Abstract
Dapagliflozin (DAPA), a sodium–glucose co-transporter 2 (SGLT2) inhibitor, is known to have a beneficial diuretic effect, in addition to a glucose-lowering effect. Although SGLT2 inhibitor has been reported, the increase of hyperkalemia in patients treated with renin–angiotensin–aldosterone system (RAAS) inhibitors, their mechanism of action is unclear. We report the first case of a type 2 diabetes (T2DM) patient with potential mineralocorticoid deficiency who developed hyperkalemia after administration of DAPA. A 79-year-old woman underwent bilateral adrenalectomy for uncontrolled hypercortisolism due to an inoperable recurrence of Cushing’s disease, and she was subsequently maintained on replacement therapy with glucocorticoid. She was diagnosed as having T2DM at 71 years of age and was treated with sitagliptin and miglitol. Since she presented with weight gain of about 5 kg over 6 months and her HbAlc level increased over 12%, 5 mg/day DAPA was added to her daily regimen. After the start of DAPA treatment, she developed hyperkalemia (6.5 mEq/L) with increased plasma renin activity of 53.1 ng/mL/h. She was diagnosed with aldosterone deficiency and started on fludrocortisone 0.1 mg daily, after which the hyperkalemia improved immediately. In this case, DAPA treatment could potentially increase the requirement for mineralocorticoid replacement, directly suggesting that the SGLT2 inhibition-induced natriuretic effect is accompanied by compensatory activation of the RAAS axis, which is essential to keep the serum potassium level within the normal range. Therefore, physicians should be careful about the development of hyperkalemia in patients when SGLT2 and RAAS inhibitors are used in combination.
Keywords: Dapagliflozin, Type 2 diabetes, Hyperkalemia, Aldosterone deficiency, Renin–angiotensin–aldosterone system
Introduction
Dapagliflozin (DAPA), a sodium–glucose co-transporter 2 (SGLT2) inhibitor that targets hyperglycemia in type 2 diabetes (T2DM) by increasing renal glucose excretion [1]. In addition to its beneficial effects on glycemic control, DAPA elicits small reductions in blood pressure (BP) and body weight, due partly to a mild natriuretic and osmotic diuretic effect with glycosuria [2]. Similarly, an initial acute fall in eGFR has been observed during treatment with DAPA, reflecting a reduction of the circulation volume rather than worsening of structural renal function [3]. These findings suggest that SGLT2 inhibitors could influence renin–angiotensin–aldosterone system (RAAS) as part of their mechanism of action. Since SGLT2 inhibitors have been reported to cause hyperkalemia in patients treated with RAAS inhibitors [4], it is suggested that RAAS activity might be necessary to retain the urinary excretion of potassium.
Herein, we report the first case of a T2DM patient with mineralocorticoid deficiency following bilateral adrenalectomy who developed hyperkalemia after treatment with DAPA, a SGLT2 inhibitor. Furthermore, the additional administration of fludrocortisone acetate, a mineralocorticoid derivative, normalized the hyperkalemia, demonstrating that the retention of RAAS activity is essential to keep the serum potassium level within a normal range during treatment with an SGLT2 inhibitor.
Case report
A 79-year-old woman was diagnosed with Cushing’s disease at 56 years of age, and underwent transsphenoidal surgery to resect a pituitary tumor. However, the increase of serum ACTH with the resultant cortisol excess relapsed 5 years after the initial pituitary surgery. Resurgery to dissect the remnant pituitary tumor was performed but this was unsuccessful. Thereafter, persistent medical therapy with several types of inhibitors of adrenal steroidogenesis were not able to normalize her clinical and biochemical consequences of hypercortisolism, as evidenced by increased serum ACTH and cortisol below their respective upper limit. Since she subsequently developed visceral obesity, osteoporosis, impaired glucose tolerance, and hyperlipidemia, she underwent a bilateral adrenalectomy by laparoscopic surgery at the age of 70 years, and she was maintained on replacement therapy with glucocorticoid (cortisone acetate 26.25 mg twice daily, dexamethasone 0.1 mg once daily), but not mineralocorticoid. She was diagnosed with T2DM at 71 years of age, and was treated with diet, exercise and glucose-lowering agents (sitagliptin 50 mg once daily, miglitol 150 mg three times daily).
