Abstract
We report a case of smoking-related idiopathic nodular glomerulosclerosis (ING) with overexpression of glomerular advanced glycation end products (AGEs) and their receptor (RAGE). A 59-year-old Japanese man with nephrotic syndrome, who had a smoking history of one pack of cigarettes per day for approximately 40 years, presented with a 3-year history of urinalysis abnormalities without clinical evidence of diabetic mellitus. The patient’s leg edema progressively worsened over the previous 2 years, and he was admitted to our hospital. Renal biopsy showed mesangial expansion with diabetic Kimmelstiel–Wilson-like nodular lesions, glomerular basement thickening, and arteriosclerosis. No electron-dense deposits, fibrils, or microtubule deposits were seen in the glomeruli on electron microscopy. Skin AGE level measured using AGE reader was higher in this case than the average level in age-matched Caucasians. In addition, immunohistochemical analysis revealed that N-carboxymethyl lysine, one of the major AGEs, and RAGE were overexpressed and podocin expression was decreased in the peripheral area of the glomerular nodular lesions. These observations suggest that AGEs–RAGE system may be activated in smoking-related ING, possibly leading to the progression of renal dysfunction.
Keywords: Idiopathic nodular glomerulosclerosis, Cigarette smoking, Hypertension, Advanced glycation end products, RAGE
Introduction
Idiopathic nodular glomerulosclerosis (ING) is an enigmatic condition that resembles nodular diabetic glomerulosclerosis in non-diabetic patients. Nodular mesangial sclerosis, glomerular basement membrane (GBM) thickening, and a lack of immunoglobulin (Ig) deposits are known to be major histological manifestations of diabetic nephropathy. However, more than 40 cases presenting with ING without clinical evidence of diabetes mellitus (DM) have been reported in the English literature. According to these case reports, 34% of patients with ING progressed to end-stage renal disease within 2 years from diagnosis [1]. Although high rates of ING occurrence have been reported in heavy smokers, the underlying mechanism by which smoking can induce the formation of glomerular nodular lesions is not fully understood.
Advanced glycation end products (AGEs) are heterogeneous and complicated compounds formed by a non-enzymatic reaction between reduced sugar and amino acid of proteins, lipids, and DNAs [2, 3]. Several basic and clinical studies have implicated AGEs and their receptor (RAGE) in the progression of not only diabetic nephropathy, but also obesity-related glomerulosclerosis, amyloidosis, and polycystic kidney disease [4–7]. On the other hand, smoking itself produces free radicals that are capable of inducing oxidative stress in several organs, which stimulates the formation of AGEs such as N-carboxymethyl lysine (CML). Serum AGEs concentration was reportedly elevated in smokers compared with non-smokers [8–10]. These findings suggest that smoking-derived AGEs–RAGE activation may be associated with the progression of ING in hypertensive patients who smoke. Here we report a case of ING and its relation to the AGEs–RAGE system in a hypertensive patient who was a heavy smoker.
