Abstract
The World Health Organization (WHO) serves as a key organization to bring together experts along the continuum of vaccine development and regulatory approval, among its other functions. Using the revision of WHO's guidelines on prophylactic human papillomavirus (HPV) vaccine as an example, we describe the process by which (1) a need to revise the guidelines was identified; (2) a group of stakeholders with complementary expertise and key questions were identified; (3) a scientific review was conducted; (4) consensus on revisions was achieved; (5) guidelines were updated, reviewed widely, and approved. This multi-year process resulted in the consensus that regulatory agencies could consider additional endpoints, such as persistent HPV infection or immune equivalence, depending on the design of the HPV vaccine trials. Updating the guidelines will now accelerate vaccine development, reduce costs of clinical trials, and lead to faster regulatory approval.
Keywords: HPV vaccine, WHO, Policy guidelines
1. Introduction
The process to recommend a commercial vaccine has multiple steps and safeguards taken to generate international consensus among multiple stakeholders. This process ensures that recommendations are based on the quality of scientific data and are made with the health of the public in mind. Using the World Health Organization's (WHO) recent revision of its recommendations and standards for prophylactic human papillomavirus (HPV) vaccines, “Guidelines to assure the quality, safety and efficacy of recombinant human papillomavirus virus-like particle vaccines” (Technical Report Series 962, Annex 1), as an example, we describe the multi-year, multi-step process that was undertaken to arrive at the updated guidance [1].
WHO's global written standards, the Technical Report Series (TRS), serve many functions: (1) to provide guidance for national regulatory agencies and manufacturers to assure vaccine quality, safety, and efficacy; (2) to serve as the basis for national legislation; and (3) to represent WHO vaccine prequalification, thereby legitimizing vaccine procurement by United Nations agencies.
In 2006, the WHO established the technical guidelines to assure the quality, safety, and efficacy for prophylactic HPV L1-virus-like particle (VLP) vaccines (TRS 962, Annex 1) [1]. These recommendations were developed in the context of the safety and efficacy of the recently available prophylactic vaccines at that time, the bivalent (Cervarix, GlaxoSmithKline) and quadrivalent (Gardasil, Merck) recombinant VLP HPV vaccines [1]. Both of these first-generation HPV vaccines target HPV types 16 and 18, which are associated with 70% of the global cervical cancer burden, and the quadrivalent vaccine also targets non-oncogenic HPV types 6 and 11 [2]. Primary data demonstrated very strong efficacy for both vaccines against the disease endpoints for licensure: high-grade pre-invasive lesions of the cervix, vulva, and vagina [3], [4].
In 2009, the WHO published a position paper on prophylactic HPV vaccines, recommending the routine use of these vaccines in national immunization programs [5], and both vaccines were pre-qualified by the WHO that same year. These two steps enabled international global health partnerships to procure and distribute the HPV vaccine. In subsequent years, advances in HPV vaccine research compelled the WHO to consider revising its guidance on vaccine efficacy evaluation – specifically, whether or not to consider alternate clinical endpoints for next-generation vaccine trials.
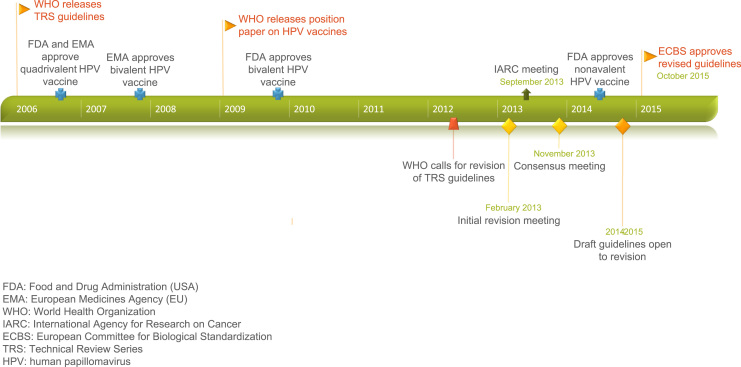
In this paper, we summarize the process implemented by the WHO to (1) identify a need for guideline revision; (2) assemble stakeholders to establish relevant questions; (3) critically review the current evidence on HPV epidemiology; (4) establish consensus on appropriate clinical endpoints for regulatory evaluation of next-generation HPV vaccines; and (5) update guidelines. Fig. 1 presents a timeline of this process. These key steps were necessary for the approval of the new guidance, named “Recommendations to assure the quality, safety, and efficacy of recombinant human papillomavirus virus-like particle vaccines” in October 2015 [6].
