Abstract
Pregnancy and membranous nephropathy (MN) can occur concurrently with nephrotic syndrome. However, the pathophysiology of MN associated with pregnancy remains unclear, including the involvement of anti-M-type phospholipase A2 receptor (PLA2R) antibody, the major antigen of idiopathic MN (iMN). A treatment for the condition is also not established. We present the case of a 43-year-old pregnant female with incidental proteinuria and hypoalbuminemia. We made a diagnosis of nephrotic syndrome at 11 week gestation. Renal biopsy revealed iMN using predominant granular staining of IgG4 along the glomerular basement membrane. No secondary cause was identified. Oral glucocorticoid therapy was started from 17 week gestation and induced complete remission at 28 week gestation. A healthy infant was born at 38 week gestation. Glucocorticoid therapy was stopped postpartum without MN relapse. Anti-PLA2R antibody was later found to be positive using serum reserved from before treatment. In conclusion, we presented the case of a pregnant woman with iMN and anti-PLA2R antibodies, whose nephrotic syndrome was successfully controlled with oral glucocorticoids to reach complete remission, even after tapering off the medication. Pregnancy per se might be associated with iMN onset.
Keywords: Membranous nephropathy, Pregnancy, Anti-phospholipase A2 receptor antibody, Case report
Introduction
When proteinuria is detected before 20 week gestation, primary or secondary renal disease should be considered rather than preeclampsia [1]. Several studies have shown that patients with chronic kidney disease are at risk for adverse pregnancy outcomes, but it is unclear how to treat gestational proteinuria and when labor should be induced [2, 3]. The decision-making process should involve the pregnant woman and the spouse in such cases.
Membranous nephropathy (MN) is a major cause of nephrotic syndrome in adults [4, 5]. Phospholipase A2 receptor (PLA2R), which is expressed in podocytes, has been reported to be the major antigen in idiopathic membranous nephropathy (iMN) [6]. A pregnant woman may have this disease before 20 week gestation. Because anti-PLA2R antibody belongs to the immunoglobulin (Ig) G subclass, this autoantibody can affect the maternal and the fetal kidney by passing through the placenta. Adequate therapy during pregnancy is associated with favorable outcomes in nephrotic women with iMN associated with anti-PLA2R antibody [7].
Here, we present the case of a pregnant woman with iMN with anti-PLA2R antibody, whose nephrotic syndrome was successfully controlled using oral glucocorticoids to reach complete remission. After delivery, the patient’s iMN did not relapse when we tapered off the therapy. This is the second reported case of a patient with anti-PLA2R antibody-positive MN during pregnancy. In the first case, the patient had MN already when pregnancy occurred [7], but the duration of the pregnancy and nephrotic syndrome was nearly identical in the present case. The severity of nephrotic syndrome and the treatment regimens were completely different. We discuss the mechanism of iMN onset during pregnancy and the potential effectiveness of measuring anti-PLA2R antibody to diagnose and manage nephrotic syndrome in pregnant women.
Case report
A 43-year-old Japanese woman (0 gravida, 0 para) was incidentally found to have severe proteinuria (3.6 g/g creatinine [g/gCr]) and hypoalbuminemia (2.0 g/dL) at a previous hospital. A diagnosis of nephrotic syndrome was made, and subsequent examination revealed that the patient was in at 11 week gestation. She was then referred to our hospital.
On admission, the patient was 148 cm tall and weighed 51 kg. Her blood pressure (BP) was 101/49 mmHg and she had mild leg edema. She had no significant change in weight and no history of a recent infection. She showed a decreased serum albumin of 2.0 g/dL, with a normal serum creatinine level of 0.51 mg/dL. Urinary protein was 2.6 g/gCr by spot urine and 1.3 g by 24-h urine. Serum antinuclear antibody was less than × 40 and Rheumatic factor was less than 8 IU/mL. Anti-hepatitis B surface antigen antibodies and Anti-hepatitis C virus antibodies were negative. No tumor was not identified by abdominal ultrasound at the previous hospital. Serum tumor markers such as carcinoembryonic antigen, Carbohydrate antigen 19-9, and Cancer antigen 125 were all negative.
