Abstract
We report a case of capillary leak that developed during treatment of antibody-mediated rejection in a kidney transplant recipient. A 53-year-old female transplant recipient experienced an increase in serum creatinine from 1.1 to 1.8 mg/dL. Antibody-mediated rejection was diagnosed by graft biopsy. She was treated with five plasmapheresis sessions (on alternate days with albumin replacement), five doses of immunoglobulin (5 g/dose at 100 mg/kg), a single dose of rituximab (500 mg), and four doses of bortezomib on days 1, 4, 7, and 10 (1.72 mg/dose at 1.3 mg/m2 body surface area). During treatment, edema, slight diarrhea, pancytopenia, hypoalbuminemia, peripheral neuropathy, and postural hypotension were noted. Despite control of liquids, she presented with edema progressing to an increase of more than 10 kg body weight. Prerenal acute graft dysfunction associated with hypotension was diagnosed on day 12, heart failure or other infectious complications being discounted. On day 13, daily hemodialysis was prescribed, and a stable volume status was reached after five hemodialysis sessions. On day 20, the patient recovered diuresis and the edema and diarrhea abated, but she remained on chronic hemodialysis. After excluding other causes of distributive shock, the diagnosis of capillary leak syndrome was based on the presence of hypotension, generalized edema, and hypoalbuminemia in the absence of significant proteinuria. The concomitant presence of diarrhea, peripheral neuropathy, and pancytopenia, suggest a possible causal role for bortezomib. Awareness by clinicians of capillary leak syndrome associated with bortezomib-based treatment of AMR is paramount, despite its rarity.
Keywords: Acute antibody-mediated rejection, Proteasome inhibitor, Graft failure, Acute kidney injury, Transplantation, Clarkson disease
Background
Antibody-mediated rejection (AMR) caused by donor-specific antibodies (DSA) against human leukocyte antigens is a major cause of graft dysfunction and loss following kidney transplantation [1]. The treatment for AMR includes plasmapheresis (PP), intravenous immunoglobulin, and agents targeting B cells (rituximab) or plasma cells (bortezomib) [2]. Nevertheless, the combined use of these highly potent immunosuppressive drugs increase the risk of severe adverse effects (e.g., infections, malignancies, haematological complications) [3].
A rare but potentially life-threatening condition associated with drug exposure is capillary leak syndrome, an unexplained episodic attack of distributive shock, characterized by a plasma extravasation with subsequent edema and vascular collapse causing severe hypotension, hypoalbumemia, and hemoconcentration followed by pulmonary edema [4].
We report herein the first case of capillary leak syndrome during treatment of AMR in a kidney transplant recipient.
Case presentation
A 53-year-old woman, resident of Mexico City, had been diagnosed with lupus nephritis 15 years earlier. After several treatments, she progressed to end-stage renal disease in 2006 and was treated with hemodialysis. In 2012, she received a kidney transplant from a deceased donor and tacrolimus, azathioprine, and prednisone were prescribed. In March 2015, the patient was admitted to our hospital with an active AMR (Banff score: glomerulitis [g]2, peritubular capillaritis [ptc]1, interstitial fibrosis, and tubular atrophy of 60%; C4d negative) diagnosed by an asymptomatic increase in her serum creatinine baseline levels from 1.1 to 1.8 mg/dL(eGFR of 31.7 mL/min/1.73 m2 by CKD-EPI equation) [5] alongside new-onset albuminuria (0.512 g/g creatinine). Donor-specific antibody detected using the Luminex was against to HLA-DR7 with a mean fluorescent intensity of 4000.
On admission, the patient was asymptomatic without edema or hypertension and adequate diuresis. She was taking prednisone 5 mg/day, tacrolimus 1 mg twice daily (drug blood level drawn on admission was 15.7 ng/mL), and azathioprine 75 mg/day. The tacrolimus dose was reduced to 1 mg/day and new levels at day 4 after admission were in 7.1 ng/mL.
