Abstract
Objectives:
This study aimed to elucidate the effects of two naps taken at night on morning waking state and performance.
Methods:
The participants were 12 women. The experiment was performed in a laboratory over 2 days (16:00-09:00). In this crossover comparative study, three experimental nap conditions were used (naps from 22:30 to 00:00 and from 02:30 to 03:00 (22:30-NAP), 00:30 to 02:00 and 04:30 to 05:00 (00:30-NAP), and no naps (NO-NAP), respectively). Measurement items were a Visual Analog Scale for sleepiness and fatigue, the Psychomotor Vigilance Test (PVT), and single-digit addition calculations (10 min) every hour for 18 h from 16:00 to 09:00, excluding nap times.
Results:
Sleep inertia and sleepiness were noted directly after napping. Less sleepiness and fatigue were noted in the nap groups between 06:00 and 09:00 in the morning than in the NO-NAP condition and PVT response times were faster. Since participants in the nap groups were able to conduct more single-digit addition calculations, the performance of these groups appeared to be superior to that of the NO-NAP condition. Furthermore, the performance of calculations was significantly better in the 00:30-NAP than in the 22:30-NAP.
Conclusions:
Taking two naps during a simulated night shift helps improve sleepiness and fatigue and maintain performance. Taking a nap in the early morning appears to be promising for improving the waking state.
Keywords: 90-min nap and 30-min nap, Night shift worker, Psychomotor vigilance task, Subjective performance
Introduction
The advanced informatization and internationalization of recent years have led to diversification of working times from the perspectives of improved production efficiency, safety, and public benefits. Shift work has become essential for modern society1). Problems with shift work include decreased sleeping time and increased fatigue produced by a day-night reversal2).
The medical profession, especially nursing, is a typical example of a profession that has adopted a shift work system. In Japan, many nurses work on two- or three-shift systems. In two-shift systems, there are two major forms of night shifts3): 12 h shifts and 16 h shifts (beginning the night shift at about 16:00). The 16 h night shifts4) are particularly burdensome for nurses both physically and mentally. Previous research has indicated that nurses on such schedules are troubled by morning sleepiness and fatigue5). Though sleep structure typically changes with age6), napping is an important fatigue and sleepiness management strategy for shift workers, including nurses7-9).
According to Japanese law, an employer shall provide workers with at least a 1 h break in the event that working hours exceed 8 h (Article 34, Labor Standards Law in Japan). Informally, however, the nursing staff at public hospitals are usually allowed to sleep or rest up to 2 h during 16 h night shifts7), and many nurses nap during the night shift10).
A brief nap of 30 min or less has demonstrated improved objective and subjective alertness, in addition to fatigue and vigilance9). With 60 min naps, there is a problem with residual sleepiness due to sleep inertia. Sleep inertia refers to the grogginess and disorientation felt upon first waking from a nap11). To minimize sleep inertia, sleeping for 90 min (i.e., one cycle) is considered appropriate5). Therefore, additional sleep taken before the effects of a nap of 90 min or longer wear off may help maintain the level of wakefulness12).
According to previous research, a 20 min nap is effective in reducing sleepiness for 1 to 2 h, and naps of 30 to 120 min are effective in reducing sleepiness for 2 to 3 h. Since sleepiness increases at 03:0013), a 120 min nap that ends at 03:00 could be effective in reducing sleepiness during night shifts. However, a single nap may not be sufficient to prevent drowsiness and fatigue until 09:00, which is the usual end time of night shifts. Previous studies have not clarified the effects of taking two naps or the timing of naps. In the case of 16 h night shifts, it is common for nurses to nap between 22:00 and 06:0012). Therefore, it is necessary to set naps during that time period for nurses on 16 h night shifts.
The aim of this study was to investigate, for the first time, performance and subjective sleepiness immediately following a 90 min and a 30 min nap at nighttime. We tested the following two hypotheses in this study.
Hypothesis 1: Naps of 90 min and 30 min positively affect the level of wakefulness and performance maintenance and the effects last for 3 h.
Hypothesis 2: Naps of 90 min and 30 min do not lead to sleep inertia.
Methods
Sample and data collection
Twelve healthy, female university student volunteers were recruited for the study. Since most nurses in Japan are women, we believed these participants to be representative of the general nurse population in Japan. Participants (mean age 22.2±0.4 years) who did not obviously conform to either morning type or evening type personalities according to the "Morningness-Eveningness Questionnaire" 14) were asked to participate in this experiment with remuneration of 15,000 Japanese yen (approximately $135 US dollars), which is the pay equivalent of one night shift. They had no previous night shift experience, and the study took place during their summer vacation from August to November in 2014. The participants were randomly assigned to the nap condition or the no-nap condition. The participants conducted the experiment three times, each of which included 90 min and 30 min naps at different times. Participants in this study were randomly assigned using counterbalancing. All participants were non-smokers, non-obese (body mass index ≤ 25), and consumed low amounts of coffee and alcohol. They had normal sleep patterns (habitual sleep ranged between 7 h and 9 h) and were not under medication. Data collection began 2 days before the experiment, with an ActiGraph worn on the wrist and a diary to record activity and sleep. We requested the participants not consume alcohol or caffeine, particularly coffee during the study, beginning the day before the experiment.
This study was approved by the Ethics Committee for Epidemiologic Research at the Prefectural University of Hiroshima. Participants gave written, informed consent to participate.
Study design
Participants resided in a windowless and sound-insulated sleep laboratory for 2 consecutive days (1 night). Ambient room temperature was maintained at 26±2°C15) according to each participant's preference, and humidity was 50%16). Light intensity was set to 200 lux at head height during all wake periods of the experiment, and the participants could adjust the level of brightness/darkness during all scheduled sleep periods according to their preference.