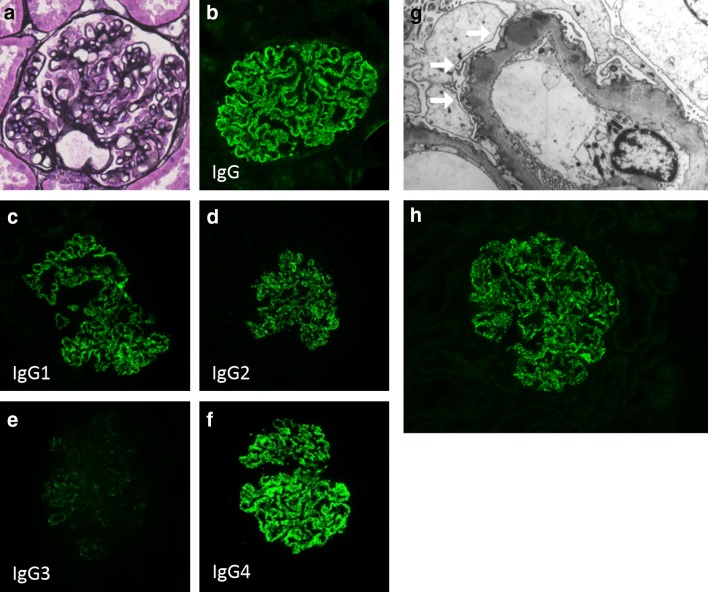
She consented to undergo further examination, and renal biopsy was performed at 15 week gestation (Fig. 1). Light microscopic examination of the renal specimen containing 27 glomeruli revealed typical histological features of membranous nephropathy, as follows: thickening of the glomerular basement membrane (GBM) and spike formation and bubbly appearance on periodic acid methenamine silver (PAM) stain (Fig. 1a). Immunofluorescence staining showed that IgG deposits along the GBM had a diffuse granular pattern (Fig. 1b). Among IgG subclasses, IgG4 staining was the most predominant (Fig. 1c–f). Electron microscopic examination revealed subepithelial dense deposits (Fig. 1g). We made a diagnosis of iMN with histological stage (Ehrenreich-Churg) II.
Fig. 1.
Representative Figure showing MN. a PAM stain showed slight thickening of the GBM (original magnification ×200). b Immunofluorescence microscopy shows granular IgG deposits in the basement membrane. c–f IgG subclass staining revealed that the dominant class is IgG4 (original magnification ×200). g Electron microscopy shows electron dense deposits in subepithelial area of the GBM (white arrows; original magnification ×5000). h Additional retrospective evaluation by immunofluorescence microscopy revealed granular anti-PLA2R1 deposits in the basement membrane
The clinical course is shown in Fig. 2. As the patient’s hypoalbuminemia progressed, oral corticosteroid therapy was started with 40 mg prednisolone daily (0.8 mg/kg/day) from 17 week gestation. After starting corticosteroid administration, there was a gradual improvement of proteinuria and hypoalbuminemia without any accompanying symptoms of clinical importance except steroid diabetes, which required temporary insulin administration by subcutaneous injection. At 22 week gestation, urinary protein excretion decreased to 0.6 g/day and serum albumin increased to 2.5 g/dL; we then began to taper off the steroid dose. The urinary protein continued to decrease to 0.2 g/day at the 28 week gestation, the prednisolone dose was then fixed at 10 mg at 32 weeks gestation. The estimated fetal weight was within the normal range.
Fig. 2.
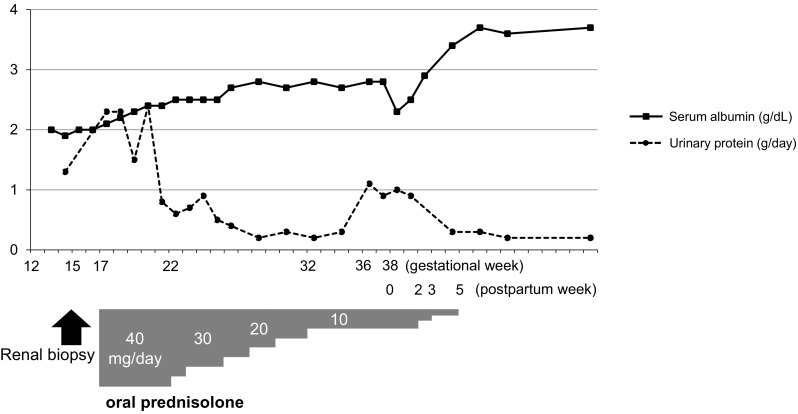
Clinical course of our patient. Serum albumin (solid line, g/dL) and 24-h urinary protein (dotted line, g/day) are plotted by the gestation week and the postpartum week. An arrow indicates the timing for the renal biopsy, and a gray bar indicates prednisolone dosage (mg/dL)
At 36 week gestation, however, proteinuria suddenly increased to 1.1 g/day, which was accompanied by an elevated BP of 130/80 mmHg. Ultrasound revealed fetal growth retardation. We induced labor at 38 week gestation, and a male infant was born (weight, 2095 g; length, 48 cm; cranial circumference, 31.5 cm). The infant’s Apgar score was 8 at 1 min and 9 at 5 min. He showed no apparent signs of nephrotic syndrome or severe proteinuria. The placenta was relatively small (weight, 220 g), but there were no histological abnormalities such as infarction, decidual vasculopathy, or accelerated villous maturation.