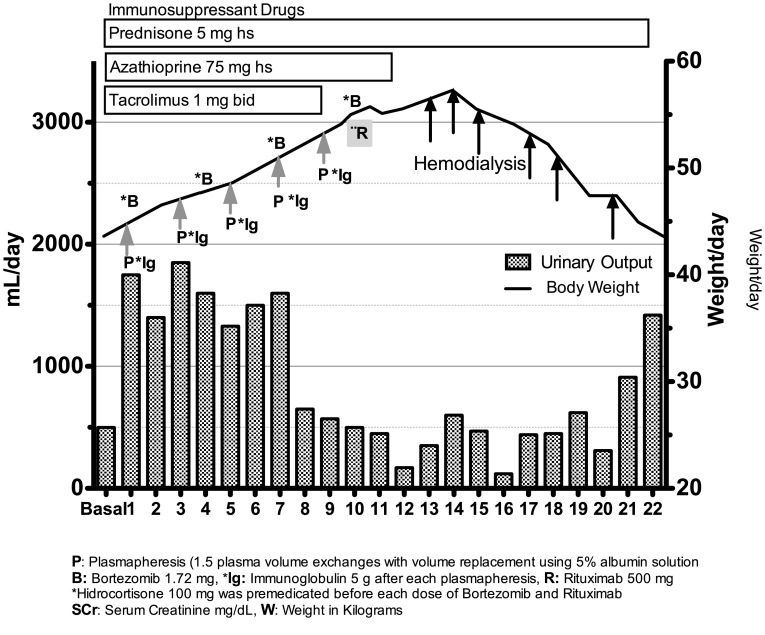
She was treated with anti-AMR treatment for 10 days: 5 PP alternate-day sessions (1.5 plasma volume exchanges with volume replacement using 5% albumin solution), 5 doses of immunoglobulin (5 g each dose at 100 mg/kg) after each PP, 4 doses of bortezomib, 1.72 mg (1.3 mg/m2 body surface area) on days 1, 4, 7, and 10, and a single dose of rituximab, 500 mg on day 10. Hydrocortisone (100 mg) was premedicated before each dose of rituximab and bortezomib. Strict monitoring of fluid balance did not show overhydration, and it was verified that all PP sessions used albumin 5% as replacement fluid. The volume of patient plasma exchanged in each session was 1.5, and only 70% of replacement (Fig. 1). From the first day of treatment the onset of edema, diarrhea, pancytopenia, peripheral neuropathy, and postural hypotension was noted. On day 10, her weight increased from 44 to 56 kg and her urine output decreased from 1750 to 500 mL per day.
Fig. 1.
Day-by-day evolution of urinary output (mL/day), body weight in kg, and serum creatinine of KTR with capillary leak syndrome
After the last dose of bortezomib, the patient had severe edema of the face, trunk, and extremities, oliguria (250 mL in 12 h) with blood pressure of 90/65 mmHg. Aggressive intravenous fluid resuscitation was indicated (20 ml/kg bolus followed by 3 ml/kg/h). There was an increase of serum creatinine level from 1.8 to 4.2 mg/dL, hyponatremia (125 mEq/L), and hypoalbuminemia (2.6 mg/dL). The hemoglobin level was 8.7 g/L with hematocrit of 26%. Complement proteins C3 and C4 were at reference values. A urinary test revealed urinary density of 1.025, urinary sodium 8 mEq/L, fractional excretion of sodium 0.19%, and microalbuminuria index of 0.454 g/g creatinine. Blood and urine cultures were negative. A graft ultrasonogram showed absence of ureter obstruction or thrombosis. A new repeated graft biopsy revealed active AMR (Banff score: ptc2, g1, calcineurin toxicity, interstitial fibrosis and tubular atrophy of 70%, and negative C4D). An electrocardiogram showed only sinus tachycardia, and an echocardiogram revealed a conserved fraction expulsion of 73%, pulmonary artery pressure of 32 mm/Hg, and low left ventricular relaxation (mild diastolic dysfunction). Troponin levels were below reference values. On day 12, pulmonary edema with basilar crackles and dyspnea appeared. A new urinary test revealed granular cast and a fractional excretion of sodium of 2%. Intravenous furosemide infusion (8 mg/hour) and albumin 60 g/day did not lead to improvement in urine output, and hemodialysis was indicated. During the following days, daily hemodialysis was required. After the beginning of hemodialysis, on day 20, the patient recovered diuresis without appropriate solute clearance, and other manifestations such as edema and diarrhea abated; however, peripheral neuropathy persisted and chronic hemodialysis was required over the following weeks. The patient gave her informed consent for publication of her case report.