At the age of 79 years, she gradually gained body weight from 64.0 to 69.4 kg over the last 6 months, and her glycemic control worsened gradually. In December 2015, her HbAlc level increased by over 12%, and she was admitted to the Department of Endocrinology, Osaka City University Hospital for glycemic control. Her current medication, except for glucocorticoid replacement and glucose-lowering agents, included pitavastatin 2 mg once daily, roxatidine 75 mg once daily, alendronic acid 35 mg once weekly. The physical findings were as follows: height 156.2 cm; weight 69.4 kg; BMI 28.6 kg/m2; pulse rate 69/min and regular; BP 141/57 mmHg; body temperatures 35.9 °C; general skin pigmentation; and slight pitting edema in the extremities. The laboratory findings upon admission are presented in Table 1. Her insulin secretion, estimated from urinary C-peptide immunoreactivity (CPR) was 47.5 μg/day, and glucagon loading test showed that her plasma glucose level changed from 146 to 160 mg/dL, her serum CPR changed from 6.29 to 8.63 ng/mL, with a ΔCPR of 2.34 ng/mL. Immunoreactive insulin (IRI) was 28.1 µU/mL. Anti-glutamic acid decarboxylase antibody (GAD) could not be detected. Thyroid function test was within normal limits. The patient’s plasma ACTH level (1170 pg/mL) was very high but her plasma cortisol (2.1 μg/dL) was low, and the 24-h urinary-free cortisol (UFC) level was 21.6 μg/day. Although her serum aldosterone was 9 pg/mL and the 24-h urinary aldosterone level was 0.4 μg/day, her fractional excretion of potassium (FEK) was in a normal range (11.2%). In addition to adrenal insufficiency, the abnormal findings detected upon admission included slight anemia, hypoalbuminemia, mild liver dysfunction, moderate renal dysfunction, insulin-independent and strong insulin resistance. Clinical examination showed stage 1 diabetic nephropathy (urinary albumin-to-creatinine ratio (UACR), 6 mg/g Cr; estimated glomerular filtration rate, 36.9 mL/min × 1.73/m2), but no diabetic neuropathy, no diabetic retinopathy, and no evidence of macrovasculopathy. Chest X-ray, abdominal CT scan, upper gastrointestinal endoscopy and fecal occult blood test showed no abnormal findings.
Table 1.
Laboratory data upon admission
| Hematology | |
| WBC | 5400 /µL |
| RBC | 388 × 104 /µL |
| Hemoglobin | 10.5 g/dL |
| Hematocrit | 32.2 % |
| Platelet | 34.1 × 104 /µL |
| Biochemistry | |
| Total protein | 6.5 mg/dL |
| Albumin | 3.2 mg/dL |
| AST | 49 IU/L |
| ALT | 52 IU/L |
| LDH | 171 IU/L |
| γ-GT | 196 IU/L |
| ALP | 221 IU/L |
| BUN | 26 mg/dL |
| Creatinine | 1.1 mg/dL |
| eGFR Cr | 36.9 mL/min/1.7 m2 |
| eGFR Cys C | 43.5 mL/min/1.7 m2 |
| UA | 6.4 mg/dL |
| Na | 136 mEq/L |
| K | 4.9 mEq/L |
| Cl | 107 mEq/L |
| TC | 166 mg/dL |
| LDL-C | 101 mg/dL |
| HDL-C | 51 mg/dL |
| Triglyceride | 91 mg/dL |
| FPG | 219 mg/dL |
| HbA1c | 12.1% |
| GA | 31.5% |
| CPR | 6.29 ng/mL |
| IRI | 28.1 µU/mL |
| FT4 | 1.35 ng/mL |
| TSH | 0.822 μIU/mL |
| Cortisol | 2.1 μg/dL |
| ACTH | 1170 pg/mL |
| Aldosterone | 9 pg/mL |
| Urinalysis | |
| Protein | – |
| Glucose | – |
| Ketone | – |
| ACR | 6 mg/gCr |
| CPR | 47.5 μg/day |
| Free cortisol | 21.6 μg/day |
| Aldosterone | 0.4 μg/day |