After delivery, the patient’s blood pressure and urinary protein returned to normal within 5 week postpartum, and prednisolone was tapered off. She had an uneventful course without any evidence of relapse, and the infant showed normal growth parameters after birth. Maternal serum that was collected before treatment was initiated was found to be weakly positive for anti-PLA2R antibody of 13.2 RU/mL (cutoff value; 2 RU/mL [8]), which was measured using a Western blot, the same method used in the previous study [9]. We also evaluated the renal deposition of PLA2R retrospectively. By immunofluorescence, glomerular deposition of PLA2R1 was detected in a frozen section using rabbit polyclonal anti-PLA2R1 antibodies (Atlas Antibodies AB, Bromma, Sweden) at a dilution of 1:100 followed by FITC-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch, Baltimore, PA) at a dilution of 1:100 (Fig. 1h).
Discussion
Although there have been many pregnant women with iMN [7, 10], there are only a few case reports where the serum anti-PLA2R antibody levels were measured in that condition (Table 1). Al-Rabadi et al. reported the first successful pregnancy where the mother had anti-PLA2R antibody seropositive MN [7]. The antibody titer (125 RU/mL) and initial proteinuria (29.2 g/day) were higher in Al-Rabadi et al.’s patient than in our patient. Our patient was positive for anti-PLA2R antibody and she had a relatively low titer. Although these titers cannot be compared, because they were measured using different systems, these results suggest that serum anti-PLA2R antibody levels also reflects MN activity in pregnant patients as well as in the overall iMN patient population [11]. However, antigens other than anti-PLA2R might include MN in pregnant women. Ope-Adenuga et al. measured anti-PLA2R antibody in a pregnant MN patient, but the anti-PLA2R antibody result was negative and their patient had a maximum proteinuria of 13 g/day [10]. These descriptions suggest that the pathophysiology of iMN in pregnancy cannot be uniformly explained by the current studies.
Table 1.
Previously reported cases of MN in pregnant women in whom serum anti-PLA2R antibody was measured
| Al-Rabadi et al. [7] | Ope-Adenuga et al. [10] | Present case | |
|---|---|---|---|
| Serum anti-PLA2R antibody | 125 RU/mL (by ELISA) | Negative | 13.2 RU/mL (by Western blot) |
| Age (years) | 39 | 28 | 43 |
| Obstetric history | Multiparous | gravida 7 para 3 (three elective terminations, one fetal death at 29th week) | gravida 0 |
| Time at diagnosis of nephrotic syndrome | Several months before pregnancy | 12 week gestation | 11 week gestation |
| Maximum urinary protein | 29.2 g/day | 18 g/day | 3.6 g/gCre, 2.4 g/day |
| Minimum serum albumin | 1.5 g/dL | 0.3 g/dL | 1.9 g/dL |
| Treatment | Lisinopril, rituximab, tacrolimus (after delivery) | Tacrolimus, oral prednisolone | Oral prednisolone |
| Pregnancy outcome | Delivery at 38 week gestation, healthy | Preterm labor at 30 week gestation, 1021 g | Inducted labor at 38 week gestation, 2095 g |
| Maternal outcome | Completely resolved after 9 month postpartum | Completely resolved after delivery | Completely resolved after 5 week postpartum |
PLA2R phospholipase A2 receptor, ELISA Enzyme-linked immunosorbent assay
Compared with Al-Rabadi et al.’s patient who already had MN when she became pregnant [7], the timing of nephrotic syndrome diagnosis and remission matched better with the duration of pregnancy in the present patient. Pregnancy per se might be associated with iMN onset. Several hypothetical scenarios involving anti-PLA2R antibody production during pregnancy could be proposed using a literature search. One scenario is PLA2R expression in the placenta. Although the previous investigation using immunohistochemistry showed that PLA2R was observed only in kidney, PLA2R mRNA expression was observed in many organs including the placenta [12]. PLA2R or possibly similar antigens that can induce cross-sensitization might be produced in the placenta and lead to disease onset. Another scenario is that changes in immune function may modify the pathophysiology in pregnant patients. For example, in systemic lupus erythematosus (SLE) recurrence during pregnancy, changes in levels of hormones such as estrogen or progesterone and the proportion of regulatory T-cells seem to be involved [13]. There was no difference in regulatory T-cell subsets between the MN patient group and the non-MN group [14], but to date, there has been no investigation in pregnant MN patients. A mechanism similar to that of SLE recurrence may also play a role in MN during pregnancy.
