Abstract
IgG4-related disease (IgG4-RD) is a newly recognized immune-mediated multisystemic disease characterized by a fibro-inflammatory condition with tissue infiltration of IgG4-positive plasma cells and often associated with elevated serum IgG4 levels. Typical renal involvement of IgG4-RD presents as tubulointerstitial nephritis (TIN), membranous or membranoproliferative nephropathy. We are presenting a case with combined IgG4 membranous nephropathy and TIN, as well as a literature review on pathophysiology, diagnosis and treatment of IgG4-RD. A 62-year-old man presented with weight loss and fatigue. Labs showed significant proteinuria and hematuria with elevated serum creatinine (2.5 mg/dL). CT/PET scan found scattered lymphadenopathy without increased FDG uptake. Kidney biopsy showed glomerular lesions as well as severe interstitial fibrosis and tubular atrophy. Immunohistochemistry study was negative for anti-phospholipase A2 receptor antibodies and showed interstitial lymphocytic infiltration with IgG4 positive plasma cells. Patient also had elevated serum IgG4 level and IgG4 to total IgG ratio. Prednisone treatment was initiated soon after the diagnosis was made, patient responded well with proteinuria and hematuria both resolved. IgG4-related disease (IgG4-RD) is a newly increasingly recognized immune-mediated multisystemic disease; IgG4-related membranous nephropathy should be included in the differential diagnosis for patients with proteinuria.
Keywords: IgG4-related disease, Membranous nephropathy, Secondary membranous nephropathy, Tubulointerstitial nephritis
Introduction
IgG4-related disease (IgG4-RD) was first recognized as a systemic disease in 2003. It is a fibroinflammatory condition characterized by dense lymphoplasmacytic infiltration of IgG4 positive plasma cells leading to storiform-pattern fibrosis and often associated with increased serum IgG4 levels [1]. IgG4-RD is an immune-mediated multisystemic disorder with unique clinical, serologic and pathological features. Many previously described conditions including Mikulicz’s syndrome, Küttner’s tumor, and Riedel’s thyroiditis are now classified to be part of IgG4-RD with the characteristic clinic, serologic and pathologic features [2]. Pancreatic involvement is the most common manifestation of IgG4-RD, high serum IgG4 concentrations with lymphoplasmacytic infiltration has also been found in multiple other tissues including salivary glands, lymph nodes, lungs, meninges, prostate, thyroid, and kidneys [3]. IgG4-related tubulointerstitial nephritis (IgG4 TIN) has been the most commonly reported renal manifestation of IgG4-RD. A very limited numbers of cases have reported glomerular lesions in IgG4-RD. Here, we described a patient who presented with acute kidney injury and was diagnosed with combined IgG4 membranous nephropathy and TIN. Prednisone was started early after diagnosis and the patient responded well. Our case presentation illustrates the importance of including IgG4-related membranous nephropathy in the differential diagnosis for patients with proteinuria.
Case report
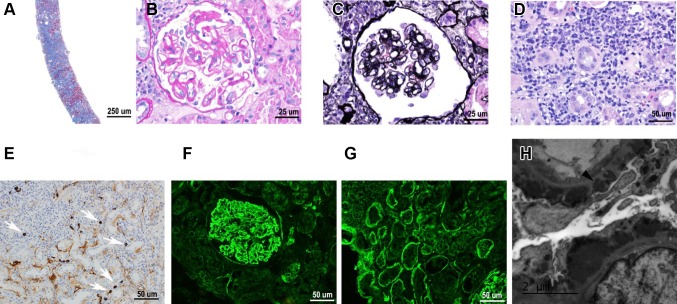
