Abstract
We explored HPV vaccination intention and its determinants among male clients of the sexually transmitted infections (STI) clinic in Amsterdam. In 2015, male clients aged ≥18 years were invited to complete a web-based questionnaire regarding HPV vaccination intention and socio-psychological determinants. Determinants (scale −3 to +3) were assessed with linear regression, stratified for men who have sex with men (MSM) (including men who have sex with men and women) and men who only have sex with women (MSW). Additionally, we explored the effect of out-of-pocket payment on intention.
Of 1490 participants (median age 33 years [IQR:25–44]), 1,053(71%) were MSM. HPV vaccination intention was high (mean 1.68, 95%CI:1.55–1.81 among MSW; mean 2.35, 95%CI:2.29–2.42 among MSM). In multivariable analyses, socio-psychological determinants had similar effects on intention in both groups (R2=0.70 among MSW; R2=0.68 among MSM), except for subjective norms, self-efficacy, and HPV knowledge (significantly stronger associations among MSW). HPV vaccination intention decreased significantly when vaccination would require out-of-pocket payment; intention was negative at the current list price (€350). HPV vaccination intention among male clients of the Amsterdam STI-clinic is high and variance in intention was mostly be explained by socio-psychological factors. Out-of-pocket payment had a strong negative effect on HPV vaccination intention.
Keywords: HPV, Vaccination, Men, Intention, Out-of-pocket payment, MSM, The Netherlands
1. Introduction
More than 80% of sexually active people get infected with the human papillomavirus (HPV) at least once in their life time [1]. Persistent HPV infections can cause anogenital warts, and cervical-, vaginal-, vulvar-, anal-, penile-, and head-and-neck cancer. Vaccination is available and found to be effective and safe for the prevention of HPV infection and many of its precancerous and cancerous sequelae [2], [3], [4], [5], [6], [7]. In many countries, girls are vaccinated against HPV in order to prevent cervical cancer [8]. Vaccinating girls influences the HPV-related disease burden among women and, indirectly via herd immunity, among men who have sex with women [9], [10]. Men who have sex with men (MSM) do not profit from vaccinating girls, but they are at increased risk of HPV-induced cancer, notably anal cancer (incidence of 5.1 per 100,000 person-years among HIV-negative MSM, rising to 77.8 per 100,000 person-years among HIV-positive MSM in the HAART-era) [11].
In the Netherlands HPV vaccination is currently only offered to girls in the year they become 13 years of age. From 2014 onwards a full course of vaccination among 12–13 year old girls requires two doses. The uptake has been low, approximately 60% [12], providing limited herd protection for men who have sex with women [9], [10]. A study on the effect of gender-neutral HPV vaccination in the Netherlands suggested that it could be cost-effective to offer HPV vaccination to boys and girls in the year they turn 13 years old [9]. An alternative strategy would be to start with targeted vaccination, in which the HPV vaccination is offered to men at Sexually Transmitted Infections (STI) clinics, like is being introduced in the UK [13], [14]. Previous studies indicate that the intention to receive an HPV vaccination differs widely among MSM and MSW (men who have sex with only women); ranging from 36% to 88% in MSM [15], [16], [17], [18], [19], [20], [21], [22] and from 37% to 70% in MSW [23], [24], [25]. Additionally, MSM seem to have a higher intention to receive HPV vaccination compared to MSW [25], [26]. In the Netherlands it is currently unknown to what degree men would be interested in getting vaccinated against HPV, and whether targeted vaccination would need different communication strategies for MSW and MSM. The aim of this study was to investigate the acceptability of HPV vaccination and determinants of vaccination intention among male clients, separate for MSW and MSM, of the STI clinic of the Public Health Service in Amsterdam, the Netherlands.
2. Methods
2.1. Study participants
From June through October 2015, male clients of the STI clinic of the Public Health Service in Amsterdam were invited to participate in the HP4V (human papillomavirus preparedness for vaccination) study. Male clients were invited to participate at three separate time points before, during, and after their visit to the STI clinic: in their appointment confirmation email, through a flyer handed out at the reception at the entrance of the STI clinic, and in the email in which they received their STI-test results.
Men were eligible for participation if they were aged 18 years or older and conversant in Dutch or English. After completing the questionnaire, men received 10 condoms to thank them for their participation. Participants provided informed consent by completing the questionnaire. Ethical clearance for this study was obtained from the Medical Ethics Committee of the Academic Medical Center (AMC) [A1 15 0120].
2.2. Data collection
Participants completed an anonymous self-administered web-based questionnaire addressing HPV vaccination intention, socio-psychological determinants of HPV vaccination intention, HPV knowledge, socio-demographic characteristics, health related characteristics and sexual behavior characteristics. Participants were able to complete the questionnaire, either on their mobile phone or on a computer, in approximately 10 min.
2.3. Questionnaire
Prior to the survey, in April and May 2015, five one-on-one interviews focusing on HPV vaccination intention and related perceptions were conducted with male clients of the STI clinic in Amsterdam. Based on the one-on-one interviews, a standardized questionnaire on HPV vaccination decision making was adapted for this study population [27], [28], [29]. The Reasoned Action Approach [30], [31], [32], Social Cognitive Theory [33], and the Health Belief Model [34] provided the framework for hypothesizing the pathways influencing HPV-vaccination decision making. Within this framework, intention is considered to directly impact actual behavior and was therefore used as a surrogate marker for behavior, as HPV vaccination is not routinely offered to males (yet). Intention was assessed with two 7-point Likert-type items (ranging from −3 up to +3) and combined to one score (Cronbach α=0.95). Most constructs were assessed by multiple Likert-type scaled items (see Supplementary Table 1 for more detail). Composite scores of constructs measured with multiple items were only computed if Cronbach's Alpha exceeded 0.60 (Supplementary Table 1). Demographic characteristics measured included age and educational level. Sexual behavior characteristics included the number of male and female sex partners in the preceding 6 months and lifetime. Health related characteristics included medical history (HIV-status and history of anogenital warts) and HPV related variables (knowledge about HPV, friends with a history of anogenital warts, friends with (a history of) penile/anal cancer).