In autoimmune diseases associated with pregnancy, fetuses or neonates can be affected by transplacental autoimmune antibody transfer. In the present case, because the infant showed no apparent signs of nephrotic syndrome or any abnormality, we did not perform a blood or urine test. This is the limitation of the present case report. In the case reported by Al-Rabadi et al., cord blood serum was only weakly positive for the anti-PLA2R antibody; thus, the antibody may have been absorbed by the placenta, or rapidly sequestered in the fetal kidney, but the effect was clinically negligible [7]. Evaluating proteinuria in neonates may lead to new insights into the pathophysiology of similar cases in the future.
Kidney disease may develop or recur during pregnancy, but it is unknown whether kidney biopsy should be considered to diagnose glomerulonephritis or preeclampsia in pregnant women [15]. Kidney biopsy has been performed from 13 to 35 week gestation using broad inclusion criteria [15], but some complication such as bleeding can be a problem especially in later in the gestational period. Our patient was positive for anti-PLA2R antibody before renal biopsy, so avoiding a renal biopsy was a good option to improve patient safety. Monitoring of titers may also be useful to determine treatment plans for patients with the disease and for continuation of pregnancy.
It has been reported that 5–9% of pregnant iMN patients have impaired renal function and 24–35% have fetal loss [16, 17]. Because renal blood flow and the glomerular filtration rate physiologically increase as gestation progresses, persistent or even progressive hypoalbuminemia was observed in the second half of our patient’s pregnancy. We decided to start immunosuppressive therapy with oral glucocorticoids, which has been reported to be effective in a Japanese population [18]. Our patient responded to glucocorticoid monotherapy, and remained in remission in the latter half of her pregnancy. With the potential exception of the first trimester, prednisolone is seen as a relatively safe therapeutic agent in pregnancy because of its lower transplacental passage than other corticosteroids, and widely used in other conditions such as rheumatic diseases [19]. The experience of its use in long-time literature also suggests that it is relatively safer during pregnancy than other immunosuppressive agents, such as cytotoxic agents [19] and calcineurin inhibitors [20]. Rituximab, which is recent possible alternative treatment option with limited toxicity, was reported to be used in a previous case [7]. It has been reported to cross the placenta and induce adverse effects including decreased B-cells and immunosuppression [21]. Its use is not contraindicated in pregnant condition but should be carefully considered.
In summary, we report the case of a pregnant woman with iMN who was seropositive for the anti-PLA2R antibody, and who was successfully treated using oral glucocorticoid monotherapy. After delivery, she remained in remission and without glucocorticoid therapy. This suggests that pregnancy may be associated with her kidney disease.
Acknowledgements
We thank Dr. Shinichi Akiyama (Department of Nephrology, Graduate School of Medicine, Nagoya University) for measuring anti-PLA2R antibody, and Dr. Sachiko Minamiguchi (Department of Diagnostic Pathology, Kyoto University Hospital) for histological diagnosis. We also thank Mr. Chris Rowthorn for the English language review.
Compliance with ethical standards
Conflict of interest
M. Yanagita is on the advisory board of Astellas and receives research grants from Astellas, Chugai, Daiichi Sankyo, Fujiyakuhin, Kyowa Hakko Kirin, Mitsubishi Tanabe Pharma Corporation, MSD, Nippon Boehringer Ingelheim, and Torii. The other authors declare no conflicts of interest.
Ethical approval
This study was exempted from institutional review board approval, because it was a case review. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Written informed consent was obtained from the patient for publication of this case and any accompanying images.