A 62-year-old man presented with acute kidney injury, proteinuria and hematuria. He had a history of unintentional weight loss of 10.4 kg over the past year associated with fatigue. Past medical history included hypertension and hyperlipidemia. Past surgical history was significant for hernia repair in 1961 and prostatectomy due to prostate cancer in 2006. Family history was unremarkable. Patient is not an alcoholic, not an illicit drug user and works as a custodian. Home medications included aspirin 81 mg, amlodipine 10 mg, simvastatin 40 mg, losartan 100 mg, carvedilol 6.25 mg twice a day, and hydralazine 20 mg twice a day. Review of system was negative for abdominal pain, arthralgia, skin rash and hemoptysis. Temperature 36.8 Celsius degree, blood pressure 174/84 mmHg, heart rate 66 bpm, respiration rate 16 breaths/min, oxygen saturation 96%, weight74.8 kg, height 182.9 cm, BMI 22.4 kg/m2. Physical exam was remarkable for a thin white male with 1 + lower extremity edema. There was no evidence of organomegaly, lymphadenopathy, abdominal tenderness or bruits, and focal neurological deficit. Initial laboratory and diagnostic workup showed: BUN 44 mg/dL, creatinine 2.5 mg/dL, estimated GFR 25 mL/min/1.73 m2, calcium 8.2 mg/dL, albumin 3.3 g/dL, alkaline phosphatase 185 IU/L, AST 33 IU/L, ALT 25 IU/L, cholesterol 139 mg/dL, triglycerides 425 mg/dL, HDL 14 mg/dL; WBC 4.8 × 103/μL, RBC 2.77 × 106/μL, hemoglobin 8.6 g/dL, hematocrit 25.6%, platelets 194 × 103/μL and eosinophils 3%; urinalysis was significant for protein 4+, WBC 6–10/hpf, RBC 11–30/hpf, granular hyaline and red cell casts present. Sedimentation rate, calculated using the Westergren method, was elevated at 132 mm/h. Urine protein was 307.7 mg/dL and urine creatinine was 138.3 mg/dL. Serological evaluation including serum immunofixation revealed no evidence of monoclonal protein, negative ANCA screening, slightly elevated kappa/lambda light chain ratio, low C3 at 79 mg/dL, low C4 at 11 mg/dL, positive anti-SSA, SSB and ANA antibodies. Immunoglobin tests showed elevated IgE at 110 IU/mL, elevated IgG3 at 138 mg/dL, and elevated IgG4 at 142 mg/dL (IgG4 to total IgG ratio 10.5%), with normal IgG1, IgG2, IgA, and IgD quantity. Due to anemia, patient underwent EGD/colonoscopy which was unrevealing. Lymphoproliferative disorders have been ruled out by hematology/oncology with CT/PET scan which found scattered lymphadenopathy in the bilateral axilla, celiac trunk (1.8 cm celiac axis lymph node), and the retroperitoneum regions without increased FDG uptake. CT-guided renal biopsy was done and pathology studies show the following results (Fig. 1): (1) light microscopy: 5 out of 16 glomeruli are globally sclerotic. The glomerular basement membranes are globally thickened and irregular with prominent mottling and small spike formation on Silver stain. There is no definitive endocapillary proliferation, fibroid necrosis, or crescent formation present. Severe interstitial fibrosis and tubular atrophy involving approximately 50% of the cortex, patchy dense interstitial lymphoplasmacytic inflammatory infiltrate admixed with few eosinophils present both within areas of scarring and focally within the intact tubulointerstitium. (2) An IgG4 immunoperoxidase stain showed patchy positive staining of the interstitium as well as locally more than 10 IgG4 positive plasma cells per high-power field (40×). (3) Immunofluorescence: the glomeruli show diffuse global, closely granular, capillary lip staining by IgA(trace to 1+), IgG(3+), IgM(trace to 1+), C3(3+), C1q(1–2+),kappa(3+),and lambda(3+) light chains. Staining for the phospholipase A2 receptor (PLA2R) within the glomerular deposits was negative. (4) Electron microscopy showed glomerular basement membrane with frequent subepithelial and occasional intramembranous immune complex-type electron dense deposits.
Fig. 1.
