Abstract
To determine the optimal method of evaluating kidney function in patients with thyroid dysfunction, this study compared the estimated glomerular filtration rate derived from serum creatinine, cystatin C, or β2-microglobulin with inulin or creatinine clearance in two pediatric patients, one with hypothyroidism and the other with hyperthyroidism. It was observed that the kidney function decreased in a hypothyroid child and enhanced in a hyperthyroid child, with their kidney function becoming normalized by treatment with drugs, which normalized their thyroid function. Kidney function cannot be accurately evaluated using cystatin C-based or β2-microglobulin-based estimated glomerular filtration rate in patients with thyroid dysfunction, as these tests overestimated glomerular filtration rate in a patient with hypothyroidism and underestimated glomerular filtration rate in a patient with hyperthyroidism, perhaps through a metabolic rate-mediated mechanism. In both our patients, 24-h urinary creatinine secretion was identical before and after treatment, suggesting that creatinine production is not altered in patients with thyroid dysfunction. Therefore, kidney function in patients with thyroid dysfunction should be evaluated using creatinine-based estimated glomerular filtration rate.
Keywords: Hypothyroid, Hyperthyroid, Estimated glomerular filtration rate, Creatinine, Cystatin C, β2-microglobulin
Introduction
Methods of evaluating kidney function in children include serum creatinine (Cr)-based estimated glomerular filtration rate (eGFR) [1, 2], serum cystatin C (cysC)-based eGFR [3], and serum β2 microglobulin (β2MG)-based eGFR [4] in Japan. These methods may be inaccurate, however, because serum Cr, cysC, or β2MG concentrations are influenced by muscle mass, administration of glucocorticoids [5], or malignant tumors [6] and inflammatory disorders [7], respectively.
GFR in both adults [8–10] and children [11–13] with thyroid diseases was shown to alter by fluctuations in serum Cr concentrations or creatinine clearance (Ccr). GFR is lower in patients with hypothyroidism, and restoration of thyroid function, by treatment for hypothyroidism, has been shown to result in recovery of kidney function, as shown by Cin [13]. Furthermore, serum concentrations of cysC [9, 10] and β2MG [14] do not accord with the change of serum Cr concentrations in patients with thyroid disease.
To determine the optimal method of evaluating kidney function in patients with thyroid dysfunction, this study compared the eGFRs derived from serum Cr, cysC, and β2MG with Cin or Ccr in two pediatric patients, one with hypothyroidism and the other with hyperthyroidism. A retrospective review of the records of a few patients, to report as a case study or case series, is not considered by our institution to be research on human subjects and did not require ethics committee approval. Informed consent of the patients involved was provided about the publication of this article.
Evaluation of kidney function
Cin was performed using the method standardized for Japanese pediatric patients [1]. Ccr-based eGFR was calculated based on 24-h Ccr, using the formula 24-h Ccr-based eGFR = 0.764 × 24-h Ccr [15]. Cr-based eGFR, cysC-based eGFR, and β2MG-based eGFR were calculated as described [1, 3, 4]. Cr-based eGFR was calculated using the formula: Cr-based eGFR = 110.2 × (reference serum Cr/patient’s serum Cr) + 2.93, where reference serum Cr is calculated as: − 1.259X5 + 7.815X4 − 18.57X3 + 21.39X2 − 11.71X + 2.628 for males and − 4.536X5 + 27.16X4 − 63.47X3 + 72.43X2 − 40.06X + 8.778 for females [X = body length (cm)]. CysC-based eGFR was calculated using the formula: cysC-based eGFR = 104.1 × 1/serum cysC (mg/L) − 7.80, and β2MG-based eGFR was calculated using the formula: β2MG-based eGFR = 149.0 × 1/serum β2MG (mg/L) + 9.15.