Case report
A 59-year-old Japanese man was admitted to our hospital because of a 3-year history of urinary abnormalities. Urinary analysis showed 3 + proteinuria, 2 + microscopic hematuria, and inactive sediment, and the urinary protein excretion level was 4.06 g/gCr. An ultrasound showed the size of both kidneys to be within the normal range (Rt: 113 × 73 mm, Lt: 120 × 69 mm), and there was no evidence of renal artery stenosis. The patient had a smoking history of one pack of cigarettes per day for approximately 40 years (Brinkman index: more than 800) and had completely quit smoking 4 months before admission. Hypertension and hyperlipidemia were detected 2 years ago; since then, he had been taking an anti-hypertensive drug (valsartan: 80 mg per day) and an HMG-CoA reductase inhibitor (atorvastatin: 10 mg per day). However, his systolic blood pressure (BP) remained around 160–170 mmHg at the time of admission. Pretibial pitting edema was observed, and his body weight had increased by 8 kg in the past 2 years. Spirometry showed that forced expiratory volume% in 1 s (FEV1%) and FEV1 were 61.9 and 58.1%, which are classified as moderate chronic obstructive pulmonary disease (COPD) [11]. His body mass index (BMI) was 25.2 kg/m2. Neither lymphadenopathy nor suspected systemic infection was found. Laboratory examinations revealed that serum creatinine was 0.75 mg/dL, creatinine clearance was 123.2 mL/min/1.73 m2, and the selectivity index was 0.284, which indicates low selectivity. Total cholesterol, low-density lipoprotein, and high-density lipoprotein were 229, 87.3, and 88.1 mg/dL, respectively. Furthermore, glycated hemoglobin (HbA1c) and glycated albumin (GA) levels were 5.6 and 10.6%, which are within the normal ranges. The 2-h plasma glucose level, measured using the 75-g oral glucose tolerance test (75-g OGTT), was 118 mg/dL, indicating normal glucose tolerance. The fasting plasma immunoreactive insulin level was 7.7 μU/mL, and the homeostasis model assessment of insulin resistance (HOMA-IR) was 2.1, indicating no evidence of insulin resistance. Serum total protein and albumin levels were 5.73 and 2.58 g/dL, respectively, and serum concentrations of IgA, IgM, and IgG were 417, 59, and 1216 mg/dL, respectively. Serum complement levels were within the normal ranges (C3: 118 mg/dL, C4: 28 mg/dL). Serum-free light chain/ratio was 1.38 (normal range 0.248–1.804). This result rules out immunotactoid/light-chain deposition disease. Neither hepatitis B virus antigen nor anti-hepatitis C virus antibody was detected. Immunological examination demonstrated that antinuclear antibody, myeloperoxidase–antineutrophil cytoplasmic antibody, proteinase-3-antineutrophil cytoplasmic antibody, and anti-GBM antibody were all negative. AGE reader, capable of measuring skin AGE accumulation, showed that autofluorescence value in this patient was higher [2.6 arbitrary units (AU)] than the average value in age-matched Caucasian subjects (2.09 ± 0.36 AU) [12] and Japanese heavy smokers (2.23 ± 0.31 AU) [13].