Pathological studies on renal biopsy sample. a Light microscopy, ×40, b light microscopy shows glomerulus with thick and irregular capillary loops, ×400, c frequent GBM spikes on silver stain, ×400, d patchy dense interstitial lymphoplasmacytic inflammatory infiltration, ×400, e an IgG4 immunoperoxidase stain shows patchy positive staining of the interstitium as well as locally more than 10 IgG4 positive plasma cells per high-power field, ×400, f immunofluorescence shows glomerular IgG, ×200, g immunofluorescence shows tubular basement membrane staining by IgG, ×200, h electron microscopy shows glomerular basement membrane with frequent subepithelial immune complex-type electron dense deposits, ×5000
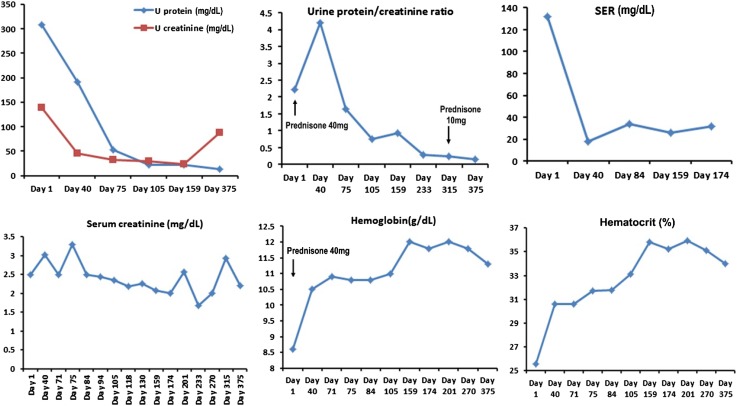
Negative immunostaining for antiphospholipase A2 receptor (PLA2R) antibody in this patient is consistent with a secondary etiology for membranous nephropathy. Immunohistochemical stain for IgG4 met the current consensus of > 10 cells/hpf is diagnostic of IgG4-RD. A diagnosis of IgG4-related membranous nephropathy and IgG4 TIN was made. As shown in Fig. 2, serum IgG4 level was elevated at 142 mg/dL with high IgG4 to total IgG ratio (10.5%) supporting the diagnosis.
Fig. 2.
Responses to glucocorticoid treatment (initiated on day 1). Prednisone 40 mg daily was started since day 1, Urine protein, urine protein to creatinine ratio and SER were quickly decreased with serum creatinine level kept stable around 2–2.5. Patient’s hemoglobin/hematocrit also steadily improved with treatment
The patient was started on prednisone 40 mg PO daily for 12 months which was slowly tapered down to 10 mg PO daily due to side effects with recurrent lower extremity swelling. Losartan 50 mg PO daily and Simvastatin 40 mg PO daily was continued throughout the treatment, after discussion with nephrologist, we decided to keep the patient on hydralazine 20 mg and start Lasix 60–80 mg PO daily. Short trial of metolazone was also administered; patient’s lower extremity edema remained improved at 3 and 6-month follow-up visits, but remained fluctuating in his follow-up visits. Patient’s kidney function has been stable with good urine output. His proteinuria improved with urine protein–creatinine ratio which decreased from 2.22 to 0.16, erythrocyte sedimentation rate improved from 132 to 26 mm/h, and his serum creatinine level slightly decreased from 2.5 to 2.0 mg/dL at 12-month visit, steroid was completely discontinued around his 24-month visit and serum creatinine was improved to 1.63 with eGFR 41 at his most recent visit by when the patient has been off steroids for 7 months. Urinalysis at 5 months of treatment was negative for protein. Repeat CT chest, abdomen and pelvis after 3 months of prednisone showed the celiac lymph node decreased to 7.2 mm compared with 18 mm previously. Hemoglobin (hematocrit) improved from 8.6 g/dL (25.6%) to around 11.3 g/dL (35.2%) and eosinophilia improved from 3 to 0.5–0.6% (Fig. 2).
Discussion
Immunoglobin G (IgG) includes 4 subclasses: IgG1, IgG2, IgG3 and IgG4. Normally, IgG1 accounts for 60–70%, IgG2 20–30%, IgG3 5–8% of the body’s production. IgG4 is the rarest IgG subclass with only 1–4%. Unlike other IgGs, IgG4 has weak binding capacity to both C1 and Fcr receptors, its unstable disulfide bonds between heavy chains allow interactions and exchanges of Fab-arms between two IgG4 molecules which result in the inability to cross-link with antigens and thus immune-complex formation is compromised. IgG4 is considered a weak activator for the classical complement pathway [4]. IgG4 production is thought to be a Th2-cell dominant immune response which is supported by the evidence that Th2 cytokines including IL-4 and IL-13 increase IgG4 secretion [5]. However, their biological roles are not completely understood.