2.4. Impact of out-of-pocket payment
We asked the intention to get vaccinated after all the socio-psychological questions were posed, and again after the concept of out-of-pocket payment for HPV vaccination was introduced. The study period was divided in four time periods of approximately six weeks. Dependent on the period of inclusion a different amount of out-of-pocket payment (€50; €100; €200; €350) was proposed.
2.5. Stratification based on sexual behavior
To assess whether MSW and MSM would profit from a tailored HPV vaccination campaign, we performed separate analyses for MSW and MSM. Based on reported sexual behavior in the preceding 6 months two risk groups were created: those who reported at least one male sex partner in the preceding 6 months were categorized as MSM, whereas those reporting sex with only female partners in the preceding 6 months were categorized as MSW. In total, 98 (7%) of all participants reported sexual contact with at least one male and one female partner in the preceding six months, this is 9.3% of the MSM population (N=1053). All individuals reported at least one sexual partner.
2.6. Statistical analyses
We assessed which proportion of male clients participated in the study. We compared the main demographics of the total population of male clients who attended the STI clinic during the study period to those participating, using Chi-squared tests and Wilcoxon rank sum tests.
Baseline characteristics of the study population and HPV vaccination intention were explored using descriptive statistics. Univariate associations between socio-psychological factors, socio-demographic characteristics, sexual risk behavioral and health related characteristics with the outcome were computed by Pearson's r for continuous social-psychological variables, Chi-squared tests for categorical variables, and Wilcoxon rank sum tests for continuous sexual behavioral variables.
Two-step multivariable linear regression analyses were used to assess the determinants of HPV vaccination intention when the vaccination was offered free of charge. In the first step, all socio-psychological variables that were associated (at P<0.2, Wald test) with intention in the univariate analyses were included in multivariable analyses. A backward selection procedure was performed, leading to a model that included socio-psychological determinants significantly associated with HPV vaccination intention (at P<0.05). In the second step, sociodemographic characteristics, health related characteristics, and sexual behavioral characteristics (univariately associated with intention at P<0.20) were added to the model obtained in step one, using a backward selection procedure. Regardless of the effect of socio-demographic characteristics, health related characteristics, and sexual behavioral characteristics on the socio-psychological determinants, socio-psychological determinants obtained in the first step remained in the model. Multicollinearity was tested by assessing the variance inflation factor of all variables included in the multivariable model. Finally, determinants found to be significantly associated with intention in the final model including MSW and in the final model including MSM where combined into one model. In this combined model we tested for interaction between these determinants and risk group. The rationale for testing for interaction was to assess whether MSW and MSM need a tailored HPV vaccination campaign.
Furthermore, we assessed the impact of a gradual increase in the amount of out-of-pocket payment on intention in multivariable analyses. For this analyses we created a dataset containing two outcome variables for each participant: one relating to vaccination intention when offered free of charge, and one relating to vaccination intention in the case of a paid HPV vaccination. This dataset contained correlated data (two records for each participant), therefore generalized estimating equations were used in univariate and multivariable linear regression analysis, using an exchangeable correlation structure and robust variance. Finally, we assessed the impact of determinants on intention in the model including out-of-pocket payment.
For all variables, continuous data, if available, were used, with the exception of age. Age was categorized in age ≤26 years, 27–40 years, and ≥41 years, because of proven safety and efficacy of HPV vaccination among males ≤26 years of age [3], [4]. All analyses were performed using Stata (version 13.1; Stata Corp, College Station, Texas, USA).
3. Results
3.1. Study participation
Of the 9,301 male clients that attended the clinic in the study period (41% MSW and 59% MSM), 1,490 (16%) participated. The participation rate was 11% among MSW and 20% among MSM. Participants were significantly older (median: 33 years [IQR: 25–44] vs. 29 [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40]), more educated (83% vs. 73% high education), more likely to be MSM (71% vs. 57%), had a higher median number of sex partners (6 [IQR: 4–12] vs. 5[IQR:3–10]), and were more often HIV positive (13% vs. 11%) than the total male clinic population (all P-values <0.001; Supplementary Table 2).
3.2. Characteristics of study population
Of the 1,490 participants, 1,053 (71%) were MSM and 437 (29%) MSW. The median age of the participants was 33 years (IQR: 25–44); MSW were younger than MSM (median age of 25 vs. 38 years; P<0.001) (Table 1). Most participants had a high educational level (83%). MSM reported significantly more sex partners in the preceding six months compared to MSW (median 9 [IQR: 5–18] vs. median 5 [IQR: 3–7]; P<0.001), as well as a higher lifetime number of sex partners (median 100 [IQR: 38–400] vs. median 23 [IQR: 10–43]; P<0.001). Thirteen percent of the participants was HIV-positive (1% of MSW and 18% of MSM; P<0.001).
Table 1.
Baseline characteristics of the study population (N=1,490), overall and stratified by risk group (HP4V men study, Amsterdam 2015).
