Abstract
Cinacalcet is an effective and safe alternative to parathyroidectomy in end stage renal disease (ESRD) patients with secondary hyperparathyroidism. Hypocalcemia is a known complication of treatment that is usually readily reversible upon discontinuation of the drug. It rarely manifests severely and symptomatically requiring hospital admission. We present the case of a 55 year old man with severe, symptomatic and prolonged hypocalcemia that occurred 2 weeks after starting cinacalcet. Cinacalcet induced a state of pharmacological parathyroidectomy with subsequent hungry bone syndrome. Serum calcium returned to normal range after 4 weeks of stopping the drug while receiving high doses of elemental calcium and vitamin D receptor activation therapy (VDRA).
Keywords: Hypocalcemia, Secondary hyperparathyroidism, Cinacalcet, Hungry bone syndrome
Background
Cinacalcet hydrochloride, a calcimimetic agent approved by the USA-FDA in 2004, is a well-established therapeutic option for secondary hyperparathyroidism in end stage renal disease (ESRD) patients [1]. It is a relatively safe drug with few serious side effects. We herein present a case of severe persistent hypocalcemia requiring hospitalization that developed 2 weeks after starting cinacalcet and persisted over 4 weeks.
Case presentation
A 55 year old man with ESRD secondary to obstructive uropathy and hypertensive nephrosclerosis presented to the emergency department with headache, nausea, muscle spasms and diffuse numbness over his extremities. His laboratory data revealed severe corrected hypocalcaemia of 5.3 mg/dL.
He has been maintained on thrice-weekly hemodialysis for three and a half years. He had secondary hyperparathyroidism (serum intact PTH 1035 pg/mL by chemiluminescence on Cobas 6000 by Roche diagnostics, Basel, Switzerland) over the past year which was initially treated with alfacalcidol 0.5 μg daily; however, it was stopped because of significant hypercalcemia and hyperphosphatemia despite concomitant use of sevelamer 1600 mg three times daily with meals. Cinacalcet (Mimpara® in the EU and Sensipar® in the USA by Amgen Inc., Thousand Oaks, CA, USA) 30 mg daily was started 2 weeks prior to his presentation. The rest of his medications included bisoprolol, bumetanide, amlodipine, and aspirin.
Upon presentation, his serum albumin level was 4.2 g/L, iPTH level was 145 pg/mL (15–76 pg/mL), phosphorus 2.3 mg/dL, magnesium 1.9 mg/dL, 25-OH vitamin D 4.8 ng/mL (deficient < 10 ng/mL) and total alkaline phosphatase 331 IU/L (normal 35–120 IU/L). His electrocardiogram showed a normal sinus rhythm. His cinacalcet was discontinued. He required a total of 12 g of intravenous calcium gluconate and 5 g of oral calcium carbonate before he became asymptomatic with a serum calcium level above 6 mg/dL. He was discharged home on maximal doses of elemental calcium (2000 mg) [2] and alfacalcidol 1 μg per day which was subsequently increased to 2 μg per day. He was being dialyzed against a 1.25 mmol/L calcium bath. Serial weekly serum calcium showed slowly improving levels: 6.3 and 7.3 mg/dL on day 10 and day 16 after stopping cinacalcet, respectively (Fig. 1). By week 4, his calcium level was 8.1 mg/dL and his intact PTH level was 230 pg/mL.
Fig. 1.
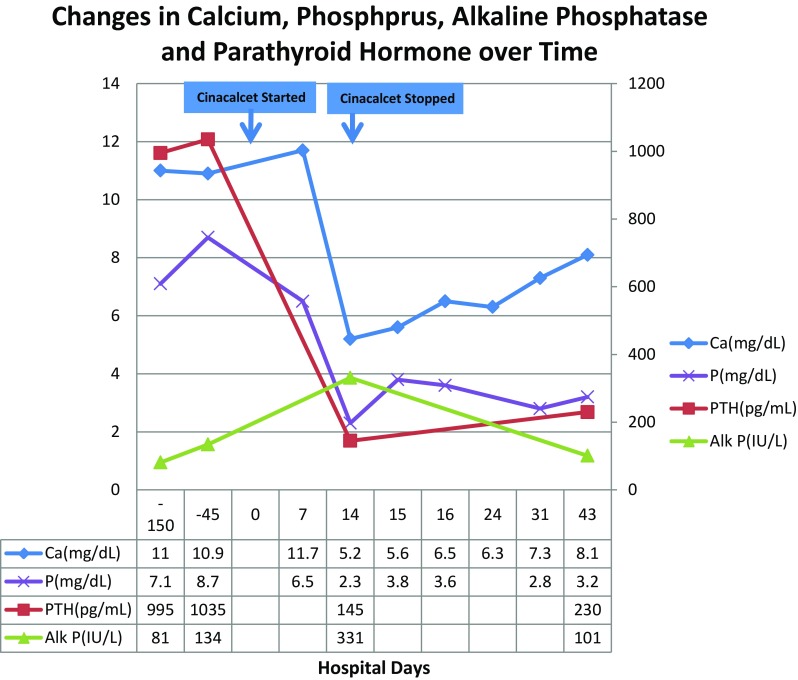
Changes in serum calcium, phosphorus, alkaline phosphatase and parathyroid hormone over time. Ca calcium (corrected), P phosphorus, Alk P alkaline phosphatase, PTH parathyroid hormone
Discussion
Hypocalcaemia is a well-known complication of cinacalcet. In the EVOLVE trial [3], a level < 7.5 mg/dL occurred in 25% of the cinacalcet group and 7% in the placebo group. It rarely manifests severely and symptomatically requiring hospital admission and discontinuation of the drug. Besides, hypocalcemia is usually readily reversible upon discontinuation of the drug.