References
- 1.Umans JG. Obstetric nephrology: preeclampsia—the nephrologist’s perspective. Clin J Am Soc Nephrol. 2012;7(12):2107–2113. doi: 10.2215/CJN.05470512. [DOI] [PubMed] [Google Scholar]
- 2.Nevis IF, Reitsma A, Dominic A, McDonald S, Thabane L, Akl EA, et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6(11):2587–2598. doi: 10.2215/CJN.10841210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piccoli GB, Cabiddu G, Attini R, Vigotti FN, Maxia S, Lepori N, et al. Risk of Adverse Pregnancy Outcomes in Women with CKD. J Am Soc Nephrol. 2015;26(8):2011–2022. doi: 10.1681/ASN.2014050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yokoyama H, Taguchi T, Sugiyama H, Sato H. Membranous nephropathy in Japan: analysis of the Japan Renal Biopsy Registry (J-RBR) Clin Exp Nephrol. 2012;16(4):557–63. doi: 10.1007/s10157-012-0593-7. [DOI] [PubMed] [Google Scholar]
- 5.Ronco P, Debiec H. Pathophysiological advances in membranous nephropathy: time for a shift in patient’s care. Lancet. 2015;385(9981):1983–1992. doi: 10.1016/S0140-6736(15)60731-0. [DOI] [PubMed] [Google Scholar]
- 6.Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Rabadi L, Ayalon R, Bonegio RG, Ballard JE, Fujii AM, Henderson JM, et al. Pregnancy in a patient with primary membranous nephropathy and circulating anti-PLA2R antibodies: a case report. Am J Kidney Dis. 2016;67(5):775–778. doi: 10.1053/j.ajkd.2015.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timmermans SA, Damoiseaux JG, Heerings-Rewinkel PT, Ayalon R, Beck LH, Jr, Schlumberger W, et al. Evaluation of anti-PLA2R1 as measured by a novel ELISA in patients with idiopathic membranous nephropathy: a cohort study. Am J Clin Pathol. 2014;142(1):29–34. doi: 10.1309/AJCP8QMOY5GLRSFP. [DOI] [PubMed] [Google Scholar]
- 9.Akiyama S, Akiyama M, Imai E, Ozaki T, Matsuo S, Maruyama S. Prevalence of anti-phospholipase A2 receptor antibodies in Japanese patients with membranous nephropathy. Clin Exp Nephrol. 2015;19(4):653–60. doi: 10.1007/s10157-014-1054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ope-Adenuga S, Moretti M, Lakhi N. Management of membranous glomerulonephritis in pregnancy: a multidisciplinary challenge. Case Rep Obstet Gynecol. 2015;2015:839376. doi: 10.1155/2015/839376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofstra JM, Beck LH, Jr, Beck DM, Wetzels JF, Salant DJ. Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2011;6(6):1286–1291. doi: 10.2215/CJN.07210810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 13.Stojan G, Baer AN. Flares of systemic lupus erythematosus during pregnancy and the puerperium: prevention, diagnosis and management. Expert Rev Clin Immunol. 2012;8(5):439–53. doi: 10.1586/eci.12.36. [DOI] [PubMed] [Google Scholar]
- 14.Fervenza FC, Abraham RS, Erickson SB, Irazabal MV, Eirin A, Specks U, et al. Rituximab therapy in idiopathic membranous nephropathy: a 2-year study. Clin J Am Soc Nephrol. 2010;5(12):2188–2198. doi: 10.2215/CJN.05080610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piccoli GB, Daidola G, Attini R, Parisi S, Fassio F, Naretto C, et al. Kidney biopsy in pregnancy: evidence for counselling? A systematic narrative review. BJOG. 2013;120(4):412–27. doi: 10.1111/1471-0528.12111. [DOI] [PubMed] [Google Scholar]
- 16.Packham DK, North RA, Fairley KF, Whitworth JA, Kincaid-Smith P. Membranous glomerulonephritis and pregnancy. Clin Nephrol. 1987;28(2):56–64. [PubMed] [Google Scholar]
- 17.Lindheimer MD, Katz AI. Gestation in women with kidney disease: prognosis and management. Bailliere’s Clin Obstet Gynaecol. 1994;8(2):387–404. doi: 10.1016/S0950-3552(05)80327-X. [DOI] [PubMed] [Google Scholar]
- 18.Shiiki H, Saito T, Nishitani Y, Mitarai T, Yorioka N, Yoshimura A, et al. Prognosis and risk factors for idiopathic membranous nephropathy with nephrotic syndrome in Japan. Kidney Int. 2004;65(4):1400–1407. doi: 10.1111/j.1523-1755.2004.00518.x. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell K, Kaul M, Clowse MEB. The management of rheumatic diseases in pregnancy. Scand J Rheumatol. 2010;39(2):99–108. doi: 10.3109/03009740903449313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paziana K, Del Monaco M, Cardonick E, Moritz M, Keller M, Smith B, et al. Ciclosporin use during pregnancy. Drug Saf. 2013;36(5):279–94. doi: 10.1007/s40264-013-0034-x. [DOI] [PubMed] [Google Scholar]
- 21.Friedrichs B, Tiemann M, Salwender H, Verpoort K, Wenger MK, Schmitz N. The effects of rituximab treatment during pregnancy on a neonate. Haematologica. 2006;91(10):1426. [PubMed] [Google Scholar]