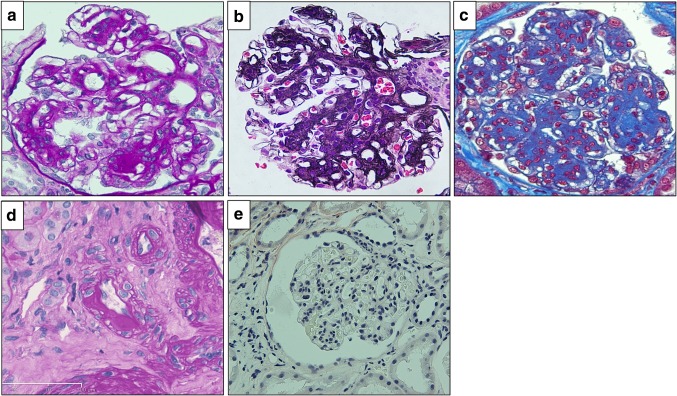
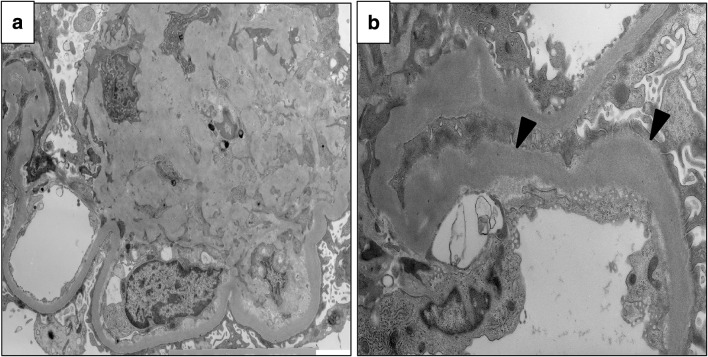
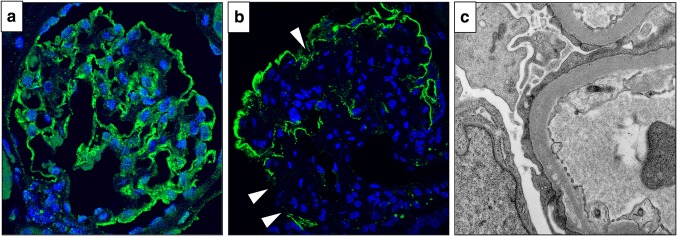
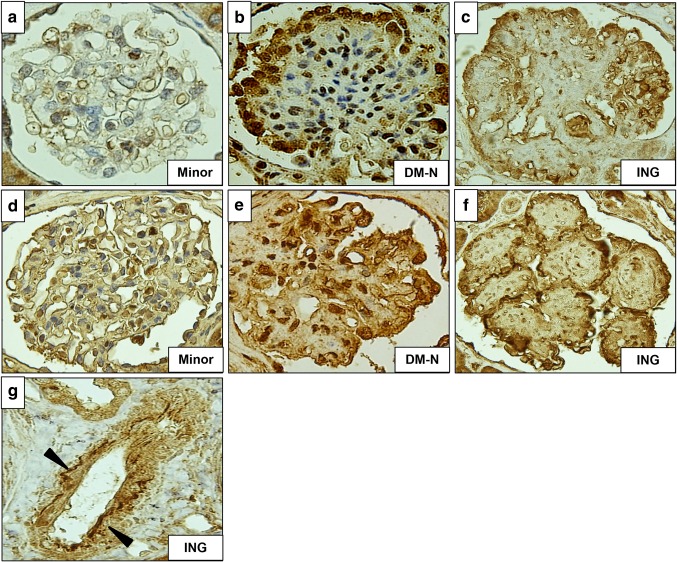
On renal biopsy, 22 glomeruli were obtained. Three glomeruli exhibited global sclerosis, one had adhesion, and all glomeruli had no crescent. All of the remaining glomeruli showed mesangial area expansion with severe mesangial hypercellularity, and some of them formed nodular lesions (Fig. 1a–c). Hyalinosis which suggested arteriosclerosis was seen in interlobular arteries, but glomerular polar vasculosis, a characteristic feature of diabetic nephropathy, was not detected (Fig. 1d). Tubular atrophy and interstitial fibrosis were mild, with less than 25% of the cortex being affected. Direct fast scarlet staining was negative, leading to exclusion of renal amyloidosis (Fig. 1e). Immunofluorescence analysis demonstrated linear staining for IgG, kappa and lambda chains on GBM (figure not shown). Electron microscopy (EM) revealed mesangial area expansion and mesangial interposition (Fig. 2a). Irregular GBM thickening without prominent evidence of electron-dense deposits was also observed through EM. Neither amyloid fibrils nor microtubular pattern of deposits was seen in the thickened GBM (Fig. 2b). Immunofluorescence analysis showed that compared with minor glomerular abnormalities, podocin expression was decreased and foot processes were effaced, as shown by EM (Fig. 3a–c), which implicated podocyte dysfunction in this patient. In addition, in our patient, CML accumulation and RAGE expression were enhanced in the peripheral area of nodular lesions such as those in diabetic nephropathy (Fig. 4a–f) compared with those of a patient with minor glomerular abnormalities. CML also accumulated in the intimal and adventitial sides of the arterial vessel wall (Fig. 4g).
Fig. 1.
Light microscopy findings Light microscopy showed mesangial area expansion and the formation of nodular lesions with severe mesangial hypercellularity following PAM, PASM and Masson trichrome stainings (a–c). d shows hyalinosis of the lobular artery. Direct fast scarlet staining revealed no amyloid deposition in this patient’s kidneys (e). PAS periodic acid–Schiff, PASM periodic Schiff–Methenamine
Fig. 2.
Electron microscopy findings Electron microscopy revealed mesangial area expansion (a) and irregular GBM thickening (indicated by black arrows, b). GBM glomerular basement membrane
Fig. 3.
Histological findings related to podocyte injury Immunofluorescence analysis for podocin in a male patient with minor glomerular abnormalities (a) and in our presented patient (indicated by white arrows, b). Electron microscopy also showed foot process effacement (c) in our presented patient
Fig. 4.