WBC white blood cell, RBC red blood cell, AST aspartate transaminase, ALT alanine transaminase, LDH lactate dehydrogenase, γ-GT γ-glutamyl transferase, ALP, alkaline phosphatase, BUN blood urea nitrogen, eGFR estimated glomerular filtration rate, Cr creatinine, Cys C cystatin C, UA urinary acid, TC total cholesterol, FPG fasting plasma glucose, GA glycoalbumin, CPR C-peptide immunoreactivity, IRI immunoreactive insulin, FT4 free thyroxine, TSH thyroid-stimulating hormone, ACTH adrenocorticotropic hormone, ACR albumin/creatinine ratio
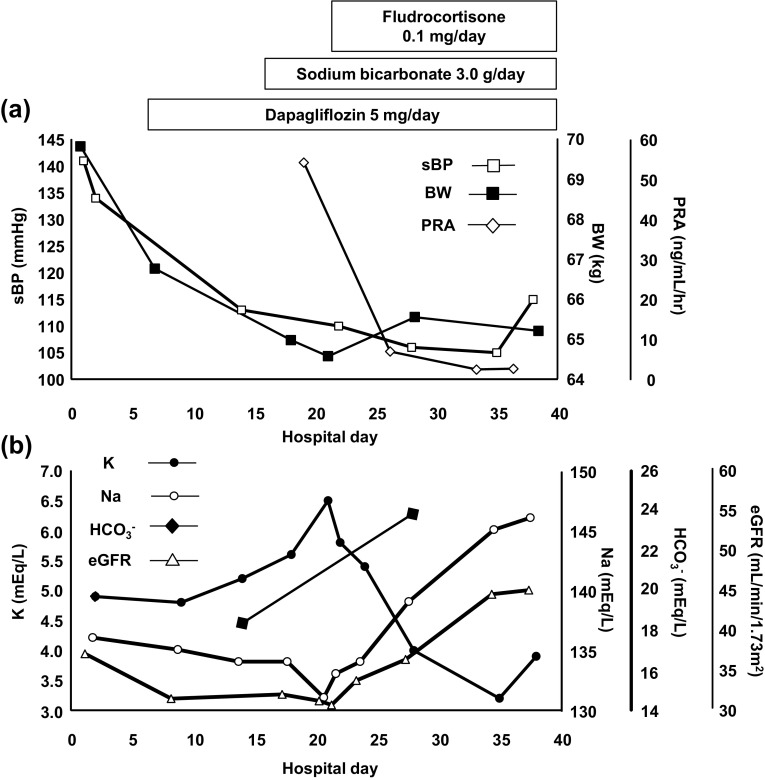
On hospital day 7, the patient was treated with 5 mg/day dapagliflozin (DAPA) once daily in the morning. Daily urinary glucose and urine volume increased from 2.8 g and 1000–1500 mL to 20 g and 2000–2500 mL after the start of DAPA treatment. The fasting plasma glucose and GA level were also reduced from 219 mg/dL and 31.5% to 105 mg/dL and 16.9%, respectively, after 28 days of treatment. As show in Fig. 1a, her body weight decreased from 66.8 to 64.9 kg after 14 days of DAPA treatment. The systolic blood pressure (sBP), and eGFR also decreased, from 141 mmHg and 36.9 mL/min × 1.73/m2 to 110 mmHg and 31.0 mL/min × 1.73/m2, respectively. Of importance, 7 days of DAPA treatment increased her urinary Na excretion from 65 mEq/day to 86 mEq/day; although she was provided with 6 g/day of NaCl-restricted diet during her admission.
Fig. 1.
Clinical course of volume reflecting factors and electrolyte levels during admission. a Although the BW and sBP were reduced gradually after DAPA treatment, both recovered along with normalization of the PRA after fludrocortisone replacement. b Although the serum potassium level increased and sodium, bicarbonate levels and eGFR decreased after DAPA treatment, both were normalized after sodium bicarbonate and fludrocortisone replacement
Surprisingly, approximately 1 week after the start of DAPA treatment (day 14), the patient developed hyperkalemia (5.2 mEq/L), hyponatremia (134 mEq/L) and metabolic acidosis with respiratory compensation (pH 7.356, BE − 5.8 mEq/L, PCO2 33.8 Torr, HCO3 − 18.4 mEq/L, Cl 105 mEq/L) (Fig. 1b). She was started on sodium bicarbonate at the daily dose of 3.0 g and the NaCl restriction was attenuated from 6 to 10 g/day on day 17, with no essential improvement in the serum potassium. On day 21, her serum potassium increased abruptly from 5.6 to 6.5 mEq/L with plasma renin activity of 53.1 ng/mL/h and FEK decreased from 11.2 to 7.6%. She was diagnosed with aldosterone deficiency and started on fludrocortisone 0.1 mg daily. The serum potassium level on the next day was 5.8 mEq/L. Seven days later (day 28), her serum potassium was reduced to 4.0 mEq/L with plasma renin activity of 5.8 ng/mL/h. Additional laboratory data results from day 28 were as follows: Na 139 mEq/L, pH 7.402, BE − 0.1 mEq/L, PCO2 39.3 Torr, HCO3 − 24.0 mEq/L, Cl 105 mEq/L, FENa 0.92%, FEK 15.3% (Fig. 1). At 4 months follow-up after discharge, her volume regulation was well-controlled (BW 67.3 kg, sBP 122 mmHg, eGFR 49.33 mL/min × 1.73/m2) with continued normalization of the electrolytes and the acid–base equilibrium imbalance (Na 144 mEq/L, K 3.8 mEq/L, Cl 109 mEq/L) with fludrocortisone 0.04 mg and sodium bicarbonate 3.0 g daily.