| Total | MSW | MSM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| (n=1490) |
(n=437) |
(n=1053) |
||||||
| No. | % | No. | % | No. | % | |||
| Socio-demographic characteristics | <0.001 | |||||||
| Median age (IQR) in years | 33 | (25–44) | 25 | (22–29) | 38 | (28–47) | ||
| Age | <0.001 | |||||||
| ≤26 years | 485 | 32 | 263 | 60 | 222 | 21 | ||
| 27–40 years | 534 | 36 | 153 | 35 | 381 | 36 | ||
| ≥41 years | 471 | 32 | 21 | 5 | 450 | 43 | ||
| Educational level | 0.314 | |||||||
| Lower education | 246 | 17 | 79 | 18 | 167 | 16 | ||
| High education | 1,235 | 83 | 357 | 82 | 878 | 84 | ||
| Medical history | ||||||||
| HIV status | <0.001 | |||||||
| HIV-negative | 1,054 | 71 | 313 | 72 | 741 | 70 | ||
| HIV-positive | 194 | 13 | 6 | 1 | 188 | 18 | ||
| Unknown or not willing to disclose | 242 | 16 | 118 | 27 | 124 | 12 | ||
| History of anogenital warts | <0.001 | |||||||
| Yes | 450 | 30 | 78 | 18 | 372 | 35 | ||
| No | 1040 | 70 | 359 | 82 | 681 | 65 | ||
| Sexual behavior | ||||||||
| Median no. of sex partners (preceding 6 months) (IQR) | 6 | (4–12) | 5 | (3–7) | 9 | (5–18) | <0.001 | |
| Median no. of lifetime sex partners (IQR) | 55 | (22–201) | 23 | (10–43) | 100 | (38–400) | <0.001 | |
| HPV related variables | ||||||||
| Knowledge about HPV (0–7) (IQR) | 5 | (4–6) | 5 | (4–6) | 6 | (5–7) | <0.001 | |
| Friends with history of anogenital warts | <0.001 | |||||||
| No | 945 | 63 | 325 | 74 | 620 | 59 | ||
| Yes | 545 | 37 | 112 | 26 | 433 | 41 | ||
| Friends with (a history of) anal/penile cancer | <0.001 | |||||||
| No | 1344 | 90 | 423 | 97 | 921 | 88 | ||
| Yes | 146 | 10 | 14 | 3 | 132 | 13 | ||
| Mean HPV vaccination intention (95% CI) (range −3 to 3) | 2.15 | (2.09–2.22) | 1.68 | (1.55–1.81) | 2.35 | (2.29–2.42) | <0.001 | |
| HPV vaccination intention | <0.001 | |||||||
| Certainly not (−3) | 17 | 1 | 7 | 2 | 10 | 1 | ||
| Probably not (−2) | 12 | 1 | 7 | 2 | 5 | 1 | ||
| Maybe not (−1) | 33 | 2 | 21 | 5 | 12 | 1 | ||
| Maybe/Maybe not (0) | 86 | 6 | 46 | 11 | 40 | 4 | ||
| Maybe (+1) | 167 | 11 | 75 | 17 | 92 | 9 | ||
| Probably (+2) | 298 | 20 | 96 | 22 | 202 | 19 | ||
| Definitely (+3) | 877 | 59 | 185 | 42 | 692 | 66 | ||
Data was missing for: educational level (n=9).
Educational level is measured according to the Dutch education levels. Participants were asked what their highest completed level of education was. They could choose: no educational degree (did not finish any education), Primary school, Lower general secondary school, Higher general secondary school, Preuniversity school, Secondary vocational school, Higher vocational school, University, Different. Lower education contains: no educational degree, Primary school, Lower general secondary school, Higher general secondary school and Secondary vocational school. Higher education included: Preuniversity school, Higher vocational school, and University.
Abbreviations: IQR=Interquartile range; HIV=human immunodeficiency virus; HPV=human papillomavirus; MSW=men who have sex with only women; MSM=men who have sex with men.
3.2.1. Vaccination intention
Vaccination intention among the participants was very high with a mean vaccination intention of 2.15 (95%CI: 2.09–2.22; range −3 up to +3). MSW had a lower vaccination intention score (mean=1.68; 95%CI: 1.55–1.81) compared to MSM (mean=2.35; 95%CI: 2.29–2.42; P<0.001) (Table 1).
3.2.2. Univariate linear regression analyses
Univariate linear regression analyses stratified by risk group (MSW vs. MSM) are presented in Table 2. The following factors were significantly associated with HPV-vaccination intention among MSW: all socio-psychological determinants, knowledge about HPV, a history of anogenital warts, and having friends with a history anogenital warts. Among MSM, all socio-psychological determinants, all socio-demographic characteristics, and sexual behavioral variables, were found to be significantly associated with intention, except educational level, having friends with (a history of) anogenital warts, and having friends with (a history of) anal or penile cancer (Table 2).
Table 2.
Association between socio-psychological determinants, socio-demographic characteristics, health related characteristics, and sexual behavioral characteristics with vaccination intention by risk group (HP4V men study, Amsterdam 2015).
| MSW (N=437) |
MSM (N=1053) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable linear regression analysis |
Multivariable linear regression analysisa |
Univariable linear regression analysis |
Multivariable linear regression analysisa |
||||||||||
| Beta | (95%CI) | P-value | Beta | (95%CI) | P-value | Beta | (95%CI) | P-value | Beta | (95%CI) | P-value | ||
| Sociopsychological determinants | |||||||||||||
| Risk perception | 0.30 | (0.21–0.40) | <0.001 | 0.20 | (0.16–0.25) | <0.001 | |||||||
| Attitude towards HPV vaccination | 0.90 | (0.83–0.97) | <0.001 | 0.57 | (0.49–0.66) | <0.001 | 0.81 | (0.78–0.85) | <0.001 | 0.66 | (0.61–0.71) | <0.001 | |
| Negative outcome expectancies of HPV vaccination | 0.24 | (0.15–0.33) | <0.001 | 0.15 | (0.10–0.19) | <0.001 | −0.03 | (−0.06 to 0.00) | 0.031 | ||||
| Anticipated regret about rejecting HPV vaccination | 0.55 | (0.44–0.65) | <0.001 | 0.12 | (0.05–0.19) | 0.001 | 0.47 | (0.42–0.52) | <0.001 | 0.09 | (0.05–0.13) | <0.001 | |
| Beliefs about HPV vaccination | 0.22 | (0.12–0.32) | <0.001 | 0.11 | (0.05–0.17) | <0.001 | |||||||
| Subjective norm towards HPV vaccination | 0.55 | (0.45–0.66) | <0.001 | 0.12 | (0.05–0.19) | 0.002 | 0.38 | (0.32–0.43) | <0.001 | ||||