Immunohistochemical analysis for AGEs–RAGE axis Immunohistochemical analysis for CML accumulation in the male patient with minor glomerular abnormalities (a), diabetic nephropathy (b), and the peripheral area of nodular lesions in our presented patient (c). CML accumulation was also seen in the arterial vessel wall as shown in g (indicated by black arrows). RAGE expression in the patient with minor glomerular abnormalities (d), diabetic nephropathy (e) and in our presented patient (f). CML N-carboxymethyl lysine, RAGE receptor for advanced glycation end products
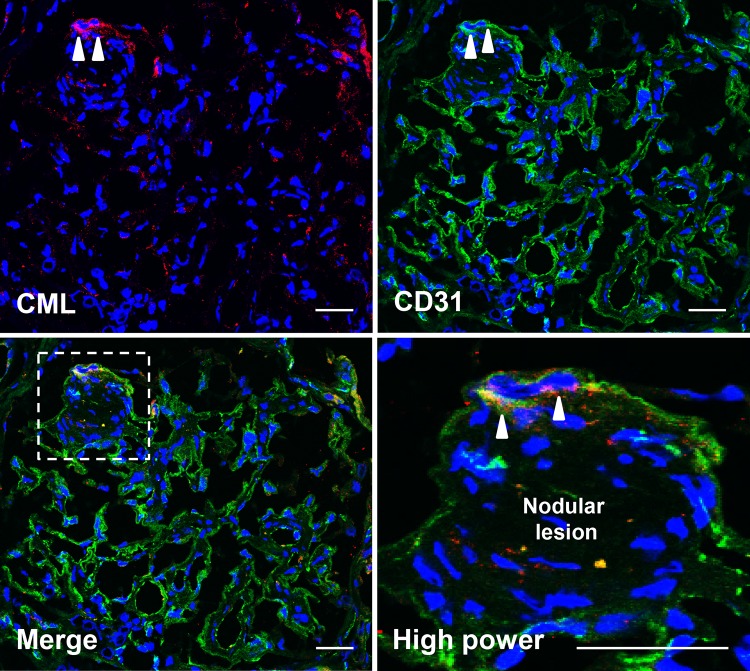
To determine the underlying mechanism of ING formation, we performed immunofluorescence staining for CML and CD31, one of the endothelial markers, in the glomeruli of the presented patient with ING. As shown in Fig. 5, we found CML accumulation in the glomeruli, which was partly merged with CD31 expression at the tuft around the nodular lesions.
Fig. 5.
Immunofluorescence staining for CML and CD31 Double-staining for CML and CD31, one of the endothelial markers, revealed that CML accumulated at the endothelium around nodular lesions in the glomerulus of the presented patient in this case, suggesting that CML accumulation from blood stream into endothelium may be a potent pathogenic mechanism for the formation of nodular lesion in ING patients. Scale bar 10 μm
Discussion
ING is clinically diagnosed on the basis of histological findings such as nodular mesangial sclerosis, irregular GBM thickening, and a lack of Ig deposits without clinical evidence of DM. In this case, HbA1c, GA levels, and 75-g OGTT showed no glucose intolerance, and his serum insulin and HOMA-IR levels were within the normal ranges. These observations suggest that the development of ING could be independent of DM and insulin resistance in this patient. Although the patient’s BMI was 25.2 kg/m2, defined as slightly overweight, because the criterion for the diagnosis of obesity-related glomerulopathy (ORG) is BMI > 30 kg/m2 and the median BMI in ORG patients is 41.7 kg/m2, ORG should be excluded as well [14]. Ischemic nephropathy was excluded because of the absence of renal artery stenosis. Since ING is highly prevalent in heavy smokers, smoking-derived harmful substances have been thought to correlate with ING progression. It has been demonstrated that nicotine, a major component of tobacco, increases mesangial cell proliferation [15]. Although hypercellular nodules in this case are not common findings of ING, similar nodules with increasing mesangial cells have been presented as smoking-related glomerulopathy with focal segmental glomerulosclerosis(FSGS)-like lesion [16]. In this case, the mechanism underlying the mesangial cell proliferation in the patient with ING is unclear. Smoking-induced CML accumulation in the endothelium and RAGE overexpression in podocytes may be correlated with each other, thereby activating mesangial cell proliferation. Skin AGE level in this patient was higher than the average level in the age-matched Japanese heavy smokers. CML was strongly expressed in nodular lesions and the arterial vessel wall. Indeed, immunofluorescence double staining for CML and CD31 revealed that CML strongly accumulated in the endothelium of the tuft around nodular lesions in the ING patient, which was significantly different from that of DM-N. In addition, the patient in this case suffered from COPD. Since AGE expression in the serum and lung tissue has been shown to be much higher in COPD patients than in age-matched subjects with the same smoking history [17], the combination of longstanding hypertension, smoking history, and COPD could be implicated in the development of ING.