Studies have indicated multi-factorial mechanisms for IgG4-RD including genetic risk factors, bacterial infection and molecular mimicry and autoimmunity. IgG4-RD should be considered in patients with a characteristic pattern of tissue involvement. This includes pancreatitis of unknown etiology, lymphadenopathy, sclerosing cholangitis, bilateral salivary and/or lacrimal gland enlargement, retroperitoneal fibrosis, orbital pseudotumor or proptosis and renal dysfunction. Many previously described conditions including Mikulicz’s syndrome, Küttner’s tumor, and Riedel’s thyroiditis are now classified to be part of IgG4-RD [2].The diagnosis of IgG4-RD requires histopathology study of biopsy sample. According to the international consensus statement proposed in 2012, the histopathological findings of a dense lymphoplasmacytic infiltrate, storiform fibrosis, and obliterative phlebitis are critical features for establishing the diagnosis. These findings often present together with mild tissue eosinophilia and increased numbers of IgG4-positive plasma cells [6, 7]. Semi-quantitative analysis of IgG4 immunostain is also being used, the cutoff points range from more than 10 to more than 50 IgG4 positive plasma cells per high power field [8, 9]. The ratio of IgG4 positive plasma cells to IgG positive plasma cells can be calculated with a ratio greater than 50% strongly indicating the diagnosis. Previous studies have identified electron dense granular deposits at the basement membrane of renal tubules in patients with IgG4-RD and the deposits mainly consist of IgG4 and C3.
Our patient presented with acute kidney injury associated with unintentional weight loss and fatigue, imaging studies found generalized lymphadenopathy. His follow-up studies revealed typical histopathology presentation with interstitial fibrosis, lymphoplasmacytic inflammatory infiltration with more than 10 IgG4 positive plasma cells per hpf (40×), immune complex-type electron dense deposits and elevated IgG4 level. The patient also consistently had eosinophilia, elevated IgE, low C3 and C4 level which were reported as common findings in IgG4-RD [6].
Membranous nephropathy may be primary or secondary to a variety of causes. One of the major causes of primary membranous nephropathy is the development of antibodies against a podocyte antigen, M-type PLA2R [10]. Anti-PLA2R antibody staining is a useful marker for differentiating primary from secondary membranous nephropathy, which was negative in our patient, as is expected in a case of secondary membranous nephropathy.
Autoimmune disease, infections, neoplasms and drugs are the main etiologic factors of secondary membranous nephropathy. Work-up on our patient did not reveal evidence of underlying autoimmune disease, infection or malignancy. No drug history was revealing. Recently, a series of published literature suggested that IgG4-RD is an etiological factor for secondary membranous nephropathy [11–13]. Membranous nephropathy secondary to IgG4-RD has been termed as “IgG4-related membranous nephropathy” in the 2011 international symposium [14].
Diagnosis of “IgG4-related membranous nephropathy and TIN” in our patient is supported by the following diagnostic criteria [15]:
Clinical criteria of nephrotic syndrome with renal dysfunction
High serum IgG4 level (142 mg/dL) and high IgG4/IgG ratio (10.5%)
Histological evidence of membranous nephropathy confirmed by electron microscopy study along with interstitial fibrosis and dense infiltration of IgG4 positive plasma cells (more than 10 per hpf).
The etiopathogenesis of IgG4-related membranous nephropathy remains unclear. A hypothesis by Fervenza et al. proposed that the proliferating plasma cells produce autoreactive IgG4 against podocyte antigens [16]. Kuroki et al. also showed that cytokines of type 2 helper T-cell cause IgG4 production by increasing and stimulating B-cells in membranous nephropathy as well as in other IgG4-related sclerosing lesions [17]. These researches not only advanced our understanding on the pathophysiology of IgG-RD but also raised novel therapeutic pathways as discussed below.