Discussion
We report a case of a T2DM patient who had undergone bilateral adrenalectomy and developed hyperkalemia following treatment with DAPA. This patient developed hyperkalemia following a decrease in body weight, BP and eGFR, which suggested that the SGLT2 inhibition-induced natriuretic effect could not be compensated by increasing aldosterone levels, leading to the impairment of potassium excretion due to the manifestation of aldosterone deficiency. Thereafter, the hyperkalemia improved immediately after the addition of mineralocorticoid replacement.
In this case report, the increased requirement for mineralocorticoid replacement after SGLT2 inhibition has important clinical implications. Similar to both loop and thiazide diuretics [5], SGLT2 inhibitors could influence renal potassium excretion as part of their mechanism of action. Specifically, defective sodium absorption in the proximal convolute tubule (PCT) leads to an increased sodium load to the distal tubule, with compensatory activation of the RAAS axis induced by volume depletion, stimulating sodium reabsorption along the collecting duct (CD), leading to potassium secretion. In fact, several articles have indicated the relationship between SGLT2 inhibition and RAAS mediators. Cherney et al. [6] reported that SGLT2 inhibition with empagliflozin-attenuated renal hyperfiltration in subjects with type 1 diabetes. After 8 weeks of empagliflozin treatment in patients with baseline hyperfiltration, there was a 20% reduction in GFR, which was not mediated by the RAAS, as there was an increase in both angiotensin II and aldosterone levels as consequence of the diuretic effect. Another study in subjects with T2DM and inadequate BP control [7] showed that SGLT2 inhibition with DAPA was associated with reductions in body weight, BP, GFR and possibly plasma volume. After 12 weeks of DAPA treatment, plasma renin activity and serum aldosterone increased, consistent with the observed changes in body weight and plasma volume. These observations of an increase in volume-regulating hormones can be explained as compensatory mechanisms to restore fluid balance and serve as further evidence for a reduction of the effective circulating volume after SGLT2 inhibition.
In the present case, the lack of endogenous aldosterone secretion due to bilateral adrenalectomy, similar to the pathophysiological condition observed in cases treated with RAAS inhibitors; although it is an extreme situation, provides novel insight into renal potassium homeostasis after SGLT2 inhibition. The final potassium excretion along the CD is the main determinant of external potassium handling, and is responsible for hyperkalemia [8]. Disturbances of renal potassium excretion were caused by reduced delivery of sodium to the distal nephron, aldosterone deficiency, and abnormal functioning of the cortical collecting tubule [9]. In the clinical setting, common risk factors for hyperkalemia include chronic kidney disease (CKD), diabetes mellitus, decompensated congestive heart failure, volume depletion, advanced age and drugs that interfere in renal potassium exertion. Similar to this case, hyperkalemia may develop as a complication of therapy with SGLT2 inhibitors in patients with one or more defect in renal potassium excretion. In fact, hyperkalemia has been reported in patients who undergoing treatment with canagliflozin, particularly those predisposed to hyperkalemia due to moderate renal impairment (eGFR 45–60 mL/min × 1.73/m2) and in patients treated with potassium-sparing diuretics or inhibitors of the RAAS [4]. On the other hand, a pooled analysis [10] showed that DAPA is not associated with an increased risk of hyperkalemia in patients with T2DM, including patients at a higher risk of hyperkalemia, such as those with moderate renal impairment (eGFR 30–60 mL/min × 1.73/m2) or undergoing treatment with potassium-sparing diuretics or inhibitors of the RAAS. We believe that the true diagnosis of hyperkalemia might have been overlooked in the previous study because measurement of post-potassium levels was only performed 1 and 4 weeks after DAPA administration. Although further precise studies should examine whether SGLT2 inhibitors affect the risk of hyperkalemia, this case suggested that frequent potassium monitoring is clinically important after initiating treatment with SGLT2 inhibitors in patients with the use of RAAS inhibitors or adrenal insufficiency.