The aberrant mesangial expansion and increased nodular lesions in this case may have reduced the glomerular capillary area, leading to high intracapillary pressure. Podocyte injury may be induced by exposure to hyperfiltration-induced mechanical forces and fluid flow shear stress [18], which resulted in nephrotic-range proteinuria in this case. Since AGEs themselves induce podocyte detachment via the stimulation of angiotensin II production, which is attenuated by angiotensin II blockade (AIIB) therapy [19], the control of BP, especially with AIIB therapy, may help prevent podocyte injury in patients with ING.
CML accumulated in the intimal and adventitial sides of the arterial vessel wall (Fig. 4g). AGEs form non-specific cross-link in several organs such as the kidney and arteries. AGEs are strongly detected in the intimal and adventitial layers of the aorta in diabetic rats [20]. Therefore, CML accumulation in the intimal and adventitial sides may accelerate endothelial dysfunction, which may have led to arteriosclerosis in this patient.
We observed the co-localization of CD31 and CML in this patient (Fig. 5). These findings are supported by a previous report showing that the predominant site of AGEs staining within glomeruli was endothelial cells in control and diabetic rats [20]. Furthermore, previous reports have demonstrated that smoking results in oxidative stress, accelerating AGEs production inside the body [13] and tobacco leaves/cigarette smoke contain high level of AGEs that are inhaled and then transported into the bloodstream [8]. Therefore, smoking-derived AGEs may enter the glomerulus tissue through the endothelium from the blood stream, leading to the formation of nodular lesion, which contributes to renal dysfunction.
In conclusion, this case highlights the possible role of smoking-derived AGEs–RAGE activation in the development of ING without glucose intolerance. Hypertensive patients with AGEs–RAGE overexpression possibly due to smoking and COPD are more prone to develop ING. Although the management of hypertension with AIIB therapy is an ideal therapeutic strategy, the longstanding smoking-derived AGEs–RAGE overexpression may not be fully negated by AIIB therapy. Therefore, direct intervention targeting the AGEs–RAGE axis in addition to discontinuation of smoking may be a potent therapeutic strategy for the prevention of ING progression.
Acknowledgements
The authors would like to thank the members of the Kyusyu Okinawa Kidney Biopsy forum for their useful discussion. The authors also would like to thank Enago (http://www.enago.jp) for English language review.
Compliance with ethical standards
Informed consent
Informed consent was obtained from the patient in this study.
Conflict of interest
The authors declare that no conflict of interest exists.
References
- 1.Markowitz GS, Lin J, Valeri AM, Avila C, Nasr SH, D’Agati VD. Idiopathic nodular glomerulosclerosis is a distinct clinicopathologic entity linked to hypertension and smoking. Hum Pathol. 2002;33(8):826–835. doi: 10.1053/hupa.2002.126189. [DOI] [PubMed] [Google Scholar]
- 2.Stinghen AE, Massy ZA, Vlassara H, Striker GE, Boullier A. Uremic toxicity of advanced glycation end products in CKD. J Am Soc Nephrol. 2016;27(2):354–370. doi: 10.1681/ASN.2014101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao Y, Ootaka T, Saito T, Sato H, Sato T, Ito S. The involvement of advanced glycation endproducts (AGEs) in renal injury of diabetic glomerulosclerosis: association with phenotypic change in renal cells and infiltration of immune cells. Clin Exp Nephrol. 2003;7(3):201–209. doi: 10.1007/s10157-003-0245-z. [DOI] [PubMed] [Google Scholar]