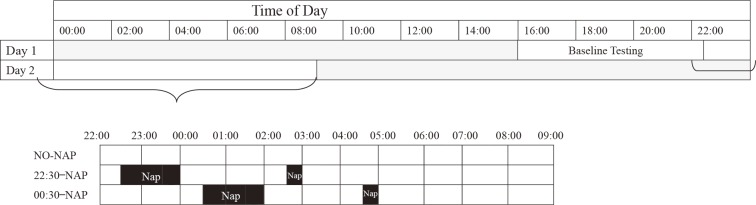
A timeline diagram of the study design is shown in Fig. 1. The measurements for one experiment were conducted over 2 days, between 16:00 and 09:00. Participants arrived at the laboratory at 15:00 and took practice measurements such as the Psychomotor Vigilance Test (PVT) until 16:00; they spent the rest of the day adapting to the setting and practicing various performance tasks. The three conditions were two naps from 22:30-00:00 and 02:30-03:00 (22:30-NAP), 00:30-02:00 and 04:30-05:00 (00:30-NAP), and no-nap (NO-NAP). During wake periods, participants performed neurobehavioral test batteries approximately every hour and were permitted to read books, play cards, interact with each other and study staff, or listen to music between test sessions. For each hour throughout the experiment, 25 min were measurement time in which the participants completed a Visual Analog Scale (VAS) on sleepiness and fatigue and performed neurobehavioral testing. The next 35 min were free time. Participants were given a meal of the same amount and contents at 19:25-19:40 during every experimental period. Since menstruation can greatly affect sleepiness17), participants did not take part in the experiment during their periods. A researcher observed the participants and performed measurements from the start of the experiment to its completion. At the end of each scheduled nap time, the researcher notified the participants that it was time to wake up. There was an interval of at least 3 to 4 weeks (average 29.75±15.25 days, range 20-72 days) between the two test conditions.
Fig. 1.
Schematic of the study protocol. Each row represents 24 h.
The black areas indicate nap times. The diagonal hatching shows all three conditions during that time period.
Measurements
1) Performance test
Unless napping, participants performed single-digit mental addition calculations for 10 min every hour. The number of calculations carried out in 10 min was considered to be the participant's workload for that time.
2) Subjective measures
Sleepiness and fatigue were assessed subjectively using a VAS18). Participants rated their sleepiness and fatigue on a 100 mm line every hour except when they were napping. The values ranged from 0 mm (not at all sleepy or tired) to 100 mm (extremely sleepy or tired).
3) Neurobehavioral test
Neurobehavioral function was assessed using the PVT, a portable reaction time test based on the Dinges and Powell Digital Test19). The PVT has been shown to be sensitive to sleep deprivation and circadian effects19). To avoid problems of uncertainty regarding the accuracy of timing of the test platform, we utilized a precise computer-based version of the 10 min PVT20). Participants were instructed to watch the computer screen and press a response button as soon as a white circular edge appeared on the screen, which stopped the counter and displayed the response time (RT) in ms for a 1 s period. The PVT records RT to visual stimuli that occur at random 2 to 10 s inter-stimulus intervals over a 10 min period20). The acceptable time frame to react was >100 ms to <500 ms, and the number of omission errors (RT ≥ 500 ms) plus commission errors (premature responses or RT < 100 ms) were measured21).
4) Physiological measures
The ActiGraph is a reliable and valid measure for detecting sleep in normal, healthy adult populations22). Individual sleep periods were calculated from actigraphically determined sleep onset to actigraphically determined sleep offset, with correction for waking after sleep onset. Total sleep time (TST) was the sum in min of all periods scored as sleep periods in a 24 h day, including naps. The data were analyzed using the software package AW2 (Ambulatory Monitoring Inc.).
Statistical analysis
Mean and standard deviations were calculated for VAS, PVT, and performance test scores every hour. The analysis was done with the experimental period divided into 00:00-02:00, 02:00-06:00, and 06:00-09:00 time blocks.
In order to test the effect of a nap on neurobehavioral outcomes during the sleepiness and fatigue measurement period, a fully saturated, linear mixed-effects analysis of variance (ANOVA)23) with a between-participants fixed effect of condition (22:30-NAP, 00:30-NAP, NO-NAP) and a within-participant fixed effect of time and random intercept were used. Simple planned contrasts (00:00, 02:00, or 06:00 versus each post-nap test point) were conducted to further investigate significant interaction effects within conditions. Within-condition comparisons were chosen in order to minimize the influence of individual differences. As a secondary analysis, between-condition comparisons were also assessed for each time block. To compare baseline sleep variables between groups, one-way ANOVA was used with a between-participants fixed effect of condition (22:30-NAP, 00:30-NAP, NO-NAP). Sleep variables during the nap conditions were analyzed using one-way ANOVA with a between-participants fixed effect of condition (22:30-NAP, 00:30-NAP). To evaluate patterns of change in all three nap conditions, multiple comparisons were assessed using the Bonferroni correction.
Statistical analyses were performed using IBM SPSS statistics software version 20.0J (IBM, Tokyo, Japan). The hypothesis rejection level for all tests was set at p < 0.05.
Results
There were no significant differences between conditions for any sleep or baseline variables (at 22:00) on the day of the experiment (Table 1).
Table 1.
