]. In contrast, the prevalence of HPV 6 was similar to the prevalence of HPV 11 in a Dutch study [7].
HPV high-risk types were detected in a large proportion of the samples and were similar to what has been reported in other European studies, both regarding types detected and prevalence [7], [8], [20]. The prevalence increased with age and was found to be two- to three times higher in 21-year old women compared to 17-year olds. Increasing prevalence with age is in line with several studies [20], [21], [22], [23]. All together, these observations confirm that infection with HPV vaccine types or other high-risk types is common in young Norwegian women.
The HPV prevalence differed between the two birth cohorts of 17-year olds. The prevalence was significantly higher among girls in the 1994 birth cohort as compared to the 1996 birth cohort. The regional participation pattern was similar in the two birth cohorts (results not shown), thus regional differences in HPV prevalence do not explain the difference between these two birth cohorts. The finding may be a result of natural fluctuations in the prevalence of HPV. Also, a few of the participants in the study may have received the HPV-vaccine outside the national childhood immunization program. According to data from the Norwegian immunization register, approximately 3% of all girls born in 1996 and 2% of all girls born in 1994 have been vaccinated with three doses of the 4-valent vaccine (unpublished data). We do not suspect the small proportion of individuals in the cohorts already vaccinated to differentially affect the HPV results. The assumption that the difference in HPV prevalence between the two cohorts of 17-year olds is not due to vaccination is supported by the prevalence of non-vaccine HPV types which is also generally lower in the 1996 birth cohort compared to the 1994 birth cohort.
A major strength of the current study is the population-based design and large sample. However, the low participation rate may cause selection bias if willingness to participate is systematically associated with certain sexual behaviours increasing the risk of HPV, or vaccination status which would reduce the risk of HPV. Nevertheless, the aim of the HPV surveillance program is to monitor changes in prevalence and type distribution over time and we believe this potential bias to be comparable from year to year, so the comparison of HPV prevalence's across birth cohorts is still expected to be valid.
Considering the young age of the study subjects, taking a less intrusive urine sample is for ethical reasons preferred over a cervical sample.
Testing for HPV in urine samples may not be comparable to testing cervical smears, as detection of HPV in urine may not be representative for HPV infections in the cervix. This has been shown in several studies where in general the HPV prevalence is lower when HPV DNA is isolated from urine compared to when HPV DNA is isolated from the cervical smears [8], [24]. Accordingly, the HPV prevalence observed in our study is most likely an underestimate of the true prevalence in cervical specimens. Nevertheless, our large study confirms that HPV testing in urine samples is easy to implement and highly feasible for epidemiological studies and vaccine surveillance in young women, as also stated in other studies [25], [26], [27], [28].
Further surveillance of the early impact of the HPV vaccination program in Norway will include urine samples from both vaccinated and not vaccinated birth cohorts. Changes in the HPV prevalence over time will be documented. Additionally, the surveillance program is planned to include routine HPV-testing of cervical histological samples with cancerous and pre-cancerous lesions. So far, only girls in the 7th grade in Norway has been offered the HPV vaccine. Thus, it will take several more years before the vaccine effectiveness including these endpoints can be estimated.
5. Conclusions
In conclusion, this large population based study confirms that HPV testing in urine samples is easy and highly feasible for epidemiological studies and vaccine surveillance in young women. We have assessed the prevalence and genotype distribution of HPV in urine specimens from young women from a largely unvaccinated population, providing important baseline data for early estimation of the impact of the HPV vaccination program in Norway. HPV was frequently detected. A broad spectrum of HPV types was identified and multiple infections were prevalent. HPV was detected two to three fold more frequently in 21-year old women compared to 17-year old women, and there were also differences between the two 17-year old birth cohorts. The vaccine specific HPV types 6, 16 and 18 were quite common in young Norwegian women, whereas the vaccine type HPV 11 was quite rare.
Conflict of interests
None.
Sources of support
This research received funding from the Norwegian Ministry of Health and Care Services.
Authors’contributions
TM contributed with preparation of data files, analysis and interpretation of the data, and drafting the article. OHA and IKC contributed with laboratory analysis, analysis and interpretation of the data and revising the article for important intellectual content. MH and EM contributed to conception and design of the study, laboratory analysis, interpretation of the data, and revising the article for important intellectual content. IL contributed with analysis and interpretation of the data and revising the article for important intellectual content. RM contributed with laboratory analyses and revising the article for important intellectual content. BF, CMJ and LT contributed to conception and design of the study, interpretation of the data, and revising the article for important intellectual content. All authors have given final approval of the version to be published.