Discussion
The classical diagnosis of drug-induced capillary leak syndrome is one of exclusion. It is based on the presence of generalized edema, hypoalbuminemia, prerenal azotemia, and hypotension after certain medications. This leak phase reverse quickly with massive fluid recruitment from tissues into circulation followed by acute pulmonary edema (postleak phase) [4]. In the present case, edema and weight gain were obvious in our patient since exposure to treatment, but frank oliguria, and evident arterial hypotension did not occur until 2 days after AMR treatment was completed. It is possible that with the initiation of intravenous hydration, the clinical data of hemoconcentration may not have been detected.
Although many diseases have characteristics similar to those of capillary leak syndrome, the distinguishing features of the present case excluded the following diagnoses: nephrotic syndrome by the absence of nephrotic proteinuria; idiopathic anaphylaxis by the absence of acute hypotension, flushing, hives, laryngeal edema, or hypoalbuminemia; and sepsis by the absence of an evident site of infection, negative cultures, and resolution of disease without the use of antibiotics. Other rare diseases such as acquired C1 inhibitor deficiency were improbable, based on the absence of angioedema, laryngeal edema, or family history, and C4 level within the normal range.
The concomitant presence of diarrhea, peripheral neuropathy, and pancytopenia, all of which are common toxicities of bortezomib, suggest a possible causal role for this drug. Many side effects of bortezomib, such as thrombocytopenia, neuropathy, anemia, hypotension, and diarrhea, have a high incidence in the kidney transplant population [6]. Adverse effects related to bortezomib may differ according to kidney function; patients on dialysis in a desensitization protocol have neuropathy rates higher than those of AMR patients, who have better kidney function than patients on desensitization [7]. We have knowledge of only one case of recurrent capillary leak syndrome after exposure to bortezomib in a 65-year-old woman with myeloma [8]. It is possible that a very low graft function may be one of the principal predisposing factors for this complication.
The other treatments used in this patient, such as albumin, PP, prednisone, rituximab, and intravenous immunoglobulin, are recommended for treatment or prophylaxis of capillary leak syndrome [4] and may have provided protection against rapidly evolving symptoms during the first days. Other drugs that the patient had taken such as tacrolimus and azathioprine were considered to be unlikely culprits. According to the Naranjo adverse drug reaction probability scale (6 points), it is “probable” that the capillary leak syndrome was induced by bortezomib [9].
Intravenous immunoglobulin dosage of 2 g/kg per month or over 2 days have been reported as the optimal prophylaxis for capillary leak syndrome in descriptive case series [4]. The low doses of immunoglobulin prescribed in our patient may have attenuated the symptoms but may not have been sufficient to prevent them.
Thus far, there is no evidence relating to the mechanism of how bortezomib-based AMR treatment may precipitate a capillary leak syndrome. Several hypotheses exist regarding how capillary leak syndrome may be related to bortezomib action, such as downregulation of the expression of nuclear factor κB-dependent adhesion molecules expressed in endothelial cells that could induce endothelial apoptosis and release of inflammatory cytokines such as interferon gamma, TNF-alpha, interleukin-1, 2, 6, among others [10]. The pathophysiology of this devastating complication remains elusive.