Early aggressive treatment is required to prevent serious multiple organ dysfunction or failure. Glucocorticoid has been employed as first-line therapy [18]. A consensus statement from Japan suggested an initial dose of 0.6 mg/kg/day for 2–4 weeks followed by 3–6 months of tapering period to 5 mg/day and continue 2.5–5 mg per day for up to 3 years [19]. Alternative approach is to discontinue glucocorticoids completely within 3 months. Nevertheless, the optimal dose and period of glucocorticoid therapy has not been investigated by large trials yet. Our patient received prednisone 40 mg PO daily for 12 months and improved significantly after 1 month of treatment with labs continued to be improved throughout the rest of treatment period. Repeat CT at 3 months showed the celiac lymph node decreased from 18 to 7.2 mm. As glucocorticoid tapering and discontinuation are both associated with a high risk of disease relapse, our patient received long-term prednisone treatment as well as a very-slow tapering process with steroid completely discontinued at around his 24-month follow-up visit. His kidney function continued to improve with serum creatinine 1.63 mg/dL and eGFR 41 mL/min/1.73 m2 at his most recent visit (off steroid for 7 months). Glucocorticoid-sparing therapy including azathioprine, mycophenolate mofetil and methotrexate are also suggested as maintenance medications after glucocorticoid-induced remissions. Previous case series have reported B cell depletion with rituximab as an effective therapy in patients with IgG4-RD who are refractory to glucocorticoids and other medications [20]. Recent understanding on interactions between B and T cell lineage in the pathophysiology of IgG4-RD has led to new possible treatment strategies [21]. Development of therapies targeting certain B cell and T cell lineage has been proposed as a major goal in the next a few years, for example, B cell depletion with anti-CD20 monoclonal antibodies showed rapid and favorable efficacy, and plasmablast-directed therapy with a CD19 monoclonal antibody is in clinical trials [22]. However, these approaches have not been extensively evaluated in large randomized clinical trials [18, 21].
Standard protocol for evaluating and monitoring the severity, urgency and treatment outcome of IgG4-RD remains in research. Development of such tool is facing the challenge that IgG4-RD is a multiple system involving disease and its progression differs in different tissues as well as in each individual patient. An IgG4-RD Responder Index has been developed by employing clinical investigations and laboratory-based data, which still require future validation with a multicenter study where patients can be recruited by physicians with extensive experience in the diagnosis and management of this disease [23]. Circulating plasmablasts was found to be elevated in active IgG4-RD even in patients without elevated serum IgG4 levels, which could develop into a useful biomarker for diagnosis, treatment response, and disease relapse [24, 25].
Conclusion
As a newly increasingly recognized immune-mediated multisystemic disease, IgG4-related disease (IgG4-RD) is becoming a more common clinical conception, patient usually presents with unspecific clinical symptoms, early diagnosis and interventions are critical and require expertise from both primary care and sub-specialties. Pathophysiological mechanisms and evaluation/monitor tool for IgG4-RD remain as poorly understood questions for researchers and clinicians, optimal treatment strategy is controversial, novel treatment approaches are emerging, for which accumulation and communication of clinical cases should be continued and encouraged.
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interests to disclose. Consent, for the publication of this case report and any additional related information, was taken from the patient involved in the study. This article does not contain any studies with human participants or animals performed by any of the authors.
Contributor Information
Wei Zhang, Email: weiweiuab@gmail.com.
David Wynne, Email: summitmd@bellsouth.net.
References
- 1.Kamisawa T, Funata N, Hayashi Y, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38(10):982–984. doi: 10.1007/s00535-003-1175-y. [DOI] [PubMed] [Google Scholar]
- 2.Geyer JT, Deshpande V. IgG4-associated sialadenitis. Curr Opin Rheumatol. 2011;23(1):95–101. doi: 10.1097/BOR.0b013e3283413011. [DOI] [PubMed] [Google Scholar]
- 3.Kamisawa T, Takuma K, Egawa N, et al. Autoimmune pancreatitis and IgG4-related sclerosing disease. Nat Rev Gastroenterol Hepatol. 2010;7(7):401–409. doi: 10.1038/nrgastro.2010.81. [DOI] [PubMed] [Google Scholar]