Acknowledgments
We thank the HPVnorvaks study group for their contribution in making this study manageable. Special thanks to Alexander Eieland and Nermin Zecic at the HPV reference laboratory for technical support, Jeanette Stålkrantz, Nina Hovland, Patricia Schreuder, at Norwegian Institute of Public Health (NIPH) for their work with invitations letter and informed consent forms, Ole-Martin Kvinge at NIPH for data management, Nina Kristin Stensrud and Kari Harbak at the NIPH biobank for management of sampling kit and urine samples.
Contributor Information
Tor Molden, Email: tor.molden@fhi.no.
Berit Feiring, Email: berit.feiring@fhi.no.
Ole Herman Ambur, Email: ole.herman.ambur@ahus.no.
Irene K. Christiansen, Email: irene.kraus.christiansen@ahus.no.
Mona Hansen, Email: mona.lindsethmo.hansen@ahus.no.
Ida Laake, Email: ida.laake@fhi.no.
Roger Meisal, Email: roger.meisal@ahus.no.
Ellen Myrvang, Email: ellen.myrvang@ahus.no.
Christine Monceyron Jonassen, Email: christine.monceyron.jonassen@so-hf.no.
Lill Trogstad, Email: lill.trogstad@fhi.no.
References
- 1.Munoz N., Castellsague X., de Gonzalez A.B., Gissmann L. HPV in the etiology of human cancer. Vaccine. 2006;24(Suppl. 3/S3):S1–S10. doi: 10.1016/j.vaccine.2006.05.115. (Chapter 1) [DOI] [PubMed] [Google Scholar]
- 2.IARC Biological agents. IARC Monogr. Eval. Carcinog. Risks Hum. 2012;100:1–441. [PMC free article] [PubMed] [Google Scholar]
- 3.IARC Human papillomaviruses. IARC Monogr. Eval. Carcinog. Risks Hum. 2007;90:1–636. [PMC free article] [PubMed] [Google Scholar]
- 4.Li N., Franceschi S., Howell-Jones R., Snijders P.J., Clifford G.M. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int. J. Cancer. 2011;128:927–935. doi: 10.1002/ijc.25396. [DOI] [PubMed] [Google Scholar]
- 5.Gissmann L., deVilliers E.M., zur Hausen H. Analysis of human genital warts (Condylomata acuminata) and other genital tumors for human papillomavirus type 6 DNA. Int. J. Cancer. 1982;29:143–146. doi: 10.1002/ijc.2910290205. [DOI] [PubMed] [Google Scholar]
- 6.Brown D.R., Bryan J.T., Cramer H., Fife K.H. Analysis of human papillomavirus types in exophytic condylomata acuminata by hybrid capture and Southern blot techniques. J. Clin. Microbiol. 1993;31:2667–2673. doi: 10.1128/jcm.31.10.2667-2673.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mollers M., Scherpenisse M., van der Klis F.R., King A.J., van Rossum T.G., van Logchem E.M. Prevalence of genital HPV infections and HPV serology in adolescent girls, prior to vaccination. Cancer Epidemiol. 2012;36:519–524. doi: 10.1016/j.canep.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Kavanagh K., Sinka K., Cuschieri K., Love J., Potts A., Pollock K.G. Estimation of HPV prevalence in young women in Scotland; monitoring of future vaccine impact. BMC. Infect. Dis. 2013;13:519. doi: 10.1186/1471-2334-13-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kristiansen E., Jenkins A., Kristensen G., Ask E., Kaern J., Abeler V. Human papillomavirus infection in Norwegian women with cervical cancer. APMIS. 1994;102:122–128. doi: 10.1111/j.1699-0463.1994.tb04856.x. [DOI] [PubMed] [Google Scholar]
- 10.Gjooen K., Olsen A.O., Magnus P., Grinde B., Sauer T., Orstavik I. Prevalence of human papillomavirus in cervical scrapes, as analyzed by PCR, in a population-based sample of women with and without cervical dysplasia. APMIS. 1996;104:68–74. doi: 10.1111/j.1699-0463.1996.tb00688.x. [DOI] [PubMed] [Google Scholar]
- 11.Kraus I., Molden T., Erno L.E., Skomedal H., Karlsen F., Hagmar B. Human papillomavirus oncogenic expression in the dysplastic portio: an investigation of biopsies from 190 cervical cones. Br. J. Cancer. 2004;90:1407–1413. doi: 10.1038/sj.bjc.6601691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molden T., Kraus I., Karlsen F., Skomedal H., Nygard J.F., Hagmar B. Comparison of human papillomavirus messenger RNA and DNA detection: a cross-sectional study of 4136 women >30 years of age with a 2-year follow-up of high-grade squamous intraepithelial lesion. Cancer Epidemiol. Biomark. Prev. 2005;14:367–372. doi: 10.1158/1055-9965.EPI-04-0410. [DOI] [PubMed] [Google Scholar]
