Abstract
We had encountered the case of membranous glomerulonephritis (MGN) with dilated cardiomyopathy due to LMNA gene mutation. LMNA mutation was known as a cause of ‘laminopathy’ such as dilated cardiomyopathy, muscular dystrophy, neuropathy and so on. LMNA gene might be a candidate of genetic basis in cryptogenic MGN.
Keywords: Membranous glomerulonephritis, Dilated cardiomyopathy, Lamin A/C, LMNA
Introduction
We witnessed the case of a patient with membranous glomerulonephritis (MGN) with dilated cardiomyopathy caused by an LMNA mutation. LMNA mutations are known to cause “laminopathies,” such as dilated cardiomyopathy, muscular dystrophy, and neuropathy [1]. LMNA mutations might be a genetic cause of cryptogenic MGN.
Case report
A 65-year-old man was admitted to our hospital for renal biopsy to identify the cause of proteinuria. The patient had been diagnosed with dilated cardiomyopathy (DCM) following an episode of syncope 8 years earlier and was treated with amiodarone and valsartan. A cardioverter defibrillator was implanted in him 7 years ago and he had undergone catheter ablation 1 year ago. He had experienced hypereosinophilia 2 years ago. Although the cause of hypereosinophilia was considered to be drug-induced allergy, medication had not been changed, because of the necessity to control cardiac disease.
His cardiac function worsened, and subsequently, he developed proteinuria. 1 year ago, during admission his serum creatinine was 1.6 mg/dL.
The patient’s height, weight, blood pressure, pulse rate, and body temperature were 162 cm, 57.8 kg, 108/68 mmHg, 60 bpm, and 36.2 °C, respectively. His consciousness was alart. Auscultation of his heart sounds revealed a gallop rhythm. No remarkable abnormalities were found in the lungs or abdomen. Pitting edema was present in his lower extremities.
The results of laboratory investigations were as follows: hemoglobin, 11.7 g/dL; hematocrit, 36.0%; platelets, 323,000/µL; white blood cells, 12,400/µL (lymphocytes 9.5%, monocytes 3.5%, eosinophils 42.5%, segmented neutrophils 43.5%, band neutrophils 1.0%); C-reactive protein, 0.6 mg/dL; blood urea nitrogen, 25.4 mg/dL; serum creatinine, 1.6 mg/dL; total cholesterol, 187 mg/dL; total protein, 6.4 g/dL; albumin, 2.8 g/dL; lactate dehydrogenase, 289 IU/L; aspartate aminotransferase, 29 IU/L; alanine aminotransferase, 20 IU/L; total bilirubin, 0.4 mg/dL; alkaline phosphatase, 387 IU/L; Na, 141 mmol/L; K, 4.5 mmol/L; and Cl, 106 mmol/L; BNP, 483.1 pg/mL; IgG, 1640 mg/dL; IgA, 243 mg/dL; IgM, 98 mg/dL; IgE, 2500 IU/ml; CH50, 36.0 U/mL; C3, 97.4 mg/dL; C4, 56.2 mg/dL; and anti-dsDNA antibody, 31 IU/mL. HBs antigen and HCV antibody were negative.
Urinalysis revealed 3+ proteinuria (1.79 g/day), 2+ hematuria, and 10–20 erythrocytes per high-power field. RBC cast was absent. Urinary beta 2-microglobulin, 28.0 µg/L; urinary NAG, 30.0 IU/L.