Since SGLT-2 inhibitors do not exhibit glucose-lowing efficacy in patients with moderate renal impairment, DAPA is not recommended for use in patients with an eGFR < 60 mL/min × 1.73/m2, and similarly canagliflozin and empagliflozin should not be used in patients with an eGFR < 45 mL/min × 1.73/m2 [11]. However, SGLT-2 inhibitors could be indicated in patients with impaired renal function in terms of nephroprotection [12]. Kohen et al. [13] reported an independent reduction in UACR with DAPA in patients with stage 3 CKD. Moreover, on a subgroup analysis of the EMPA-REG OUTCOME trial [14], CKD patients treated with empagliflozin experienced a 44% significant reduction in the relative risk of incident or worsening nephropathy compared with those receiving placebo. These outcomes provide strong support for a nephroprotective effect of SGLT2 inhibitors. The combined strategy of dual SGLT2 and RAAS inhibition may be considered to be essential for nephroprotection in patients with T2DM and CKD; as such, the combined use of SGLT2 and RAAS inhibitors is expected to increase. Of note, the risk of hyperkalemia is potentially higher in these populations, as in the current case.
In conclusion, this is the first report to directly demonstrate that an SGLT2 inhibitor potentially increased the need for mineralocorticoid replacement in a patient after bilateral adrenalectomy. This case suggested that the SGLT2 inhibition-induced natriuretic effect is accompanied by compensatory activation of the RAAS axis, which is essential to keep the serum potassium level within a normal range. Therefore, physicians should routinely evaluate the patients’ pathological condition, especially the RAAS status before prescribing an SGLT2 inhibitor and monitor the patient’s potassium level after initiating or modifying medications that block the SGLT2 and RAAS.
Compliance with ethical standards
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was obtained from the patient for the publication of this case report.
References
- 1.Ferrannini E, et al. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33:2217–2224. doi: 10.2337/dc10-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imprialos KP, et al. Sodium-glucose cotransporter-2 inhibitors and blood pressure decrease: a valuable effect of a novel antidiabetic class? J Hypertens. 2015;33:2185–2197. doi: 10.1097/HJH.0000000000000719. [DOI] [PubMed] [Google Scholar]
- 3.Heerspink HL, et al. Composite renal endpoints: was ACCOMPLISH accomplished? The Lancet. 2010;375:1140–1142. doi: 10.1016/S0140-6736(10)60098-0. [DOI] [PubMed] [Google Scholar]
- 4.Weir MR, et al. Effect of canagliflozin on serum electrolytes in patients with type 2 diabetes in relation to estimated glomerular filtration rate (eGFR) Curr Med Res Opin. 2014;30:1759–1768. doi: 10.1185/03007995.2014.919907. [DOI] [PubMed] [Google Scholar]
- 5.Ernst ME, et al. Use of diuretics in patients with hypertension. N Engl J Med. 2010;361:2153–2164. doi: 10.1056/NEJMra0907219. [DOI] [PubMed] [Google Scholar]
- 6.Cherney DZ, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–597. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 7.Lambers Heerspink HJ, et al. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–862. doi: 10.1111/dom.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zacchia M, et al. Potassium: from physiology to clinical implications. Kidney Dis (Basel) 2016;2:72–79. doi: 10.1159/000446268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med. 2004;351:585–592. doi: 10.1056/NEJMra035279. [DOI] [PubMed] [Google Scholar]
- 10.Yavin Y, et al. Effect of the SGLT2 inhibitor dapagliflozin on potassium levels in patients with type 2 diabetes mellitus: a pooled analysis. Diabetes Ther. 2016;7:125–137. doi: 10.1007/s13300-015-0150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vivian EM. Sodium-glucose co-transporter 2 (SGLT2) inhibitors: a growing class of antidiabetic agents. Drugs Context. 2014;3:212264. doi: 10.7573/dic.212264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fioretto P, et al. SGLT2 inhibitors and the diabetic kidney. Diabetes Care. 2016;39(Suppl 2):S165-171. doi: 10.2337/dcS15-3006. [DOI] [PubMed] [Google Scholar]
- 13.Kohan DE, et al. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962–971. doi: 10.1038/ki.2013.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wanner C, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]