Baseline sleep variables, subjective outcomes and objective task performances.
| Variable | NO-NAP | 22:30-NAP | 00:30-NAP | F (2, 33) | p |
|---|---|---|---|---|---|
| Data are means (standard deviation). The 22:30-NAP condition had two naps at 22:30-00:00 and 02:30-03:00. The 00:30-NAP condition had two naps at 00:30-02:00 and 04:30-05:00. PVT: response speed. Proportion: proportion of correct responses on the PVT. Based on measurements of sleepiness, fatigue, PVT, Proportion in PVT and number of calculations performed at 22:00. TST, total sleep time; SE, sleep efficiency; WASO, wake after sleep onset; PVT, psychomotor vigilance test. | |||||
| Wake up time | 08:12 (01:17) | 08:14 (01:00) | 08:03 (01:04) | 0.086 | 0.917 |
| TST (min) | 436.3 (69.2) | 417.8 (94.9) | 426.9 (90.2) | 0.139 | 0.871 |
| SE (%) | 97.3 (2.9) | 97.0 (2.7) | 97.5 (2.2) | 0.137 | 0.873 |
| WASO (min) | 10.3 (14.3) | 13.8 (12.8) | 12.3 (12.8) | 0.208 | 0.813 |
| Sleepiness | 26.7 (19.5) | 17.1 (16.9) | 31.0 (23.9) | 1.475 | 0.243 |
| Fatigue | 25.1 (16.6) | 26.5 (19.1) | 35.5 (22.7) | 0.998 | 0.381 |
| PVT | 322.4 (29.1) | 338.8 (36.9) | 340.0 (35.4) | 1.007 | 0.376 |
| Proportion | 86.8 (16.0) | 86.8 (11.7) | 87.0 (8.1) | 0.001 | 0.999 |
| Calculations | 883.8 (128.2) | 888.9 (125.2) | 821.5 (136.6) | 0.915 | 0.411 |
Among the 12 participants assigned to the nap condition, two participants could not sleep during the 30 min nap in the 22:30-NAP, and their sleep latency as measured by the ActiGraph was over 30 min.
Subjective measures
Table 2 shows the distribution of sleep state for the 90 min and 30 min naps. The 30 min nap in the 22:30-NAP was not significantly different from the 30 min nap in the 00:30-NAP, but the 90 min nap in the 22:30-NAP had a significantly lower TST than the 90 min nap in the 00:30-NAP (66.9 min versus 80.5 min; p = 0.019). With regard to sleep variables before and during the experiment, there were no differences between summer (August and September) and fall (October and November).
Table 2.
Sleep variables.
| Variable | 22:30-NAP | 00:30-NAP | F (1, 22) | p |
|---|---|---|---|---|
| 22:30-00:00 nap | 00:30-02:00 nap | |||
| Data are means (standard deviation). The 22:30-NAP condition had two naps at 22:30-00:00 and 02:30-03:00. The 00:30-NAP condition had two naps at 00:30-02:00 and 04:30-05:00. TST, total sleep time; SE, sleep efficiency; WASO, wake after sleep onset. | ||||
| TST (min) | 66.9 (18.0) | 80.5 (4.9) | 6.369 | 0.019 |
| SE (%) | 96.2 (8.7) | 99.3 (1.6) | 1.422 | 0.246 |
| WASO (min) | 0.5 (0.9) | 0.6 (1.2) | 0.035 | 0.853 |
| 02:30-03:00 nap | 04:30-05:00 nap | F (1, 9) | p | |
| TST (min) | 18.8 (9.0) | 22.9 (2.7) | 2.286 | 0.145 |
| SE (%) | 82.6 (38.6) | 99.8 (0.7) | 2.388 | 0.137 |
| WASO (min) | 5.2 (11.6) | 10.8 (2.3) | 1.672 | 0.209 |
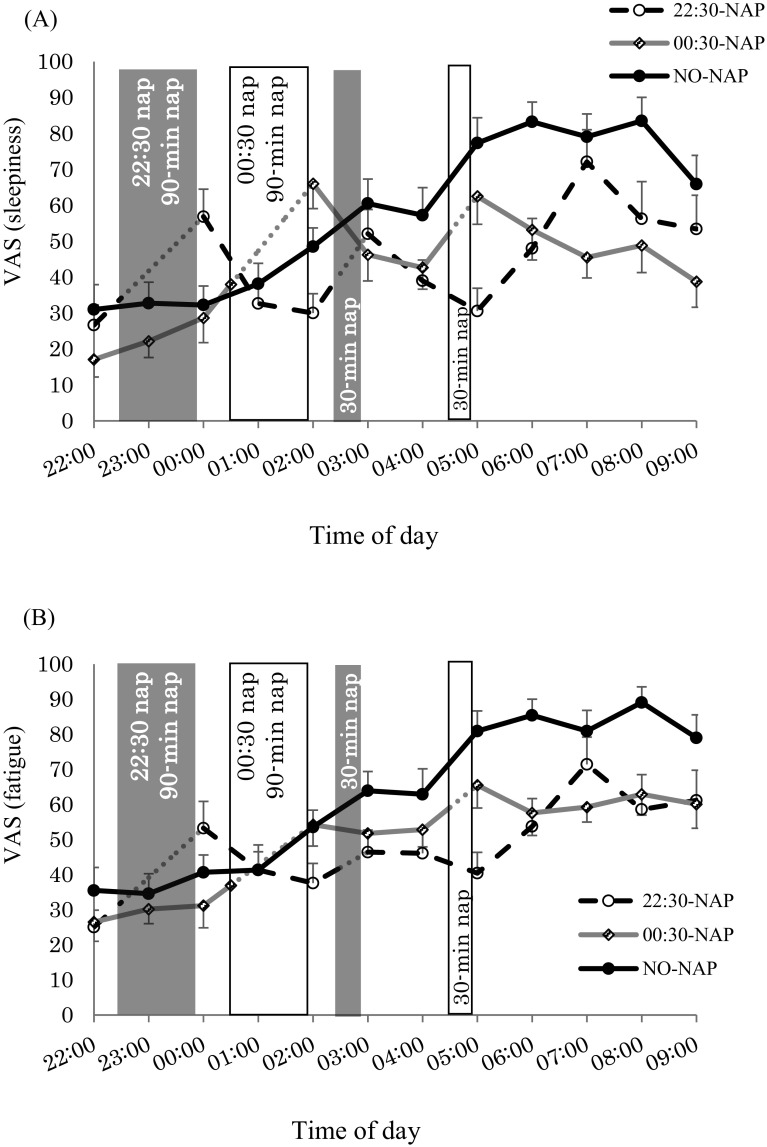
Fig. 2 shows the scores for each subjective scale. For sleepiness and fatigue (Table 3) from 00:00 to 02:00 and 02:00 to 06:00, there was a significant condition*time interaction. With the NO-NAP condition, there was a significant increase in both sleepiness and fatigue at 02:00, 05:00 and 06:00 (p < 0.05). For the 22:30-NAP, there were significant decreases in both sleepiness and fatigue at 01:00-02:00 (p < 0.05), but sleepiness increased at 03:00 and 06:00 (p<0.05), and fatigue increased at 06:00 (p = 0.028). Similarly, with the 00:30-NAP, sleepiness significantly decreased at 03:00 and 04:00 (p < 0.05); however, no main effect of time was observed for fatigue. From 06:00 to 09:00 in the 22:30-NAP and 00:30-NAP, sleepiness decreased compared with the NO-NAP condition (p < 0.001). Comparing before and after naps, there were significant increases in sleepiness and fatigue after the 90 min naps in both nap conditions (p < 0.05). Sleepiness significantly increased after the 30 min naps in both nap conditions (p < 0.05), but there were no significant differences in fatigue after the 30 min naps in either of the two nap conditions.