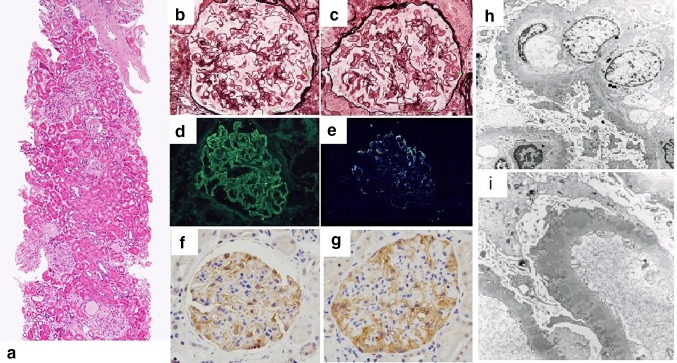
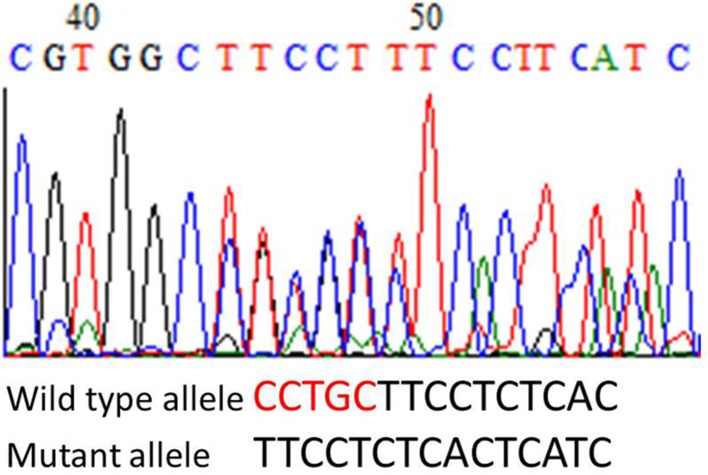
Renal biopsy was performed (Fig. 1). 50 glomeruli could be evaluated, 17 of which showed global sclerosis. Histological findings included diffused spike appearance in the subepithelial lesions and focal mesangial cell proliferation in the glomeruli. Inflammatory cell infiltration was sparse and eosinophils were almost never observed. Arteriolosclerotic changes were also observed. Immunofluorescence staining was strongly positive for IgG with a granular pattern and weakly positive for C3. PLA2R1 was positive along capillaries identified immunohistochemically. Electron microscopy revealed multiple, large electron-dense deposits in the subepithelial lesions with a few intramembranous deposits. He was diagnosed with primary membranous glomerulonephritis. Administration of 0.6 mg/kg/day prednisolone was started. His renal function remained stable, but the proteinuria persisted. His hypereosinophilia had got solved soon after prednisolone administration. One year later, genetic evaluation for cardiomyopathy was performed and mutation of the LMNA gene was established (Fig. 2).
Fig. 1.
a–c Micrographs of the glomeruli showing subepithelial spike formations. (a HE stains, b, c PAM stains), d, e immunoglobulin G and C3 deposition along the capillaries identified by immunostaining, f, g PLA2R1 was positive along capillaries identified immunohistochemically, h, i electron-dense deposits in the subepithelial and intramembranous region (3000×)
Fig. 2.
The DNA sequencing chromatogram revealed a deletion of CCTGC in the mutant allele
Discussion
LMNA (1q21.2–q21.3) encodes lamin A/C protein that belongs to the family of intermediate filaments. The protein lines the inner nuclear membrane of somatic cells, and is associated with the stabilization of nuclear membrane and regulation of cell division.
LMNA mutations cause various diseases, which are collectively called “laminopathy” and include dilated cardiomyopathy, muscular dystrophy, neuropathy, progeria, and partial lipodystrophy [1]. This patient presented with dilated cardiomyopathy associated with LMNA mutation and complicated by glomerulonephritis with, mainly, membranous lesions. MGN is divided into primary and secondary types. Secondary MGN can be due to various factor such as collagen vascular diseases, viral infections, drugs, and malignancies. Though the titer of anti-dsDNA antibody in this patient was slightly high, he did not fulfill the diagnostic criteria of systemic lupus erythematosus. No evidence of infection or malignancy were found. His medication history included amiodarone and valsartan, which have been hardly reported to cause glomerulonephritis. We could identify only few papers describing the possibility of membranous glomerulonephritis induced by the drugs [2]. We speculate that there are too few cases to clarify the relation between the drugs and glomerular lesions. Major histological findings in drug-induced renal diseases, such as tubulointerstitial nephritis or eosinophilic infiltration were not observed in our patient, although hypereosinophilia could be a manifestation of drug allergy.
MGN due to unknown etiology is classified as primary MGN. Phospholipase A2 receptor (PLA2R) expressed in podocytes was found to be the target antigen in about 70% of cases of primary MGN [3]. The importance of HLA-DQA11 in the onset of primary MGN was also described by Stranescu et al. [4]. Our case was proved PLA2R1 expression, and was considered to be primary MGN. The genetic background of primary MGN is still not completely understood in many cases. This is the first case report of cryptogenic MGN in a patient with an LMNA mutation.