Fig. 1.
A timeline of HPV vaccine adoption and corresponding WHO guidelines, with a focus on guideline revision.
2. Identifying a need for guideline revision
Pivotal trials for the current L1-VLP vaccines against HPV types 16 and 18 were powered for efficacy against a disease endpoint, CIN2+. This outcome was chosen due to the unethical and impractical nature of waiting for development of cervical cancer. Given high vaccine efficacy among vaccine-type naïve patients, these vaccines reduce the incidence of preinvasive lesions significantly [4].
With proven efficacy of current vaccines, increasing understanding of HPV epidemiology, and ongoing innovation of next-generation HPV vaccines, the WHO initiated a process to evaluate additional trial endpoints for new prophylactic HPV vaccine trials in 2012. The WHO recognized the need for next-generation HPV vaccines. New vaccines could allow for several innovations: increased coverage of non-16/18 oncogenic disease, new production and delivery platforms, potential use in younger age ranges, simpler administration schedules and routes, and prevention of non-cervical HPV disease. Secondary outcomes concurrently collected during the L1-VLP trials included persistent HPV infection for 6 and 12 months and immunogenicity up to 48 months after vaccination. Similar efficacy rates for the prevention of CIN2+ and the prevention of persistent HPV infection were found from these trials [4]. These findings suggested the possibility of additional appropriate clinical endpoints for next-generation vaccines. In order to facilitate vaccine development, reduce efficacy trial costs, and simplify regulatory approval, the WHO aimed to evaluate alternate clinical trial endpoints in parallel with the development of new vaccine candidates.
3. Identifying stakeholders and key questions
In February 2013, WHO held an initial scoping meeting in Geneva, Switzerland, to identify the key stakeholders, process steps, and data necessary to evaluate the evidence for potential alternate endpoints for HPV vaccine trials. Participants at the scoping meeting included a small gathering of experts across the WHO Secretariat, academia, governmental scientific organizations, and regulatory agencies, along with representation from the Bill and Melinda Gates Foundation (BMGF). In order to determine whether there was adequate evidence available to evaluate alternate clinical endpoints for HPV vaccine trials, the following topics were briefly reviewed: (1) the efficacy of currently approved prophylactic vaccinations; (2) an overview of HPV vaccine candidates along the development pipeline [7]; (3) candidates for alternate endpoints; (4) potential regulatory challenges; and (5) the logistics of updating the WHO technical guidelines. The scope of this particular meeting did not include other important aspects of HPV vaccination, such as efficacy as a therapeutic vaccination or a comprehensive review of vaccine safety.
As first-generation vaccines have proven highly efficacious against HPV-16 and 18-associated cervical dysplasia [4], there was consensus that placebo-controlled trials for next-generation vaccines would be unethical. Consequently, powering a trial comparing current L1-VLP vaccines to next-generation candidates with cervical dysplasia as the primary outcome would likely prove prohibitive to vaccine development, particularly if the trial were to take place among populations having achieved herd immunity with current vaccines. Notably, during the time period of these discussions, the phase III clinical trial for the nonavalent vaccine (Gardasil-9, Merck) which covers HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58, was conducted using the quadrivalent vaccine as the control group [8]. Although it demonstrated greater efficacy, this trial required over 14,000 women and 6 years to complete.
Given the challenges with the current trial outcomes and the significant potential next-generation vaccines represent, the stage was set to investigate alternative clinical endpoints that would prove efficacy and serve as surrogate outcomes for pre-cancer, but also would allow for vaccine innovation, feasible clinical trials, and regulatory approval. The discussions ultimately identified persistent HPV infection and vaccine titers as possible clinical endpoints.
The group identified individuals from the WHO, NIH, Centers for Disease Control (CDC), and academic institutions representing international expertise in HPV epidemiology, natural history, laboratory methods, and vaccine trials. Regulatory agencies from the United States, China, European Union, and Canada, and pharmaceutical companies currently developing vaccines, were also invited to be crucial members of the discussion.