Fig. 2.
Mean (± standard error of the mean) for subjective scales. (A) Visual Analog Scale (VAS) (sleepiness); (B) VAS (fatigue).
Table 3.
Results from the linear mixed-effects analysis of variance for neurobehavioral outcomes.
| 00:00-02:00 | 02:00-06:00 | 06:00-09:00 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | F | p | ||
| PVT: Psychomotor Vigilance Test. Proportion: proportion of correct reactions on the PVT. | ||||||||||
| Sleepiness | ||||||||||
| Condition | 1,55 | 0.003 | 0.959 | 2,154 | 26.517 | <0.001 | 2,121 | 19.829 | <0.001 | |
| Time | 2,55 | 2.017 | 0.143 | 4,154 | 3.776 | 0.006 | 3,121 | 1.513 | 0.215 | |
| Condition*Time | 2,55 | 11.728 | <0.001 | 8,154 | 4.812 | <0.001 | 6,121 | 1.204 | 0.309 | |
| Fatigue | ||||||||||
| Condition | 1,55 | 0.106 | 0.746 | 2,154 | 31.139 | <0.001 | 2,121 | 23.024 | <0.001 | |
| Time | 2,55 | 0.940 | 0.397 | 4,154 | 5.890 | <0.001 | 3,121 | 0.596 | 0.619 | |
| Condition*Time | 2,55 | 5.609 | 0.006 | 8,154 | 2.141 | 0.035 | 6,121 | 1.022 | 0.415 | |
| PVT | ||||||||||
| Condition | 1,55 | 5.554 | 0.022 | 2,154 | 21.975 | <0.001 | 2,121 | 20.140 | <0.001 | |
| Time | 2,55 | 1.092 | 0.343 | 4,154 | 6.117 | <0.001 | 3,121 | 0.753 | 0.523 | |
| Condition*Time | 2,55 | 2.091 | 0.133 | 8,154 | 2.286 | 0.024 | 6,121 | 1.077 | 0.380 | |
| Proportion | ||||||||||
| Condition | 1,55 | 0.190 | 0.665 | 2,154 | 12.265 | <0.001 | 2,121 | 18.035 | <0.001 | |
| Time | 2,55 | 0.597 | 0.554 | 4,154 | 8.513 | <0.001 | 3,121 | 3.239 | 0.025 | |
| Condition*Time | 2,55 | 0.214 | 0.808 | 8,154 | 1.328 | 0.234 | 6,121 | 0.347 | 0.910 | |
| Calculations | ||||||||||
| Condition | 1,55 | 1.862 | 0.178 | 2,154 | 23.456 | <0.001 | 2,121 | 32.465 | <0.001 | |
| Time | 2,55 | 0.767 | 0.470 | 4,154 | 5.006 | 0.001 | 3,121 | 4.202 | 0.007 | |
| Condition*Time | 2,55 | 2.455 | 0.095 | 8,154 | 2.032 | 0.046 | 6,121 | 0.510 | 0.800 | |
Neurobehavioral measures
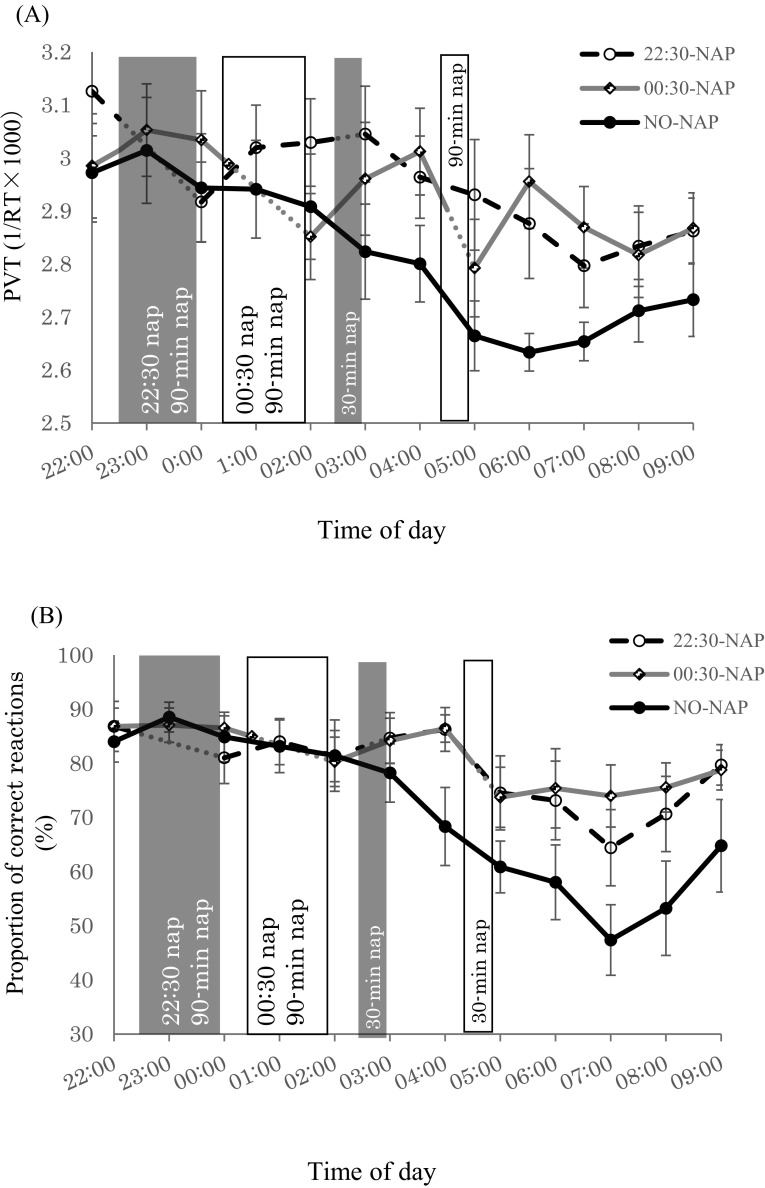
Fig. 3A shows RT data for the PVT. There was a significant main effect of condition from 00:00 to 02:00. RT in the 22:30-NAP was significantly faster than the NO-NAP condition (p = 0.022). From 02:00 to 06:00, there was a significant main effect of condition, time, and condition*time interaction. The NO-NAP condition showed significantly slower RT at 05:00 and 06:00 (p < 0.001). The 22:30-NAP showed significantly slower RT at 06:00 after the 30 min nap (p < 0.05). The 00:30-NAP showed no significant difference in RT. From 06:00 to 09:00, RT