Reported cases of glomerulonephritis with LMNA mutations were rare. However, partial lipodystrophy, which is a laminopathy, was reported to be associated with glomerulonephritis. The type of glomerulonephritis associated with partial lipodystrophy is believed to be type 2 membranoproliferative glomerulonephritis (MPGN), or C3 nephropathy, according to recent reports. Only one case report by Chartier S et al. had suggested a relationship between partial lipodystrophy and type 3 MPGN [5]. Owen KR et al. had reported the case of a patient with type 2 MPGN with an LMNA mutation [6]. Activation of the alternative complement pathway by C3 nephritic factor had been hypothesized as an important factor for the coexistence of lipodystrophy and MPGN [7]. Javor et al. argued for association with lipodystrophy and focal segmental glomerulosclerosis, but they could not comment on the etiology of the phenomenon completely [8].
Although lipodystrophy was not found in this case, the glomerulonephritis in this case might be similar to that associated with lipodystrophy based on an LMNA mutation. LMNA gene might be candidate of genetic basis in cryptogenic MGN. The association of glomerulonephritis and dilated cardiomyopathy has been hardly reported. Further studies to investigate the possibility of an LMNA mutation as an etiology of membranous glomerulonephritis or other types of renal disease might be valuable.
Acknowledgements
We would like to thank Dr. Kazuaki Kaitani and Dr. Naoaki Onishi, department of cardiology, Japanese Red Cross Otsu Hospital, Japan, for their great contribution. Furthermore, we thank Dr. Yoichiro Kobashi and Mr. Shiomi Yoshida, Tenri Institute of Medical Research, Japan for their help with the histological analyses.
Compliance with ethical standards
Conflict of interest
All the authors have declared that no conflict of interest exists.
Human and animal rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from the patient for being included in the study.
References
- 1.Broers JLV, Ramaekers FCS, Bonne G, Yaou RB, Hutchison AC. Nuclear lamins: laminopathies and their role in premature ageing. Physiol Rev. 2006;86:967–1008. doi: 10.1152/physrev.00047.2005. [DOI] [PubMed] [Google Scholar]
- 2.Kimura T, Kuramochi S, Katayama T, Hoshikawa T, Yamada T, Ueda Y, Okada Y. Amiodarone-related pulmonary mass and unique membranous glomerulonephritis in a patient with valvular heart disease: diagnostic pitfall and new findings. Pathol Int. 2008;58:657–663. doi: 10.1111/j.1440-1827.2008.02286.x. [DOI] [PubMed] [Google Scholar]
- 3.Beck LH, Bonegio RGB, Lambeau G, Beck DM, Powell DW, Cummins TD, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanescu HC, Arcos-Burgos M, Mediar A, Bockenhauer D, Kottgen A, Dragomirescu L, et al. Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med. 2011;364:616–626. doi: 10.1056/NEJMoa1009742. [DOI] [PubMed] [Google Scholar]
- 5.Chartier S, Buzzanga JB, Paquin F. Parial lipodystrophy associated with a type 3 form of membranoproliferative glomerulonephritis. J Am Acad Dermatol. 1987;16:201–205. doi: 10.1016/S0190-9622(87)80062-2. [DOI] [PubMed] [Google Scholar]
- 6.Owen KR, Donohoe M, Ellard S, Clarke TJ, Nicholls AJ, Hattersley AT, Bingham C. Mesangiocapillary glomerulonephritis type 2 associated with familial partial lipodystrophy (Dunnigan-Kobberling syndrome) Nephron Clin pract. 2004;96:c35-c38. doi: 10.1159/000076396. [DOI] [PubMed] [Google Scholar]
- 7.Peters DK, Charlesworth JA, Sissons JG, Williams DG, Boulton-Jones JM, Evasn DJ, et al. Mesangiocapillary nephritis, partial lipodystrophy, and hypocomplementaemia. Lancet. 1973;2:535–538. doi: 10.1016/S0140-6736(73)92351-9. [DOI] [PubMed] [Google Scholar]
- 8.Javor ED, Moran SA, Young JR, Cochran EK, Depaoli AM, Oral EA, et al. Proteinuric nephropathy in acquired and congenital generalized lipodystrophy: base line characteristics and course during recombinant leptin therapy. J Clin Endocrinol Metab. 2004;89:3199–3207. doi: 10.1210/jc.2003-032140. [DOI] [PubMed] [Google Scholar]