- 4.Fukami K, Taguchi K, Yamagishi S, Okuda S. Receptor for advanced glycation endproducts and progressive kidney disease. Curr Opin Nephrol Hypertens. 2015;24(1):54–60. doi: 10.1097/MNH.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 5.Rhee H, Song SH, Kwak IS, Kim IY, Seong EY, Lee DW, et al. An explorative analysis of secretory receptor for advanced glycation endproducts in primary focal segmental glomerulosclerosis. Clin Exp Nephrol. 2012;16(4):589–595. doi: 10.1007/s10157-012-0599-1. [DOI] [PubMed] [Google Scholar]
- 6.Tomino Y, Hagiwara S, Gohda T. AGE-RAGE interaction and oxidative stress in obesity-related renal dysfunction. Kidney Int. 2011;80(2):133–135. doi: 10.1038/ki.2011.86. [DOI] [PubMed] [Google Scholar]
- 7.Park EY, Kim BH, Lee EJ, Chang E, Kim DW, Choi SY, et al. Targeting of receptor for advanced glycation end products suppresses cyst growth in polycystic kidney disease. J Biol Chem. 2014;289(13):9254–9262. doi: 10.1074/jbc.M113.514166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerami C, Founds H, Nicholl I, Mitsuhashi T, Giordano D, Vanpatten S, et al. Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci USA. 1997;94(25):13915–13920. doi: 10.1073/pnas.94.25.13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biswas SK, Mudi SR, Mollah FH, Bierhaus A, Arslan MI. Serum soluble receptor for advanced glycation end products (sRAGE) is independently associated with cigarette smoking in non-diabetic healthy subjects. Diab Vasc Dis Res. 2013;10(4):380–382. doi: 10.1177/1479164113479618. [DOI] [PubMed] [Google Scholar]
- 10.Prasad K, Dhar I, Caspar-Bell G. Role of advanced glycation end products and its receptors in the pathogenesis of cigarette smoke-induced cardiovascular disease. Int J Angiol. 2015;24(2):75–80. doi: 10.1055/s-0034-1396413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 12.Koetsier M, Lutgers HL, de Jonge C, Links TP, Smit AJ, Graaff R. Reference values of skin autofluorescence. Diabetes Technol Ther. 2010;12(5):399–403. doi: 10.1089/dia.2009.0113. [DOI] [PubMed] [Google Scholar]
- 13.Nomoto K, Yagi M, Arita S, Ogura M, Yonei Y. Skin accumulation of advanced glycation end products and lifestyle behaviors in Japanese. Anti Aging Med. 2012;9(6):165–173. [Google Scholar]
- 14.D’Agati VD, Chagnac A, de Vries AP, Levi M, Porrini E, Herman-Edelstein M, et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol. 2016;12(8):453–471. doi: 10.1038/nrneph.2016.75. [DOI] [PubMed] [Google Scholar]
- 15.Jaimes EA, Tian RX, Joshi MS, Raij L. Nicotine augments glomerular injury in a rat model of acute nephritis. Am J Nephrol. 2009;29(4):319–26. doi: 10.1159/000163593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salvatore SP, Troxell ML, Hecox D, Sperling KR, Seshan SV. Smoking-related glomerulopathy: expanding the morphologic spectrum. Am J Nephrol. 2015;41(1):66–72. doi: 10.1159/000371727. [DOI] [PubMed] [Google Scholar]
- 17.Wu L, Ma L, Nicholson LF, Black PN. Advanced glycation end products and its receptor (RAGE) are increased in patients with COPD. Respir Med. 2011;105(3):329–336. doi: 10.1016/j.rmed.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Durvasula RV, Petermann AT, Hiromura K, Blonski M, Pippin J, Mundel P, et al. Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int. 2004;65(1):30–39. doi: 10.1111/j.1523-1755.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- 19.Fukami K, Yamagishi S, Kaifu K, Matsui T, Kaida Y, Ueda S, et al. Telmisartan inhibits AGE-induced podocyte damage and detachment. Microvasc Res. 2013;88:79–83. doi: 10.1016/j.mvr.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Soulis T, Thallas V, Youssef S, Gilbert RE, McWilliams BG, Murray-McIntosh RP, et al. Advanced glycation end products and their receptors co-localise in rat organs susceptible to diabetic microvascular injury. Diabetologia. 1997;40(6):619–628. doi: 10.1007/s001250050725. [DOI] [PubMed] [Google Scholar]