in the 22:30-NAP and 00:30-NAP was not significantly different, but both nap conditions had significantly faster RT than the NO-NAP condition (p < 0.001). Comparing before and after naps, RT became significantly slower after the 90 min naps in both nap conditions (p < 0.01). RT before and after the 30 min nap in the 22:30-NAP was not significantly different, but it was significantly slower after the 30 min nap in the 00:30-NAP (p < 0.001).
Fig. 3.
Mean (± standard error of the mean) for neurobehavioral function (A) Psychomotor Vigilance Task (PVT) response time (RT); (B) proportion of correct responses (i.e., rate of <100 ms or ≥500 ms).
Fig. 3B shows the proportion of correct responses on the PVT. There was no significant main effect of condition, time, or condition*time interaction from 00:00 to 02:00, 02:00 to 06:00, and 06:00 to 09:00. From 02:00 to 06:00, the proportion of correct responses on the PVT was significantly lower in the NO-NAP condition than in the 22:30-NAP and 00:30-NAP (p < 0.05). The proportion of correct responses was also significantly lower at 05:00 and 06:00 (p < 0.01). From 06:00 to 09:00, no difference was observed between the 22:30-NAP and the 00:30-NAP. The proportion of correct responses in the two nap conditions was significantly higher than in the NO-NAP condition (p < 0.001). There was no significant difference before and after the 90 min naps in either of the two nap conditions. No difference in the proportion of correct responses was observed after the 30 min nap in the 22:30-NAP, but it was significantly lower after the 30 min nap in the 00:30-NAP (p = 0.003).
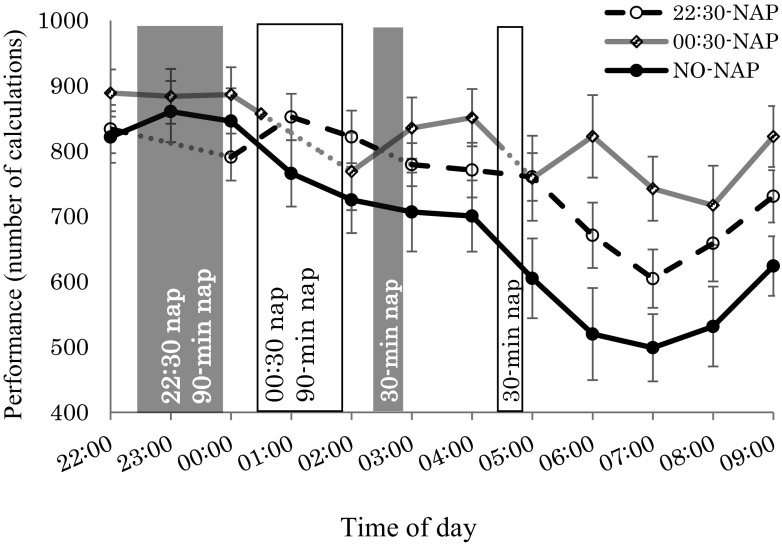
Fig. 4 shows the number of single-digit calculations performed by the participants. From 00:00 to 02:00, there was no significant main effect of condition, time, or condition*time interaction. From 02:00 to 06:00, main effects were observed in condition, time, and condition*time interaction. With the NO-NAP condition, there was a significant decrease at 05:00 and 06:00 (p < 0.05). Similarly, with the 22:30-NAP, there was a significant decrease at 06:00 (p = 0.001). On the other hand, with the 00:30-NAP, there was a significant decrease at 04:00 (p = 0.043). From 06:00 to 09:00, the number of calculations performed was significantly higher with the 00:30-NAP than with the 22:30-NAP (p = 0.001). It was also significantly higher with the two nap conditions than with the NO-NAP condition, and work efficiency was better (p < 0.001).