Bortezomib-based AMR treatment will be prescribed in multicenter clinical trials for the treatment of kidney transplant recipients with DSA and AMR [11] in an increasing number of patients. In the wake of the evidence that bortezomib effectively reduces DSA levels associated with histologic improvement on repeat graft biopsies, [12] prescription of this drug and, hence, its toxicity, is expected to increase in the following years [13]. The present case raises awareness of this rare complication, especially in patients with uremia or low graft function. An early diagnosis of capillary leak syndrome is useful in establishing therapeutic measures such as lower doses of bortezomib, higher doses of steroids as prophylaxis, or higher doses of immunoglobulin during AMR treatment.
In summary, this case report shows that treatment of AMR may be related to capillary leak syndrome. Awareness of this complication should help in early diagnosis and prevention of disease.
Compliance with ethical standards
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted (IRB approval number SALUD-2006-C01-45085 for project: “Evaluación de la seguridad y eficacia del bortezomib en pacientes receptores de trasplante renal con rechazo agudo mediado por anticuerpos”) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Stegall MD, Dean PG, Gloor J. Mechanisms of alloantibody production in sensitized renal allograft recipients. Am J Transplant. 2009;9:998–1005. doi: 10.1111/j.1600-6143.2009.02612.x. [DOI] [PubMed] [Google Scholar]
- 2.Sin Y-H, Kim Y-J, Oh JS, Lee JH, Kim SM, Kim JK. Treatment of acute antibody-mediated rejection using bortezomib: a case report. Nephrology. 2015;20:86–89. doi: 10.1111/nep.12459. [DOI] [PubMed] [Google Scholar]
- 3.Kim M-G, Kim YJ, Kwon HY, Park HC, Koo TY, Jeong JC, et al. Outcomes of combination therapy for chronic antibody-mediated rejection in renal transplantation. Nephrology. 2013;18:820–826. doi: 10.1111/nep.12157. [DOI] [PubMed] [Google Scholar]
- 4.Druey KM, Greipp PR. Narrative review: the systemic capillary leak syndrome. Ann Intern Med. 2010;153:90–98. doi: 10.7326/0003-4819-153-2-201007200-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levey AS, Stevens L, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Everly MJ, Everly JJ, Susskind B, Brailey P, Arend LJ, Alloway RR, et al. Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation. 2008;86:1754–1761. doi: 10.1097/TP.0b013e318190af83. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt N, Alloway RR, Walsh RC, Sadaka B, Shields AR, Girnita AL, et al. Prospective evaluation of the toxicity profile of proteasome inhibitor-based therapy in renal transplant candidates and recipients. Transplantation. 2012;94:352–361. doi: 10.1097/TP.0b013e318257acf6. [DOI] [PubMed] [Google Scholar]
- 8.Hsiao SC, Wang MC, Chang H, Pei SN. Recurrent capillary leak syndrome following bortezomib therapy in a patient with relapsed myeloma. Ann Pharmacother. 2010;44:587–589. doi: 10.1345/aph.1M585. [DOI] [PubMed] [Google Scholar]
- 9.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 10.Shi WY, Wang L, Xiao D, Yao Y, Yang F, Jiang XX, et al. Proteasome inhibitor bortezomib targeted tumor-endothelial cell interaction in T-cell leukemia/lymphoma. Ann Hematol. 2011;90:53–58. doi: 10.1007/s00277-010-1022-1. [DOI] [PubMed] [Google Scholar]
- 11.Eskandary F, Bond G, Schwaiger E, Kikic Z, Winzer C, Wahrmann M, et al. Bortezomib in late antibody-mediated kidney transplant rejection (BORTEJECT study): study protocol for a randomized controlled trial. Trials. 2014;15:107. doi: 10.1186/1745-6215-15-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leyva S, Marino L, Alberú J, Morales-Buenrostro LE. Bortezomib for acute humoral rejection treatment at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán in Mexico City: an update. Clin Transpl. 2010;35:369–82. [PubMed]
- 13.Yang KS, Jeon H, Park Y, Jo IH, Kim J-I, Moon IS, et al. Use of bortezomib as anti-humoral therapy in kidney transplantation. J Korean Med Sci. 2014;29:648–651. doi: 10.3346/jkms.2014.29.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]