4. Scientific review on HPV epidemiology
The scoping group tasked the International Agency for Research on Cancer (IARC), the specialized cancer agency of the WHO, and the National Cancer Institute (NCI), a branch of the U.S. National Institutes of Health (NIH), to jointly hold a scientific forum among HPV experts to review the current knowledge on HPV epidemiology and HPV-attributable disease, and consider persistent HPV infection and immunogenicity as possible clinical endpoints. This meeting was held in September 2013. The scientific opinion was established that use of persistent HPV infection or immunogenicity, or rarely a disease endpoint, for next-generation HPV vaccine trials was dependent on the type of trial being designed [9]. The purpose of this manuscript is not to summarize that report, but rather focus on how the scientific consensus was translated into policy.
5. How was consensus achieved?
Subsequent to the IARC/NCI meeting, a second meeting was convened at the WHO in November 2013 with key experts and stakeholders. A summary of the scientific review conducted by IARC/NCI was first presented [9]. Recommendations for clinical trial endpoints from the IARC working group based on age group (<16, 16–26, >26), anatomic site (cervix, anus, vulva, vagina, oropharynx), and vaccine type (VLP, non-VLP) were discussed at length at the consensus meeting [9]. As part of the discussion regarding appropriateness of virologic endpoints, the importance of assay standardization used in the clinical evaluation of HPV vaccines, e.g. virological (including tissue/sample collection) and serological assays, was discussed. The WHO Human Papillomavirus Laboratory Manual, which was developed by the former WHO HPV LabNet provided useful guidance and information regarding assay standardization [10].
Given the strength of the scientific evidence, the group reached consensus that persistent HPV infection greater than 6 months is an appropriate clinical endpoint for HPV vaccine trials for L1 VLP vaccines. The 6-month timeframe was agreed upon as clinical trial data has shown the absolute risk of CIN3 is the same for persistent infection for 6 or 12 months. A virologic endpoint would confer several benefits:
-
(1)
smaller sample size for clinical trials;
-
(2)
shorter trial length as incident HPV is more common than incident dysplasia;
-
(3)
applicability to various anatomic sites (i.e., cervix, anus, vulvar).
The group agreed on persistent HPV infection as a better endpoint than a serologic endpoint for several reasons. Most notably, given rare breakthrough infections with the current vaccines, the threshold of titer level that confers immunity (immune correlate of protection) is currently unknown [11]. This was felt to be one of the most significant drawbacks of a serologic endpoint, as the group agreed that future vaccine trials should be designed as non-inferiority trials, which would not be possible without an immune correlate of protection. In addition, with various vaccine types (L2 VLP, non-VLP) in development and plans for different routes of administration, it is likely that the protective antibody type and response would be different by vaccine type, thus requiring individual immune correlates of protection to be established. However, in the longer term, as immunogenicity data continues to mature especially with post-licensure data monitoring, establishment of an immune correlate of protection may become possible. Moreover, serologic non-inferiority for comparable vaccines and immunobridging studies to younger age groups will continue to serve as a core part of HPV vaccine trial research.
To further frame the discussion of applicable vaccine trial endpoints, regulators from the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) shared their perspective. They agreed the scientific evidence was unequivocal that HPV is necessary but not sufficient for cervical cancer and pointed to the virologic endpoint data from prior vaccine trials as ample evidence for adopting a new primary outcome for vaccine trials. They also highlighted the a priori need for ethical trials, prior to considering a vaccine for licensure, thus ensuring that placebo-controlled trials would not be an option.
Pharmaceutical companies innovating next-generation prophylactic HPV vaccines shared information about the various candidates along the development pipeline. They shared their support for virologic primary endpoints, in part driven by the discussion of the long and challenging nature of nonavalent vaccine trial design, powered on disease endpoints.
At the conclusion of the meeting, persistent HPV infection greater than 6 months was an acceptable and favored primary endpoint among all interested parties.
6. Updating current WHO guidelines
When a need to establish or revise the standards is identified, WHO organizes a consensus conference of relevant stakeholders (as delineated in this document). In light of these formative discussions, a drafting group preliminarily updates the guidelines, which are opened for review for several months at a time and allow for public commentary. The draft is then iteratively revised and reopened for feedback. The drafting group is comprised of a multidisciplinary and international group of experts and stakeholders, similar to the consensus meeting but on a larger scale. Upon finalizing the guidelines, they are presented to the WHO Expert Committee on Biological Standardization (ECBS) for consideration. The ECBS helps guide the process that allows countries to adopt the recommendations for the production and control of vaccines. With approval, the updated guidelines become TRS.