| Descriptive norm towards HPV vaccination | 0.74 | (0.62–0.88) | <0.001 | 0.11 | (0.01–0.21) | 0.024 | 0.49 | (0.43–0.56) | <0.001 | 0.10 | (0.06–0.15) | <0.001 | |
| Self-efficacy expectations towards HPV vaccination | 0.61 | (0.54–0.68) | <0.001 | 0.29 | (0.23–0.35) | <0.001 | 0.52 | (0.45–0.58) | <0.001 | 0.19 | (0.15–0.24) | <0.001 | |
| Socio-demographic characteristics | |||||||||||||
| Age | 0.128 | 0.019 | |||||||||||
| ≤26 years | REF | REF | REF | REF | |||||||||
| 27–40 years | 0.26 | (−0.02 to 0.54) | −0.14 | (−0.31 to 0.03) | 0.103 | 0.26 | (0.08–0.43) | 0.09 | (−0.02 to 0.19) | 0.111 | |||
| ≥41 years | 0.38 | (−0.024 to 1.00) | −0.43 | (−0.79 to −0.07) | 0.021 | 0.17 | (−0.00 to 0.34) | −0.02 | (−0.13 to 0.09) | 0.711 | |||
| Educational level | 0.150 | 0.753 | |||||||||||
| Lower education | REF | REF | |||||||||||
| High education | 0.25 | (−0.09 to 0.59) | −0.03 | (−0.20 to 0.15) | |||||||||
| Health related characteristics | |||||||||||||
| HIV status | 0.405 | <0.001 | |||||||||||
| HIV-negative | REF | REF | |||||||||||
| HIV-positive | 0.73 | (−0.41 to 1.87) | 0.37 | (0.20–0.54) | |||||||||
| Unknown or not willing to disclose | −0.05 | (−0.35 to 0.24) | −0.21 | (−0.42 to −0.02) | |||||||||
| History of anogenital warts | <0.001 | 0.007 | |||||||||||
| No | REF | REF | |||||||||||
| Yes | 0.75 | (0.41–1.09) | 0.18 | (0.05–0.32) | |||||||||
| Sexual behavior | |||||||||||||
| Number of sexpartners in preceding 6 monthsb | 0.00 | (−0.17 to 0.18) | 0.993 | 0.08 | (0.01–0.15) | <0.001 | |||||||
| Lifetime number of sexpartnersb,c | 0.11 | (0.00–0.23) | 0.059 | 0.07 | (−0.00 to 0.14) | 0.051 | 0.09 | (0.06–0.13) | <0.001 | 0.03 | (0.01–0.05) | 0.018 | |
| HPV related variables | |||||||||||||
| Knowledge about HPV | 0.17 | (0.10–0.23) | <0.001 | 0.06 | (0.02 to 0.10) | 0.005 | 0.06 | (0.02–0.09) | 0.003 | ||||
| Friends with a history anogenital warts | 0.017 | 0.100 | |||||||||||
| No | REF | REF | |||||||||||
| Yes | 0.37 | (0.07–0.67) | 0.11 | (−0.02 to 0.24) | |||||||||
| Friends with (a history of) anal/penile cancer | 0.921 | 0.194 | |||||||||||
| No | REF | REF | |||||||||||
| Yes | 0.38 | (−0.71 to 0.79) | 0.13 | (−0.07 to 0.33) | |||||||||
Abbreviations: HP4V=human papillomavirus preparedness for vaccination; CI=confidence interval; HIV=human immunodeficiency virus; HPV=human papillomavirus; MSW=men who have sex with only women only; MSM=men who have sex with men.
The multivariable analyses were executed by adding variables in two steps: (step 1) socio-psychological determinants, (step 2) medical history, sexual behavioral characteristics and HPV related variables. R squared (R2) is a measure that indicates how well the data fit a linear regression model. We found in MSW that after step 1 the R2 was 0.70 and after step 2 the R2 was 0.71. We found in MSM the R2 to be 0.68 after step 1 and this did not change after adding the variables significant in step 2.
For analyses purposes lifetime number of sexual partner was log transformed.
in MSW: number of female sex partners; in MSM: number of male sex partners.
3.2.3. Multivariable linear regression analyses
In multivariable linear regression analysis we found that among MSW attitude was one of the strongest predictors of HPV vaccination intention (Beta 0.57; 95%CI 0.49–0.66). Additionally, self-efficacy (Beta 0.29; 95%CI 0.23–0.35), anticipated regret (Beta 0.12; 95%CI 0.05–0.19), subjective norm (Beta 0.12; 95%CI 0.05–0.19), and descriptive norm (Beta 0.11; 95%CI 0.01–0.21) significantly improved the model, which altogether had a high degree of explained variance (R2=0.70). When demographics and sexual behavior variables were added, age, lifetime number of sex partners, and knowledge about HPV increased the explained variance in MSW somewhat (R2=0.71) (Table 2). Multicollinearity was not a problem for this model, since the variance inflation factor was <2.0 for all variables.
Also among MSM, attitude showed the strongest association with HPV vaccination intention (Beta 0.66; 95%CI 0.61–0.71; P<0.001). In addition to attitude, self-efficacy (Beta 0.19; 95%CI 0.15–0.24), descriptive norm (Beta 0.10; 95%CI 0.06–0.15), anticipated regret (Beta 0.09; 95%CI 0.05–0.13), and negative outcome expectancies (Beta −0.03; 95%CI −0.06 to 0.00) were significantly associated with HPV vaccination intention. Together, these socio-psychological determinants had a high explained variance (R2=0.68). Age and the lifetime number of sexual partners were also associated with HPV vaccination intention among MSM but did not increased the explained variance (R2=0.68) (Table 2). The variance inflation factor was <2.0 for all variables, indicating that multicollinearity was not posing problems in this model.
To assess whether the association of this final set of determinants of intention varied by risk group we tested for interaction in a model combining MSW and MSM. Most determinants had similar effects on intention, except for subjective norm (P=0.002), self-efficacy (P=0.002) and knowledge (P<0.001) which all three had a stronger effect in the MSW group than in the MSM group.
We also analyzed predictors of intention in a model combining MSW and MSM. Overall a similar pattern of important determinants was seen in stratified analysis. Notably, sexual behavior, being MSM or MSW, was statistically significant associated with HPV vaccination intention in the multivariable model (Supplementary Table 3).