- 4.Tao MH, Smith RI, Morrison SL. Structural features of human immunoglobulin G that determine isotype-specific differences in complement activation. J Exp Med. 1993;178(2):661–667. doi: 10.1084/jem.178.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nirula A, Glaser SM, Kalled SL, et al. What is IgG4? A review of the biology of a unique immunoglobulin subtype. Curr Opin Rheumatol. 2011;23(1):119–124. doi: 10.1097/BOR.0b013e3283412fd4. [DOI] [PubMed] [Google Scholar]
- 6.Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25(9):1181–1192. doi: 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]
- 7.Khosroshahi A, Wallace ZS, Crowe JL, et al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol. 2015;67(7):1688–1699. doi: 10.1002/art.39132. [DOI] [PubMed] [Google Scholar]
- 8.Dhall D, Suriawinata AA, Tang LH, et al. Use of immunohistochemistry for IgG4 in the distinction of autoimmune pancreatitis from peritumoral pancreatitis. Hum Pathol. 2010;41(5):643–652. doi: 10.1016/j.humpath.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Chari ST. Diagnosis of autoimmune pancreatitis using its five cardinal features: introducing the Mayo Clinic’s HISORt criteria. J Gastroenterol. 2007;42(Suppl 18):39–41. doi: 10.1007/s00535-007-2046-8. [DOI] [PubMed] [Google Scholar]
- 10.Beck LH, Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander MP, Larsen CP, Gibson IW, et al. Membranous glomerulonephritis is a manifestation of IgG4-related disease. Kidney Int. 2013;83(3):455–462. doi: 10.1038/ki.2012.382. [DOI] [PubMed] [Google Scholar]
- 12.Kurien AA, Raychaudhury A, Walker PD. Membranous nephropathy as a rare renal manifestation of IgG4-related disease. Indian J Nephrol. 2015;25(3):164–167. doi: 10.4103/0971-4065.143300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jindal N, Yadav D, Passero C, et al. Membranous nephropathy: a rare renal manifestation of IgG4-related systemic disease. Clin Nephrol. 2012;77(4):321–328. doi: 10.5414/CN107037. [DOI] [PubMed] [Google Scholar]
- 14.Stone JH, Khosroshahi A, Deshpande V, et al. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheumat. 2012;64(10):3061–3067. doi: 10.1002/art.34593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okazaki K, Umehara H. Are classification criteria for IgG4-RD now possible? The concept of IgG4-related disease and proposal of comprehensive diagnostic criteria in Japan. Int J Rheumatol. 2012;2012:357071. doi: 10.1155/2012/357071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fervenza FC, Downer G, Beck LH, Jr, et al. IgG4-related tubulointerstitial nephritis with membranous nephropathy. Am J Kidney Dis. 2011;58(2):320–324. doi: 10.1053/j.ajkd.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Kuroki A, Iyoda M, Shibata T, et al. Th2 cytokines increase and stimulate B cells to produce IgG4 in idiopathic membranous nephropathy. Kidney Int. 2005;68(1):302–310. doi: 10.1111/j.1523-1755.2005.00415.x. [DOI] [PubMed] [Google Scholar]
- 18.Khosroshahi A, Stone JH. Treatment approaches to IgG4-related systemic disease. Curr Opin Rheumatol. 2011;23(1):67–71. doi: 10.1097/BOR.0b013e328341a240. [DOI] [PubMed] [Google Scholar]
- 19.Kamisawa T, Okazaki K, Kawa S, et al. Japanese consensus guidelines for management of autoimmune pancreatitis: III. Treatment and prognosis of AIP. J Gastroenterol. 2010;45(5):471–477. doi: 10.1007/s00535-010-0221-9. [DOI] [PubMed] [Google Scholar]
- 20.Khosroshahi A, Carruthers MN, Deshpande V, et al. Rituximab for the treatment of IgG4-related disease: lessons from 10 consecutive patients. Medicine. 2012;91(1):57–66. doi: 10.1097/MD.0b013e3182431ef6. [DOI] [PubMed] [Google Scholar]
- 21.Stone JH. IgG4-related disease: pathophysiologic insights drive emerging treatment approaches. Clin Exp Rheumatol. 2016;34(4 Suppl 98):66–68. [PubMed] [Google Scholar]
- 22.Perugino CA, Stone JH. Treatment of IgG4-related disease: current and future approaches. Z Rheumatol. 2016;75(7):681–686. doi: 10.1007/s00393-016-0142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carruthers MN, Stone JH, Deshpande V, et al. Development of an IgG4-RD responder index. Int J Rheumatol. 2012;2012:259408. doi: 10.1155/2012/259408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallace ZS, Mattoo H, Carruthers M, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheumat Dis. 2015;74(1):190–195. doi: 10.1136/annrheumdis-2014-205233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanzillotta M, Della-Torre E, Stone JH. Roles of plasmablasts and B cells in IgG4-related disease: implications for therapy and early treatment outcomes. Curr Top Microbiol Immunol. 2017;401:85–92. doi: 10.1007/82_2016_58. [DOI] [PubMed] [Google Scholar]