Fig. 4.
Mean (± standard error of the mean) number of calculations performed.
Comparing before and after naps, there were significant decreases in the number of calculations performed and significantly slower RT after the 90 min naps in both nap conditions (p < 0.01). There were significant decreases in the number of calculations performed, RT, and the proportion of correct responses on the PVT at 05:00 after the 30 min nap in the 00:30-NAP (p < 0.05).
Discussion
This study is the first to examine the effects of combined 90 min and 30 min naps at night on sleepiness, fatigue, and performance. In what follows, we discuss the most important contributions of the present study.
Generally, sleepiness levels can be explained by the three-process model of circadian rhythms, prior wakeful time, and sleep inertia24). When the awake time passes 17 h, work performance declines linearly25), and the low point of circadian rhythms is a time of great sleepiness26). Sleeping time becomes longer with longer prior wakeful time27), and since wake time was longer with the 90 min nap in the 00:30-NAP than in the 22:30-NAP, sleepiness was stronger and TST was also thought to have been significantly longer. Wake time after a nap was longer toward morning with the 22:30-NAP than the 00:30-NAP, and therefore, sleepiness probably increased at 06:00. On the other hand, the 30 min naps in both nap conditions showed no difference in TST since the awake times from the end of the 90 min naps were similar between the two conditions.
The most effective combination of nap timing and nap length remains to be determined. The most favorable nap timing to sustain performance and alertness during the night shift is around the nadir of body temperature (i.e., 04:00 or 05:00)7,28). Dinges et al.29) recommended taking a nap before feeling sleepiness. In the NO-NAP condition, sleepiness increased with the time awake and at 02:00 and 05:00. Therefore, in the 22:30-NAP and 00:30-NAP, the first nap was taken by 02:00 and the next by 05:00. This facilitated napping, and an effect could thus be anticipated. The 22:30-NAP inhibited sleepiness through 05:00. A 60 min or 120 min nap taken between 23:00 and 02:00 has been found to reduce sleepiness through 04:007,28). Hence, it appears that the 30 min nap (ending at 03:00) made it possible to prolong the decrease in sleepiness by 1 h. In the 00:30-NAP on the other hand, the effect of napping was sustained through 06:00. Between 06:00 and 09:00, the number of calculations performed was significantly better in the 00:30-NAP than in the 22:30-NAP, showing that performance was sustained in the former condition. Thus, the subjective fatigue at 02:00 to 06:00 suggests that a later nap will better sustain performance in the early morning compared to an earlier nap30). Moreover, if decreases in sleepiness and fatigue and sustained performance are to be expected from 06:00 to 09:00 in the 22:30-NAP, it may be necessary to add a 30 min nap between 05:00 and 06:00.
The present study did not show a difference in the subjective measures for the 22:30-NAP or 00:30-NAP. One possible explanation is that the interval between the two nap conditions was short (i.e., 2 h)30). When taking a nocturnal nap during the night shift, the effect of nap timing on early morning subjective measures may not be as great as that of nap length.
A potential downside to on-shift napping, however, is the possibility of sleep inertia following the nap. With naps of 30 min or longer, sleep inertia facilitates slow-wave sleep and is manifested by strong sleepiness and a deterioration of work performance during wakefulness31). Moreover, sleep inertia can continue for 5 min32) to over 30 min29) after waking.
The results of this study suggested that 90 min naps ending at 00:00 (22:30-NAP) and 02:00 (00:30-NAP) were associated with substantial sleep inertia as measured by increased sleepiness after waking. The 30 min naps of the 22:30-NAP and 00:30-NAP, taken 2 h after the 90 min nap, were associated with substantial sleep inertia as measured by increased sleepiness and fatigue after waking. However, performance on calculations, RT, and the proportion of correct responses after waking were not lower after the 30 min nap in the 22:30-NAP (ending at 03:00). Based on these results, if tasks requiring rapid responses to stimuli to ensure safety are scheduled between 03:00 and 05:00 during a night shift, a 30 min nap ending at 03:00 is recommended. The 22:30-NAP attenuated performance impairment from cumulative hours of wakefulness while avoiding the pitfall of sleep inertia following the nap from 03:00 to 05:00.
One possible explanation for why performance on calculations, RT, and the proportion of correct responses were not lower after the 30 min nap in the 22:30-NAP is the interval (i.e., 2.5 h) between the first nap and the second nap. That is, the 30 min nap in the 22:30-NAP (ending at 03:00) was the second nap and sleep pressure was therefore decreased, making it difficult to fall asleep and resulting in weakened sleep inertia. Although sleep pressure was similarly reduced at the time of the 30 min nap in the 00:30-NAP, it was surmised that circadian rhythms played a role in the fact that sleep inertia was observed in this case. Sleep inertia has an endogenous circadian rhythm, with the most impaired cognitive performance upon awakening occurring when an individual takes a nap during the biological night (23:00-03:00)33).
In workplace scenarios where there is an opportunity for long breaks (>2 h), longer naps may produce benefits immediately and over longer times. However, such long breaks cannot be taken at most work places (e.g., labor and delivery nursing), so the effect is limited. From the results of this study, taking a 90 min nap to maintain long-term performance and later taking a 30 min nap to maintain the level of wakefulness is thought to be valuable in terms of work efficiency and safety in the morning. With a 30 min nap, there is a temporary decrease in subjective alertness after the nap5), but excluding this, the effect of the nap is reported to last for 3 h34). During night work (e.g., 16:00 to 09:00), naps from 22:30 to 00:00 and 02:30 to 03:00 are thought to be effective in cases when tasks with a high level of safety requiring quick responses are scheduled for 03:00 to 06:00. Especially since there is no decline in performance after a nap from 02:30 to 03:00, such naps are also thought to be effective for tasks after 03:00. When tasks that require a high level of safety are scheduled for 06:00 to 09:00 (i.e., the busiest time in night work35)), a greater effect can be expected with two naps compared with no naps. The importance of effective measures to reduce the sleepiness and fatigue experienced by shift workers is highlighted by the decreased alertness in the early morning shown in the NO-NAP condition throughout this study.