3.3. The effect of payment on vaccination intention
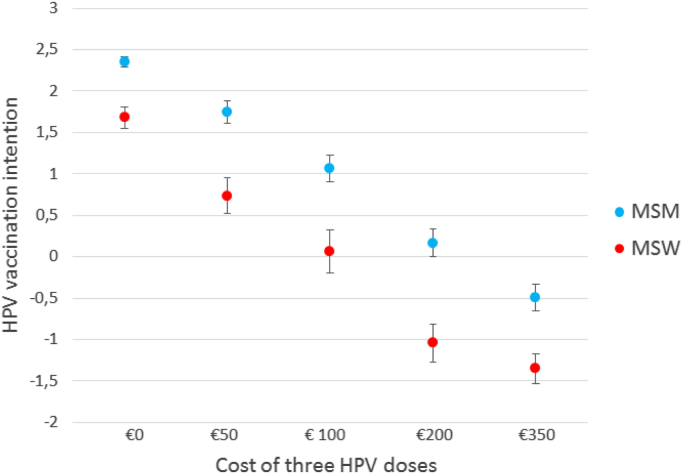
All socio-psychological questions up to the payment question implied that HPV vaccination would be free of charge (as is currently the case in the Netherlands for all 12–13 year old girls). During four different study periods different amount of out-of-pocket payments were proposed. Participants from these four periods were comparable concerning demographics, sexual behavioral characteristics, and HPV vaccination intention (data not shown). For each subsequent increase in the required amount of out-of-pocket payment for HPV vaccination, the mean HPV vaccination intention decreased significantly (Fig. 1). The mean vaccination intention among MSW was: €0: 1.68 (95%CI 1.55–1.81); €50: 0.73 (95%CI 0.52–0.95); €100: 0.06 (95%CI −0.20 to 0.32); €200: −1.04(95%CI −1.27 to −0.81); €350: −1.35 (95%CI −1.53 to −1.17). The mean vaccination intention among MSM was: €0: 2.35 (95%CI 2.29–2.42); €50: 1.75 (95%CI 1.61–1.88); €100: 1.07 (95%CI 0.90–1.23); €200: 0.16(95%CI 0.00–0.33); €350: −0.49 (95%CI −0.65 to −0.32). The drop in intention was significantly stronger among MSW compared to MSM (P=0.003). Interestingly, the intention became negative if the three doses would cost €200 or more in the MSW group (mean −1.04: 95%CI: −0.81 to −1.27) and became negative in the MSM group when the proposed cost was €350 (mean −0.49: 95%CI: −0.32 to −0.65). The effect of out-of-pocket payment on intention was similar in univariate analysis and in multivariable analysis (when it was added to the model obtained previously) (Supplementary Table 4). All other associations remained similar, except for age among MSM which had a significantly stronger impact on intention when compared to the model when HPV vaccination was offered free of charge.
Fig. 1.
Mean (95% CI) HPV vaccination intention by amount of out-of-pocket payment of three HPV vaccination doses. Mean vaccination intention among MSW €0: 1.68 (95%CI 1.55–1.81); €50: 0.73 (95%CI 0.52–0.95); €100: 0.06 (95%CI 0.32 to −0.20); €200: −1.04(95%CI −0.81 to −1.27); €350: −1.35 (95%CI −1.17 to −1.53). Mean vaccination intention among MSM €0: 2.35 (95%CI 2.29–2.42); €50: 1.75 (95%CI 1.61–1.88); €100: 1.07 (95%CI 0.90–1.23); €200: 0.16(95%CI 0.00–0.33); €350: −0.49 (95%CI −0.32 to −0.65).
4. Discussion
HPV vaccination intention among male clients of the STI clinic in Amsterdam, the Netherlands, was high: a mean vaccination intention of 2.15 (95%CI: 2.09–2.22). Furthermore, MSM (including both men who have sex with only men and men who have sex with both men and women) included in this study had a higher vaccination intention than included MSW. This study revealed that attitude towards HPV vaccination and self-efficacy (to what extent does one consider oneself able to come to the clinic three times to get vaccinated) were the most important determinants of HPV vaccination intention among both MSW and MSM. Socio-psychological determinants explained the variance of HPV vaccination intention among male clients of the STI outpatient clinic in Amsterdam to a large degree. Overall, most determinants had the same effect on intention in the MSW and MSM groups, except for subjective norm, self-efficacy, and knowledge which all three were stronger in the MSW group than in the MSM group. When intention was assessed in the situation where an out-of-pocket payment would be required, HPV vaccination intentions decreased drastically. At the current list price (around €350 for a complete series of three vaccinations), the mean vaccination intention was negative for both MSW and MSM.
This study showed a higher HPV vaccination intention (90%) compared to other studies exploring the intention to receive the HPV vaccination when vaccination is offered free of charge (range 36–88%) [15], [16], [17], [18], [19], [20], [21], [22]. Nadarzynski et al. [22] attributed the lower intention to the limited awareness of HPV. In the present study, knowledge was generally high and had a significant effect on intention in the MSW group, yet no effect in the MSM group was found which may reflect a lack of awareness among a substantial proportion in this group. Furthermore, our results confirm prior research showing a higher vaccination intention among MSM compared to MSW [25], [26].
We confirm that socio-psychological determinants are the most important determinants of HPV vaccination intention [28], [29], [35], [36], [37]. Differences across studies in strength of socio-psychological determinants on the association with HPV vaccination intention could be explained by cultural differences of study populations and differences in the way vaccinations are typically offered in countries (e.g. costs, opportunistic/organized, opt-out/in). Furthermore, as found in earlier studies [28], [29], [35], [36], [37], probing the whole spectrum of socio-psychological determinants influencing intention, attitude appeared to be one of the strongest predictors of HPV vaccination intention among both MSW and MSM. In contrast to what we have found, self-efficacy is less often found to play a crucial role on HPV vaccination intention in other studies, while social influences are found to be important determinants [24], [37], [38]. Other attitudinal constructs like outcome beliefs and anticipated regret were also significantly associated but their associations were less strong. As could be expected from theory, these determinants overlapped with general attitude to a great extent, yet they remain important targets for improving HPV vaccination related attitudes.