Patient 1 (hypothyroidism)
A 12-year-old girl presented with elevated serum Cr (1.18 mg/dL) and short stature [body length, 132 cm (Z score − 3.6 SD), body weight, 30.9 kg (Z score − 1.6S D)]. She had been diagnosed with idiopathic pulmonary hemosiderosis at age 4 years, and had been treated since with once weekly dexamethasone injections and daily azathioprine internal use. On admission, she had decreased kidney function (Cin, 59.6 ml/min/1.73 m2; 24-h Ccr-based eGFR: 60.0 ml/min/1.73 m2; Cr-based eGFR, 45.8 ml/min/1.73 m2); however, her cysC-based eGFR (108.7 ml/min/1.73 m2) and β2MG-based eGFR (115.6 ml/min/1.73 m2) were normal. A kidney biopsy showed the absence of glomerular and tubular abnormalities by light microscopy and fluorescent antibody technique. Because she had a previous history of pulmonary hemorrhage, her serum concentration of anti-glomerular basement membrane antibody was checked. This antibody was not present, nor was she diagnosed with Goodpasture syndrome.
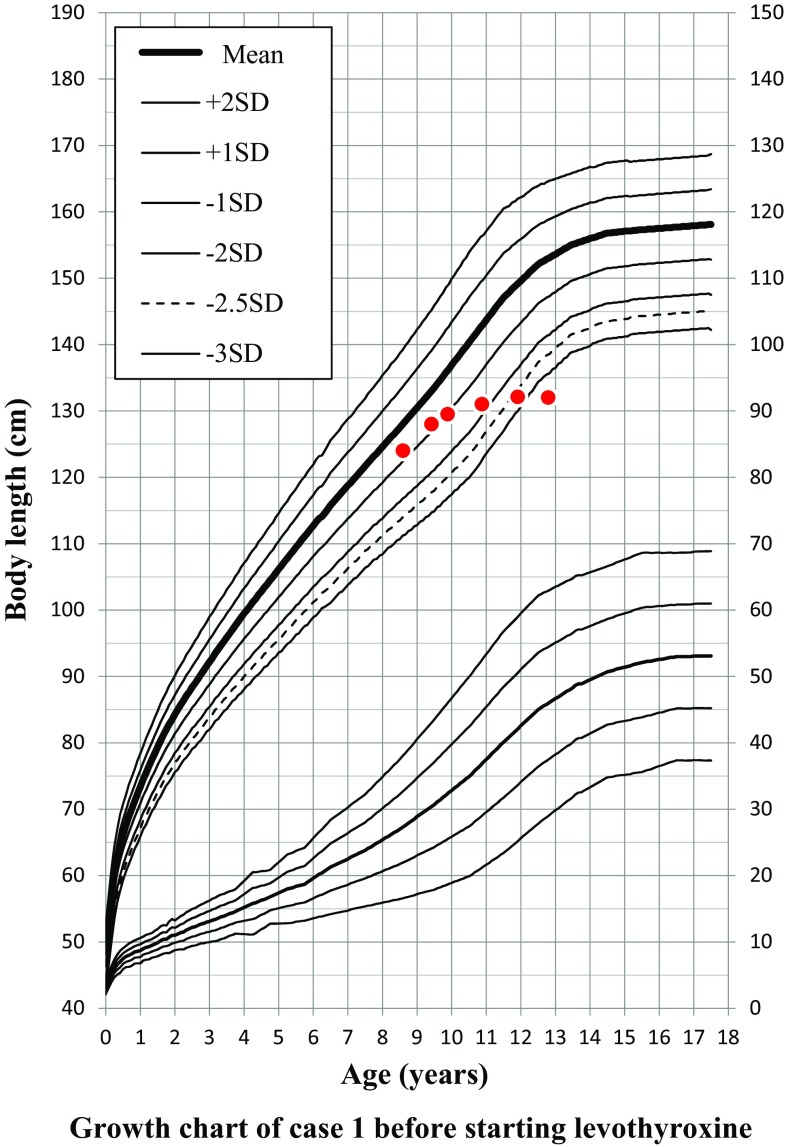
Evaluation using the Bone Age Standardized for Japanese Children (TW2-RUS scoring system) showed that her bone age was 10.6 years when she was 12.8 years old chronologically. Her body length and growth rate were 128.0 cm (Z score − 0.8 SD) and 4.9 cm/year (− 0.9 SD), respectively, at age 8.6 years; 129.5 cm (− 1.0 SD) and 3.2 cm/year (− 3.8 SD), respectively, at age 9.8 years; 131.0 cm (− 1.7 SD) and 1.5 cm/year (− 6.4 SD), respectively, at age 10.8 years; 132.1 cm (− 2.6 SD) and 1.1 cm/year (− 5.9 SD), respectively, at age 11.8 years; and 132.0 cm (− 3.6 SD) and 0 cm/year (− 4.0 SD), respectively, at age 12.8 years, indicating marked failure of growth beginning at age about 9 years (Fig. 1).