Strengths and limitations
The present study had two strengths and four limitations. With regard to the strengths, first, this was a pilot study that evaluated subjective and objective variables such as sleepiness, fatigue, and work performance, which will serve as indices for evaluation when investigating actual workplaces. According to a previous study36), when nurses' working time exceeds 12.5 h, the odds ratio of medical errors and sleepiness is 1.94. Since sleepiness increases with time, as in the 12 h night shift in the previous study36), we believe that taking two naps as introduced in the present study can be adapted to actual 16 h night shifts. Second, the working hours modeled in this study were those of nurses working a 16 h nightshift, and the effects of long periods of wakefulness on women in their 20s were analyzed. Because sleepiness is stronger in females than in males37), the findings are also likely applicable to males. Rotating shift work is predicted to increase in modern societies in the future, and the results of this study are therefore applicable to occupations other than nursing.
The first limitation is that this study was conducted under laboratory conditions. Intervention studies in actual workplaces are required to clarify measures effective for reducing sleepiness and fatigue that are tailored to the conditions of specific professions and workplaces. The second limitation is that we did not use polysomnography measurements in this study, and the sleep stage of the participants during their naps was not clearly determined. The third limitation is that the study focused on younger individuals, and there were no findings pertaining to middle-aged or elderly individuals. Härmä suggested that sleep flexibility reduces with aging38), and sleep time increases with age beginning from 40 years of age39). Consequently, whether results similar to those obtained in this study are obtained for all age groups needs to be determined. The final limitation is that the number of participants in this study was low, producing low statistical power. Therefore, further investigations using a larger sample size are necessary.
Conclusion
It was suggested that the decreased performance observed from 06:00 to 09:00 in the NO-NAP condition could be mitigated by napping twice a night. In particular, the 00:30-NAP seemed to be more effective than the 22:30-NAP. It was suggested that greater effects can be expected in maintaining the level of wakefulness and performance by selecting nap times and durations matched to the content of tasks during night shifts.
Acknowledgments: This work was supported by JSPS KAKENHI Grant Number JP26293452.
Conflicts of interest: None declared.
References
- 1). Kubo T. Estimate of the number of night shift workers in Japan. Journal of UOEH 2014; 36 (4): 273-276 (in Japanese). [DOI] [PubMed] [Google Scholar]
- 2). Fukuda H, Takahashi M, Airto H. Nurses' workload associated with 16-h night shifts on the 2-shift system. I: Comparison with the 3-shift system. Psychiatry and Clinical Neurosciences 1999; 53 (2): 219-221. [DOI] [PubMed] [Google Scholar]
- 3). Luna TD, French J, Mitcha JL. A study of USAF air traffic controller shiftwork: Sleep, fatigue, activity, and mood analyses. Aviation, Space, and Environmental Medicine 1997; 68 (1): 18-23. [PubMed] [Google Scholar]
- 4). Mastumoto M, Lee B, Tozato F, et al. Objective and subjective measures of sleep of shift-working nurses. Sangyo Eiseigaku Zasshi 2014; 56 (3): 67-73. [DOI] [PubMed] [Google Scholar]
- 5). Takahashi M, Arito H, Fukuda H. Nurses' workload associated with 16-h night shifts. II: Effects of a nap taken during the shifts. Psychiatry and Clinical Neurosciences 1999; 53 (2): 223-225. [DOI] [PubMed] [Google Scholar]
- 6). Roffwarg HP, Muzio JN, Dement WC. Ontogenic development of the human sleep-dream cycle: the prime role of dreaming sleep in early life may be in the development of the central nervous system. Science 1966; 152: 604-619. [DOI] [PubMed] [Google Scholar]
- 7). Sasaki T, Matsumoto S. Prevalence of the subjective sleepiness in nurses working 16-hour night shifts. The Journal of Science of Labour 2013; 89 (6): 218-224 (in Japanese). [Google Scholar]
- 8). Smith SS, Kinby S, Jorgensen G, et al. Napping and nightshift work: Effects of a short nap on psychomotor vigilance and subjective sleepiness in health workers. Sleep & Biological Rhythms 2007; 5: 117-125. [Google Scholar]
- 9). Smith-Coggins R, Howard SK, Mac DT, et al. Improving alertness and performance in emergency department physicians and nurses: The use of planned naps. Annals of Emergency Medicine 2006; 48 (5): 596-604. [DOI] [PubMed] [Google Scholar]
- 10). Oriyama S, Miyakoshi Y, Kobayashi T. Ways of taking rest and breaks related to night shifts in two-shift nurses and factors supporting work: ―A comparison of 12- and 16-hour night shifts―. Japan Society for Healthcare Administration 2014; 51 (1): 21-31 (in Japanese). [Google Scholar]
- 11). Tassi P, Muzet A. Sleep inertia. Sleep Medicine Reviews 2000; 4 (4): 341-353. [DOI] [PubMed] [Google Scholar]
- 12). Takahashi M, Fukuda H, Miki K, et al. Shift work-related problems in 16-h night shift nurses (2): Effects on subjective symptoms, physical activity, heart rate, and sleep. Industrial Health 1999; 37 (2): 228-236. [DOI] [PubMed] [Google Scholar]
- 13). Rajaratnem SM, Arendt J. Health in a 24-h society. Lancet 2001; 358: 999-1005. [DOI] [PubMed] [Google Scholar]