In contrast to what has been found in previous studies on vaccination acceptability in the Netherlands [28], [39], we also found self-efficacy to be a strong predictor of intention. However, this can be considered as expected, as going to the STI outpatient clinic three times to get a vaccination may pose a barrier as it can be time consuming or STI clinic visits could be perceived as embarrassing.
Not surprisingly, out-of-pocket payment had a significantly strong negative effect on HPV vaccination intention in both groups. The decrease in intention to vaccinate when out-of-pocket payment was proposed has been well established in previous studies in men [16], [17], [18], [20], [40]. When introducing costs for HPV vaccination, socio-psychological factors remained important. To our knowledge, only one other study analyzed determinants of HPV vaccination intention among men based on a certain payment for vaccination. Lau et al. [20] found that knowledge about HPV, perceived severity, and perceived vaccine efficacy were associated with HPV vaccination intention given a price of HK$1000–2000 (US$ 128–256) per shot and a total of 3 shots within six months. The increasing intention with increasing age found among MSM when out-of-pocket payment was required, can be explained by generally higher socio-economic status with increasing age, and therefore, payment for healthcare becomes more affordable at an older age. However, this increase in intention with age could also be explained by an increased risk perception for acquiring HPV-related diseases in (older) MSM, increasing the willingness to pay to avoid those diseases. Unfortunately, the chance that an individual has encountered a previous HPV infection increases with age, reducing the effectiveness of the prophylactic HPV vaccine [41].
4.1. Strengths
The strengths of this study include the large sample size, the use of a well-tested theory based questionnaire, and the relevant clinical sample of MSM and MSW attending STI clinics, allowing us to explore a diverse palette of social psychological factors influencing HPV vaccination intention. Furthermore, our final multivariable models had a large explained variance of intention (R2 of 0.71 in MSW and 0.67 in MSM), which suggests that the questionnaire used was able to cover the most important predictors of intention. Next, in contrast to most studies, we were able to test whether MSW and MSM should be approached differently when developing an HPV vaccination intervention (which we found was not necessary). Finally, this is one of the first studies that tested the effect of different amounts of out-of-pocket payment on HPV vaccination intentions, stratified by risk group.
4.2. Limitations
The response rate was relatively low (16%). This can be explained by the passive recruitment methods used in this study. Additionally, the study population differed from the total population of male clients of the STI clinic in various demographic characteristics and in sexual behavioral characteristics. Individuals participating in questionnaire-based research at the STI outpatient clinic in Amsterdam are more often higher educated [42], [43], [44] than the total client population. If selection bias occurred, this may have biased the observed mean scores upwards, but it will probably not have impacted the association between determinants and intention. Additionally, income was not assessed in this study. Income could have helped explain variance in HPV vaccination intention, especially when considering the effect of pricing on HPV vaccination intention. Lastly, this study was based on a hypothetical scenario since HPV vaccination for men is currently not offered in the Netherlands, although this may change in the future. Despite its hypothetical nature, this study may help to inform future planning strategies when introducing targeted HPV vaccinations.
4.3. Implications
In the Netherlands HPV vaccination is currently only offered free of charge to girls in the year they turn 13 years of age. Future inclusion of boys in this vaccination program is unclear, despite the predicted benefit of vaccinating boys along with girls when the vaccination coverage among girls remains at 60% [9]. However, Bogaards et al. also emphasize the high burden of anal cancer among MSM that will not be prevented even if a high HPV vaccination coverage among girls would be reached [9]. Targeted vaccination of men, especially MSM, visiting the STI clinic may therefore still be an approach to consider, even more so as we showed that intention to become vaccinated is high among men that visit the STI clinic. Therefore, high uptake can be expected if the HPV vaccination is offered free of charge. This tentative conclusion can first be evaluated by a pilot project among men visiting STI clinics in the Netherlands, to demonstrate whether offering HPV vaccination at STI clinics in the Netherlands would indeed lead to a high coverage among men at high risk. Challenges in implementing HPV vaccination among men that have been pointed out in previous studies suggest a focus on educating men about HPV infection, prevention, transmission, morbidity, and mortality is required. Emphasis should also be on health care providers to actively offer HPV vaccination to males. However, this requires men to openly identify themselves as MSM, which is a societal challenge [45], [46]. This study provides insight into what determinants should be targeted by educational interventions promoting HPV vaccination uptake among men at high risk for HPV and suggests that similar determinants can be used for both MSW and MSM, albeit with additional attention for the MSW when it comes to compliance (visiting the STI clinic three times to receive all three HPV vaccinations).
5. Conclusion
HPV vaccination intention among male clients of the Amsterdam STI clinic is very high. Most of the variance in HPV vaccination intention among men can be explained by socio-psychological factors and these determinants were largely similar in MSM and in MSW. In contrast to previous HPV vaccination acceptability studies in the Netherlands, intention was not merely attitudinally driven, but also self-efficacy played a key role. Out-of-pocket payment has a strong negative impact on HPV vaccination intention. These results suggest that if HPV vaccination would be offered to men at STI clinics free of charge, uptake of the HPV vaccination would be high.
Conflicts of interest
The institution of M. F. Schim van der Loeff received study funding from Sanofi Pasteur MSD; he is a co-investigator in a Merck-funded investigator-initiated study; he is an investigator on a Sanofi Pasteur MSD sponsored trial; he served on a vaccine advisory board of GSK; his institution received in-kind contribution for an HPV study from Stichting Pathologie Onderzoek en Ontwikkeling (SPOO); his institution receives research funding from Janssen Infectious Diseases and Vaccines. H.J.C. de Vries is a investigator in a Sanofi Pasteur MSD sponsored HPV vaccine trial.