To our knowledge, one other case was reported in the literature that describes severe hypocalcemia and cinacalcet-induced hungry bone syndrome (HBS) 2 weeks after starting therapy [4]. There is one more case in the literature with severe hypocalcemia, resulting in Torsade de pointes and cardiac arrest. It developed 3 weeks after increasing the dose of cinacalcet from 60 to 90 mg whereby the patient was maintained on cinacalcet 60 mg for 3 years [5].
In our case, cinacalcet induced a state of pharmacological parathyroidectomy with subsequent HBS characterized by prolonged hypocalcemia, mild hypophosphatemia and increased alkaline phosphatase [6]. This syndrome has been well described after parathyroidectomy in dialysis patients with severe and long standing secondary hyperparathyroidism. It is secondary to remineralization of bones and gain of new remodeling sites after the abrupt loss of parathyroid hormone. The severe hypocalcemia in our case persisted for 4 weeks and required maximum doses of calcium and increasing doses of alfacalcidol to restore acceptable level of serum calcium. In addition, iPTH level increased rather slowly after discontinuation of the cinacalcet.
Prolonged hypocalcemia after initiation of cinacalcet therapy is untypical. The underlying pathogenesis behind the prolonged hypocalcemic effect could represent an upregulation of the parathyroid calcium sensing receptors (CaSRs) resulting in prolonged over suppression of PTH and hypocalcemia [7]. Other possible explanation is a direct stimulatory effect of cinacalcet on osteoblasts via their CaSRs [8]. Risk factors for HBS include the long standing high bone turnover state, the low vitamin D level and being off any VDRA therapy [9]. Low vitamin D was found to be a risk factor for HBS in patients with primary hyperparathyroidism treated with parathyroidectomy [9]. The prolonged hypo-calcemic effect could have also been secondary to auto-infarction of the parathyroid gland as cinacalcet has been shown to decrease parathyroid gland vascularity [8], so it is possible that cinacalcet might have induced auto-infarction and thereby led to untypical prolonged suppression of PTH in our case.
Conclusion
Cinacalcet can cause acute, severe and symptomatic hypocalcaemia in susceptible patients, possibly those with prolonged high bone turnover states or those not receiving VDRA therapy. The package insert recommends checking serum calcium within 1 week of initiation or dose adjustment of cinacalcet; however, we suggest checking serum calcium level more frequently within the first month of initiating treatment especially in those at risk.
Compliance with ethical standards
Conflict of interest
Dr. Ali Abu-Alfa has consulted for Amgen in the past 12 months. The rest of the authors report no conflict of interest.
Research involving human participants/animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
An informed consent was obtained from the patient reported.
References
- 1.Goodman WG, Hladik GA, Turner SA, et al. The calcimimetic agent AMG 073 lowers plasma parathyroid hormone levels in hemodialysis patients with secondary hyperparathyroidism. J Am Soc Nephrol. 2002;13:1017–1024. doi: 10.1681/ASN.V1341017. [DOI] [PubMed] [Google Scholar]
- 2.Eknoyan G, Levin A, Levin NW. Bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:1–201. doi: 10.1016/S0272-6386(03)00905-3. [DOI] [Google Scholar]
- 3.Investigators TET. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482–2494. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 4.Lazar ES, Stankus N. Dialysis rounds: cinacalcet-induced hungry bone syndrome. Seminars in dialysis. Hoboken: Wiley; 2007. pp. 83–85. [DOI] [PubMed] [Google Scholar]
- 5.Novick T, McMahon BA, Berliner A, Jaar BG. Cinacalcet-associated severe hypocalcemia resulting in torsades de pointes and cardiac arrest: a case for caution. Eur J Clin Pharmacol. 2016;72:373–375. doi: 10.1007/s00228-015-1989-6. [DOI] [PubMed] [Google Scholar]
- 6.Cruz DN, Perazella MA. Biochemical aberrations in a dialysis patient following parathyroidectomy. Am J Kidney Dis. 1997;29:759–768. doi: 10.1016/S0272-6386(97)90131-1. [DOI] [PubMed] [Google Scholar]
- 7.Sumida K, Nakamura M, Ubara Y, et al. Cinacalcet upregulates calcium-sensing receptors of parathyroid glands in hemodialysis patients. Am J Nephrol. 2013;37:40512. doi: 10.1159/000350211. [DOI] [PubMed] [Google Scholar]
- 8.Goto S, Fujii H, Matsui Y, Fukagawa M. Marked increase in bone formation markers after cinacalcet treatment by mechanisms distinct from hungry bone syndrome in a haemodialysis patient. NDT Plus. 2010;3:71–73. doi: 10.1093/ndtplus/sfp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart ZA, Blackford A, Somervell H, et al. 25-hydroxyvitamin D deficiency is a risk factor for symptoms of postoperative hypocalcemia and secondary hyperparathyroidism after minimally invasive parathyroidectomy. Surgery. 2005;138:1018–1026. doi: 10.1016/j.surg.2005.09.018. [DOI] [PubMed] [Google Scholar]