- 13.Molden T., Kraus I., Karlsen F., Skomedal H., Hagmar B. Human papillomavirus E6/E7 mRNA expression in women younger than 30 years of age. Gynecol. Oncol. 2006;100:95–100. doi: 10.1016/j.ygyno.2005.07.108. [DOI] [PubMed] [Google Scholar]
- 14.Kraus I., Molden T., Holm R., Lie A.K., Karlsen F., Kristensen G.B. Presence of E6 and E7 mRNA from human papillomavirus types 16, 18, 31, 33, and 45 in the majority of cervical carcinomas. J. Clin. Microbiol. 2006;44:1310–1317. doi: 10.1128/JCM.44.4.1310-1317.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sjoeborg K.D., Trope A., Lie A.K., Jonassen C.M., Steinbakk M., Hansen M. HPV genotype distribution according to severity of cervical neoplasia. Gynecol. Oncol. 2010;118:29–34. doi: 10.1016/j.ygyno.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Boom R., Sol C.J., Salimans M.M., Jansen C.L., Wertheim-van Dillen P.M., van der Noordaa J. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitt M., Bravo I.G., Snijders P.J., Gissmann L., Pawlita M., Waterboer T. Bead-based multiplex genotyping of human papillomaviruses. J. Clin. Microbiol. 2006;44:504–512. doi: 10.1128/JCM.44.2.504-512.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soderlund-Strand A., Carlson J., Dillner J. Modified general primer PCR system for sensitive detection of multiple types of oncogenic human papillomavirus. J. Clin. Microbiol. 2009;47:541–546. doi: 10.1128/JCM.02007-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soderlund-Strand A., Uhnoo I., Dillner J. Change in population prevalences of human papillomavirus after initiation of vaccination: the high-throughput HPV monitoring study. Cancer Epidemiol. Biomark. Prev. 2014;23:2757–2764. doi: 10.1158/1055-9965.EPI-14-0687. [DOI] [PubMed] [Google Scholar]
- 20.Kjaer S.K., Munk C., Junge J., Iftner T. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ASCUS/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179–189. doi: 10.1007/s10552-013-0320-z. [DOI] [PubMed] [Google Scholar]
- 21.Dunne E.F., Unger E.R., Sternberg M., McQuillan G., Swan D.C., Patel S.S. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813–819. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 22.Howell-Jones R., de Silva N., Akpan M., Oakeshott P., Carder C., Coupland L. Prevalence of human papillomavirus (HPV) infections in sexually active adolescents and young women in England, prior to widespread HPV immunisation. Vaccine. 2012;30:3867–3875. doi: 10.1016/j.vaccine.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Orlando G., Fasolo M., Mazza F., Ricci E., Esposito S., Frati E. Risk of cervical HPV infection and prevalence of vaccine-type and other high-risk HPV types among sexually active teens and young women (13–26 years) enrolled in the VALHIDATE study. Hum. Vaccines Immunother. 2014;10:986–994. doi: 10.4161/hv.27682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinka K., Lacey M., Robertson C., Kavanagh K., Cuschieri K., Nicholson D. Acceptability and response to a postal survey using self-taken samples for HPV vaccine impact monitoring. Sex. Transm. Infect. 2011;87:548–552. doi: 10.1136/sextrans-2011-050211. [DOI] [PubMed] [Google Scholar]
- 25.Cuschieri K., Nandwani R., McGough P., Cook F., Hogg L., Robertson C. Urine testing as a surveillance tool to monitor the impact of HPV immunization programs. J. Med. Virol. 2011;83:1983–1987. doi: 10.1002/jmv.22183. [DOI] [PubMed] [Google Scholar]
- 26.Vorsters A., Micalessi I., Bilcke J., Ieven M., Bogers J., Van Damme P. Detection of human papillomavirus DNA in urine. A review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31:627–640. doi: 10.1007/s10096-011-1358-z. [DOI] [PubMed] [Google Scholar]
- 27.Tanzi E., Bianchi S., Fasolo M.M., Frati E.R., Mazza F., Martinelli M. High performance of a new PCR-based urine assay for HPV-DNA detection and genotyping. J. Med. Virol. 2013;85:91–98. doi: 10.1002/jmv.23434. [DOI] [PubMed] [Google Scholar]
- 28.Pathak N., Dodds J., Zamora J., Khan K. Accuracy of urinary human papillomavirus testing for presence of cervical HPV: systematic review and meta-analysis. BMJ. 2014;349:g5264. doi: 10.1136/bmj.g5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Statistics Norway. Folkemengde, etter kjønn og ettårig alder, tabell 10211, 2015, 〈www.ssb.no/statistikkbanken/〉