Acknowledgements
We gratefully acknowledge all study participants for their co-operation. Furthermore, the authors would like to thank the staff of the STI outpatient clinic in Amsterdam for their valuable contribution to data collection, in particular, Jacqueline Woutersen and Claudia Owusu, for contributing to the implementation of this study; and Martijn van Rooijen for assisting in data management. This work was supported by funding from the Public Health Service Amsterdam.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.pvr.2016.11.001.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Chesson H.W., Dunne E.F., Hariri S., Markowitz L.E. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex. Transm. Dis. 2014;41(11):660–664. doi: 10.1097/OLQ.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hildesheim A., Herrero R., Wacholder S., Rodriguez A.C., Solomon D., Bratti M.C. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298(7):743–753. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 3.Giuliano A.R., Palefsky J.M., Goldstone S., Moreira E.D., Jr., Penny M.E., Aranda C. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N. Engl. J. Med. 2011;364(5):401–411. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palefsky J.M., Giuliano A.R., Goldstone S., Moreira E.D., Jr, Aranda C., Jessen H. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N. Engl. J. Med. 2011;365(17):1576–1585. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 5.Joura E.A., Giuliano A.R., Iversen O.E., Bouchard C., Mao C., Mehlsen J. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N. Engl. J. Med. 2015;372(8):711–723. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 6.Apter D., Wheeler C.M., Paavonen J., Castellsague X., Garland S.M., Skinner S.R. Efficacy of human papillomavirus 16 and 18 (HPV-16/18) AS04-adjuvanted vaccine against cervical infection and precancer in young women: final event-driven analysis of the randomized, double-blind PATRICIA trial. Clin. Vaccine Immunol. 2015;22(4):361–373. doi: 10.1128/CVI.00591-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreimer A.R., Struyf F., Del Rosario-Raymundo M.R., Hildesheim A., Skinner S.R., Wacholder S. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA trials. Lancet Oncol. 2015;16(7):775–786. doi: 10.1016/S1470-2045(15)00047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruni L., Diaz M., Barrionuevo-Rosas L., Herrero R., Bray F., Bosch F.X. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob. Health. 2016;4(7):e453–e463. doi: 10.1016/S2214-109X(16)30099-7. [DOI] [PubMed] [Google Scholar]
- 9.Bogaards J.A., Wallinga J., Brakenhoff R.H., Meijer C.J., Berkhof J. Direct benefit of vaccinating boys along with girls against oncogenic human papillomavirus: bayesian evidence synthesis. BMJ. 2015;350:h2016. doi: 10.1136/bmj.h2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drolet M., Benard E., Boily M.C., Ali H., Baandrup L., Bauer H. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect. Dis. 2015;15(5):565–580. doi: 10.1016/S1473-3099(14)71073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machalek D.A., Poynten M., Jin F., Fairley C.K., Farnsworth A., Garland S.M. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012;13(5):487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- 12.van Lier E.A., Oomen P.J., Giesbers H., Conyn-van Spaendonck M.A.E., Drijfhout I.H., Zonnenberg-Hoff I.F. Rijksinstituut voor Volksgezondheid en Milieu; Bilthoven: 2015. Vaccinatiegraad rijksvaccinatieprogramma Nederland - Verslagjaar 2015. [Google Scholar]
- 13.JCVI . Government of United Kingdom; 2015. Joint Committee on Vaccination and Immunisation statement on HPV Vaccination of Men who Have Sex with Men. [Google Scholar]
- 14.The Scottish Governmnet - Riaghaltas na h-Alba (2016). "HPV vaccination programme." from 〈http://news.scotland.gov.uk/News/HPV-vaccination-programme-24a0.aspx〉.
- 15.Reiter P.L., Brewer N.T., McRee A.L., Gilbert P., Smith J.S. Acceptability of HPV vaccine among a national sample of gay and bisexual men. Sex. Transm. Dis. 2010;37(3):197–203. doi: 10.1097/OLQ.0b013e3181bf542c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sundstrom K., Tran T.N., Lundholm C., Young C., Sparen P., Dahlstrom L.A. Acceptability of HPV vaccination among young adults aged 18–30 years--a population based survey in Sweden. Vaccine. 2010;28(47):7492–7500. doi: 10.1016/j.vaccine.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez B.Y., Wilkens L.R., Thompson P.J., Shvetsov Y.B., Goodman M.T., Ning L. Acceptability of prophylactic human papillomavirus vaccination among adult men. Hum. Vaccine. 2010;6(6):467–475. doi: 10.4161/hv.6.6.11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wheldon C.W., Daley E.M., Buhi E.R., Nyitray A.G., Giuliano A.R. Health beliefs and attitudes associated with HPV vaccine intention among young gay and bisexual men in the Southeastern United States. Vaccine. 2011;29(45):8060–8065. doi: 10.1016/j.vaccine.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 19.Rank C., Gilbert M., Ogilvie G., Jayaraman G.C., Marchand R., Trussler T. Acceptability of human papillomavirus vaccination and sexual experience prior to disclosure to health care providers among men who have sex with men in Vancouver, Canada: implications for targeted vaccination programs. Vaccine. 2012;30(39):5755–5760. doi: 10.1016/j.vaccine.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Lau J.T., Wang Z., Kim J.H., Lau M., Lai C.H., Mo P.K. Acceptability of HPV vaccines and associations with perceptions related to HPV and HPV vaccines among men who have sex with men in Hong Kong. PLoS One. 2013;8(2):e57204. doi: 10.1371/journal.pone.0057204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadarzynski T., Smith H., Richardson D., Jones C.J., Llewellyn C.D. Human papillomavirus and vaccine-related perceptions among men who have sex with men: a systematic review. Sex. Transm. Infect. 2014;90(7):515–523. doi: 10.1136/sextrans-2013-051357. [DOI] [PubMed] [Google Scholar]