After the above consensus was reached, the draft guidelines underwent 4 rounds of commentary and revision prior to being presented to the ECBS in 2015. At the annual meeting in October 2015, 2.5 years after the initial scoping meeting took place, the revisions were adopted [6].
7. Conclusion
The WHO plays a critical role in ensuring vaccine efficacy and safety, stimulating vaccine development, and allowing vaccine uptake internationally. Although this is an important function of the WHO, the process by which they achieve these goals has not previously been delineated. With this manuscript, using the process to update guidelines on prophylactic HPV vaccines, we hope to have enhanced transparency regarding this careful and deliberate process.
Authors’ role
Serving in the roles of consultant to WHO (LE), and trainee in Obstetrics & Gynecology and policy development (MP), the authors assisted the WHO Secretariat team in the Initiative for Vaccine Research with the process of establishing expert consensus of appropriate clinical endpoints for next-generation HPV vaccines. Specifically, the authors' roles included assisting the WHO Secretariat with processes outlined in this paper, such as identifying both the scope of research for background to the HPV endpoint revision and appropriate expertise for the two WHO expert consultations (February and November 2013), and assisting in the agenda development for these consultative meetings as well as producing the meeting reports that facilitated future steps in this endpoint revision process. The authors do not represent the decisions, policy or views of the World Health Organization.
The authors have no conflicts of interest to report.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
Uli Fruth, Ph.D., and Joachim Hombach, PhD from The Initiative for Vaccine Research, Immunization, Vaccines and Biologicals, World Health Organization, Geneva, Switzerland.
Contributor Information
Malavika Prabhu, Email: mprabhu@partners.org.
Linda O. Eckert, Email: eckert@uw.edu.
References
- 1.World Health Organization. Guidelines to assure the quality, safety and efficacy of recombinant human papillomavirus-like particle vaccines. WHO Technical Report Series, Annex 1. 〈http://whqlibdoc.who.int/trs/WHO_TRS_962_eng.pdf?Ua=1〉, 2006 (accessed 15.05.15).
- 2.de Sanjose S., Quint W.G., Alemany L L. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 3.Future I.I. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861–1868. doi: 10.1016/S0140-6736(07)60852-6. (Study Group) [DOI] [PubMed] [Google Scholar]
- 4.Paavonen J., Naud P., Salmeron J. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization Human papillomavirus vaccines WHO position paper. WHO Wkly. Epidemiol. Rec. 2009;15:118–131. 〈http://www.who.int/wer/〉 (2009/wer8415.pdf?ua=1, (accessed 15.05.15) [PubMed] [Google Scholar]
- 6.World Health Organization, Recommendations to assure the quality, safety, and efficacy of recombinant human papillomavirus virus-like particle vaccines. Replacement of: TRS 962, Annex 1. 〈http://www.who.int/biologicals/HPV_Post-ECBS_ZHOU_(CLEAN)_2810〉pdf, (accessed 06.02.16), 2015.
- 7.Schiller J.T., Muller M. Next generation prophylactic human papillomavirus vaccines. Lancet Oncol. 2015;16:e217–e225. doi: 10.1016/S1470-2045(14)71179-9. [DOI] [PubMed] [Google Scholar]
- 8.Joura E.A., Guiliano A.R., Iversen O.E., Bouchard C., Mao C., Mehlsen J. For the broad spectrum HPV study. N. Engl. J. Med. 2015;372:711–723. [Google Scholar]
- 9.Lowy D.R., Herrero R., Hildesheim A. Primary endpoints for future prophylactic human papillomavirus vaccine trials: towards infection and immunobridging. Lancet Oncol. 2015;16:e226–e233. doi: 10.1016/S1470-2045(15)70075-6. [DOI] [PubMed] [Google Scholar]
- 10.Human papillomavirus laboratory manual, 1st edition, 2009. 〈www.who.int/vaccines-documents/〉, (accessed 15.05.15).
- 11.World Health Organization Human papillomavirus vaccines WHO position paper. WHO Wkly. Epidemiol. Rec. 2014;43:465–492. [Google Scholar]