- 14). Ishihara K, Miyashita A, Inugami M, et al. The results of investigation by the Japanese version of Morningness-Eveningness Questionnaire. The Japanese Journal of Psychology 1986; 57 (2): 87-91 (in Japanese). [DOI] [PubMed] [Google Scholar]
- 15). Kawashima Y, Kakitsuba N. Optimal thermal conditions during night sleep in summer. Journal of Human and Living Environment 2004; 11 (1): 17-23 (in Japanese). [Google Scholar]
- 16). Yanase T. Sleep and bedroom environment: on the correlation between the seasonal changes in bed climate and sleep. The Annals of Physiological Anthropology 1985; 4 (4): 331-333 (in Japanese). [DOI] [PubMed] [Google Scholar]
- 17). de Zambotti M, Nicholas CL, Colrain IM, et al. Autonomic regulation across phases of the menstrual cycle and sleep stages in women with premenstrual syndrome and healthy controls. Psychoneuroendocrinology 2013; 38 (11): 2618-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Monk TH. A visual analog scale technique to measure global vigor and affect. Psychological Research 1989; 27: 89-99. [DOI] [PubMed] [Google Scholar]
- 19). Thorne DR, Johnson DE, Redmond DP, et al. The Walter Reed palm-held psychomotor vigilance test. Behavior Research Methods 2005; 37 (1): 111-118. [DOI] [PubMed] [Google Scholar]
- 20). Roach GD, Dawson D, Lamond N. Can a shorter psychomotor vigilance task be used as a reasonable substitute for the ten-minute psychomotor vigilance task? Chronobiology International 2006; 23 (6): 1379-1387. [DOI] [PubMed] [Google Scholar]
- 21). Basner M, Dinges DF. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep 2011; 34 (5): 581-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Bellone GJ, Plano SA, Cardinali DP, et al. Comparative analysis of actigraphy performance in healthy young subjects. Sleep Science 2016; 9 (4): 272-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Van Dongen HP, Maislin G, Dinges DF. Dealing with inter-individual differences in the temporal dynamics of fatigue and performance: importance and techniques. Aviation, Space, and Environmental Medicine 2004; 75: 145-154. [PubMed] [Google Scholar]
- 24). Akerstedt T, Folkard S. Validation of the S and C components of the three-process model of alertness regulation. Sleep 1995; 18 (1): 1-6. [DOI] [PubMed] [Google Scholar]
- 25). Dawson D, Reid K. Fatigue, alcohol and performance impairment. Nature 1997; 388 (6639): 235-237. [DOI] [PubMed] [Google Scholar]
- 26). Folkard S, Hume KI, Minors DS, et al. Independence of the circadian rhythm in alertness from the sleep/wake cycle. Nature 1985; 313 (6004): 678-679. [DOI] [PubMed] [Google Scholar]
- 27). Gillberg M. The effects of two alternative timings of a one-hour nap on early morning performance. Biological Psychology 1984; 19 (1): 45-54. [DOI] [PubMed] [Google Scholar]
- 28). Takeyama H, Matsumoto S, Murata K, et al. Effects of the length and timing of nighttime naps on task performance and physiological function. Revista De Saude Publica 2004; 38: 32-37. [DOI] [PubMed] [Google Scholar]
- 29). Dinges DF, Orne MT, Whitehouse WG, et al. Temporal placement of a nap for alertness: contributions of circadian phase and prior wakefulness. Sleep 1987; 10 (4): 313-329. [PubMed] [Google Scholar]
- 30). Kubo T, Takeyama H, Matsumoto S, et al. Impact of nap length, nap timing and sleep quality on sustaining early morning performance. Industrial Health 2007; 45: 552-563. [DOI] [PubMed] [Google Scholar]
- 31). Ferrara M, De Gennaro L. The sleep inertia phenomenon during the sleep-wake transition: theoretical and operational issues. Aviation, Space, and Environmental Medicine 2000; 71 (8): 843-848. [PubMed] [Google Scholar]
- 32). Lubin A, Hord DJ, Tracy ML, et al. Effects of exercise, bedrest and napping on performance decrement during 40 hours. Psychophysiology 1976; 13 (4): 334-339. [DOI] [PubMed] [Google Scholar]
- 33). Scheer FA, Shea TJ, Hilton MF, et al. An endogenous circadian rhythm in sleep inertia results in greatest cognitive impairment upon awakening during the biological night. Journal of Biological Rhythms 2008; 23 (4): 353-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Tremaine R, Dorrian J, Lack L, et al. The relationship between subjective and objective sleepiness and performance during a simulated night-shift with a nap countermeasure. Applied Ergonomics 2010; 42 (1): 52-61. [DOI] [PubMed] [Google Scholar]
- 35). Oriyama S, Miyakoshi Y, Kobayashi T. Influence of night shift work on nurses; Change in activity, sleepiness, fatigue and physiological indices during night shifts. Journal of the Japan Society for Healthcare Administration 2011; 48 (3): 147-156 (in Japanese). [Google Scholar]
- 36). Scott D, Rogers AE, Hwang WT, et al. Effects of critical care nurses' work hours on vigilance and patients' safety. American Journal of Critical Care 2006; 15 (1): 30-37. [PubMed] [Google Scholar]
- 37). Doi Y, Minowa M. Gender differences in excessive daytime sleepiness among Japanese workers. Social Science & Medicine 2003; 56 (4): 883-894. [DOI] [PubMed] [Google Scholar]
- 38). Härmä M. Individual differences in tolerance to shiftwork: a review. Ergonomics 1993; 36: 101-109. [DOI] [PubMed] [Google Scholar]
- 39). Asai T, Kaneita Y, Uchiyama M, et al. Epidemiological study of the relationship between sleep disturbances and somatic and psychological complaints among the Japanese general population. Sleep and Biological Rhythms 2006; 4: 55-62. [Google Scholar]