- 22.Cummings T., Kasting M.L., Rosenberger J.G., Rosenthal S.L., Zimet G.D., Stupiansky N.W. Catching up or missing out? Human papillomavirus vaccine acceptability among 18- to 26-Year-old men who have sex with men in a US National sample. Sex. Transm. Dis. 2015;42(11):601–606. doi: 10.1097/OLQ.0000000000000358. [DOI] [PubMed] [Google Scholar]
- 23.Giuliani M., Vescio M.F., Dona M.G., Latini A., Frasca M., Colafigli M. Perceptions of Human Papillomavirus (HPV) infection and acceptability of HPV vaccine among men attending a sexual health clinic differ according to sexual orientation. Hum. Vaccine Immunother. 2016;12(6):1542–1550. doi: 10.1080/21645515.2015.1115935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ratanasiripong N.T. Factors related to Human Papillomavirus (HPV) Vaccination in college men. Public Health Nurs. 2015;32(6):645–653. doi: 10.1111/phn.12198. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert P., Brewer N.T., Reiter P.L., Ng T.W., Smith J.S. HPV vaccine acceptability in heterosexual, gay, and bisexual men. Am. J. Mens Health. 2011;5(4):297–305. doi: 10.1177/1557988310372802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newman P.A., Logie C.H., Doukas N., Asakura K. HPV vaccine acceptability among men: a systematic review and meta-analysis. Sex. Transm. Infect. 2013;89(7):568–574. doi: 10.1136/sextrans-2012-050980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waller J., Ostini R., Marlow L.A., McCaffery K., Zimet G. Validation of a measure of knowledge about human papillomavirus (HPV) using item response theory and classical test theory. Prev. Med. 2013;56(1):35–40. doi: 10.1016/j.ypmed.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 28.van Keulen H.M., Otten W., Ruiter R.A., Fekkes M., van Steenbergen J., Dusseldorp E. Determinants of HPV vaccination intentions among Dutch girls and their mothers: a cross-sectional study. BMC Public Health. 2013;13:111. doi: 10.1186/1471-2458-13-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.C. J.Alberts, M. F. S. v. d.Loeff, Y.Hazeveld, H. E. d.Melker, M. F. v. d.Wal, A.Nielen, et al. (2016). "A longitudinal study on determinants of HPV vaccination uptake in parents/guardians from different ethnic backgrounds in Amsterdam, the Netherlands." submitted. [DOI] [PMC free article] [PubMed]
- 30.Ajzen I. The theory of planned behaviour. Organ. Behav. Hum. Decis. Process. 1991;50:179–211. [Google Scholar]
- 31.I. Ajzen (2016). "TPB model." Retrieved 27-6-2016, 2016, from 〈http://people.umass.edu/aizen/tpb.html〉.
- 32.Fishbein M., Ajzen I. Psychology Press; New York: 2010. Predicting and Changing Behavior: The Reasoned Action Approach. [Google Scholar]
- 33.Bandura A. Prentice Hall; Englewoon Cliffs, NJ: 1986. Social Foundation of Thought and Action: A Social Cognitive Theory. [Google Scholar]
- 34.Becker M.H. The Health belief model and personal health behavior. Health Educ. Monogr. 1974;30:324–508. [Google Scholar]
- 35.Hofman R., van Empelen P., Richardus J.H., de Kok I.M., de Koning H.J., van Ballegooijen M. Predictors of HPV vaccination uptake: a longitudinal study among parents. Health Educ. Res. 2014;29(1):83–96. doi: 10.1093/her/cyt092. [DOI] [PubMed] [Google Scholar]
- 36.Wheldon C.W., Daley E.M., Walsh-Buhi E.R., Baldwin J.A., Nyitray A.G., Giuliano A.R. An integrative theoretical framework for hpv vaccine promotion among male sexual minorities. Am. J. Mens Health. 2016 doi: 10.1177/1557988316652937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisher W.A., Kohut T., Salisbury C.M., Salvadori M.I. Understanding human papillomavirus vaccination intentions: comparative utility of the theory of reasoned action and the theory of planned behavior in vaccine target age women and men. J. Sex. Med. 2013;10(10):2455–2464. doi: 10.1111/jsm.12211. [DOI] [PubMed] [Google Scholar]
- 38.Miller M.K., Wickliffe J., Jahnke S., Linebarger J., Humiston S.G. Views on human papillomavirus vaccination: a mixed-methods study of urban youth. J. Community Health. 2014;39(5):835–841. doi: 10.1007/s10900-014-9858-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paulussen T.G., Hoekstra F., Lanting C.I., Buijs G.B., Hirasing R.A. Determinants of Dutch parents' decisions to vaccinate their child. Vaccine. 2006;24(5):644–651. doi: 10.1016/j.vaccine.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 40.Zou H., Grulich A.E., Cornall A.M., Tabrizi S.N., Garland S.M., Prestage G. How very young men who have sex with men view vaccination against human papillomavirus. Vaccine. 2014;32(31):3936–3941. doi: 10.1016/j.vaccine.2014.05.043. [DOI] [PubMed] [Google Scholar]
- 41.Skinner S.R., Szarewski A., Romanowski B., Garland S.M., Lazcano-Ponce E., Salmeron J. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 4-year interim follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet. 2014;384(9961):2213–2227. doi: 10.1016/S0140-6736(14)60920-X. [DOI] [PubMed] [Google Scholar]
- 42.Alberts C.J., van Rooijen M.S., Prins M., Pawlita M., Schim van der Loeff M.F., Waterboer T. HIV is an important risk factor for human papillomavirus types 16 and 18 seropositivity among sexually active men who have sex with men. Sex. Transm. Dis. 2015;42(3):129–134. doi: 10.1097/OLQ.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 43.Matser A., Luu N., Geskus R., Heijman T., Heiligenberg M., van Veen M. Higher Chlamydia trachomatis prevalence in ethnic minorities does not always reflect higher sexual risk behaviour. PLoS One. 2013;8(6):e67287. doi: 10.1371/journal.pone.0067287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matser A., Heiligenberg M., Geskus R., Heijman T., Low N., Kretzschmar M. The importance of partnership factors and individual factors associated with absent or inconsistent condom use in heterosexuals: a cross-sectional study. Sex. Transm. Infect. 2014;90(4):325–331. doi: 10.1136/sextrans-2013-051087. [DOI] [PubMed] [Google Scholar]
- 45.Zimet G.D., Rosenthal S.L. HPV vaccine and males: issues and challenges. Gynecol. Oncol. 2010;117(2 Suppl):S26–S31. doi: 10.1016/j.ygyno.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 46.Shapiro G.K., Perez S., Rosberger Z. Including males in Canadian human papillomavirus vaccination programs: a policy analysis. CMAJ. 2016;188(12):881–886. doi: 10.1503/cmaj.150451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material