Fig. 1.
The growth rate declined when the hypothyroidism was acquired at age about 9 years
Her Cr-based eGFRs at ages 9.8, 10.8, and 11.8 years were 84.3, 49.4, and 46.2 mL/min/1.73 m2, respectively, showing that her Cr-based eGFR paralleled her failure of growth. Other physical findings included low blood pressure (94/40 mmHg) and bradycardia (57/min). These findings, along with short stature and reduction of growth rate, suggested hypothyroidism. Blood examination revealed hypothyroidism caused by Hashimoto’s disease (TSH, 1416 µIU/mL; fT3, 0.78 pg/mL; fT4, < 0.1 ng/dL; thyroglobulin, 1.1 ng/mL; anti-thyroglobulin antibody, 563 U/mL; anti-thyroid peroxidase antibody, 32.0 U/mL; anti-thyroid microsomal antibody, 1:25,600; TSH stimulating antibody (TSAb),7.6%).
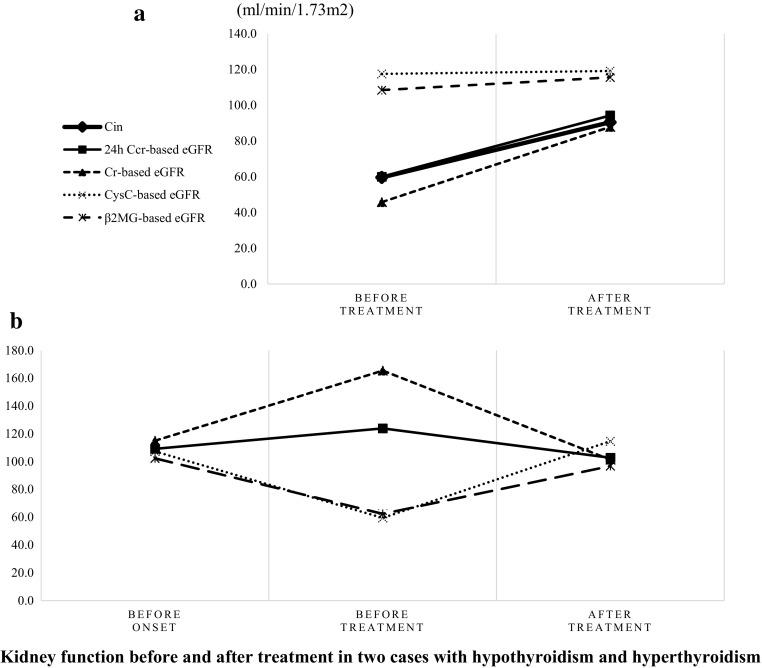
She was started on treatment with levothyroxine sodium. After 3 months of treatment, her thyroid function was restored (TSH, 33.6 µIU/mL; fT4, 0.87 ng/dL), and her kidney function recovered to 90.5 ml/min/1.73 m2 by Cin. Her 24-h Ccr-based eGFR and Cr-based eGFR were 94.3 and 86.9 ml/min/1.73 m2, respectively, consistent with the results of Cin. However, her cysC-based eGFR (108.7 ml/min/1.73 m2) and β2MG-based eGFR (115.6 ml/min/1.73 m2) remained unchanged (Table 1; Fig. 2a).
Table 1.
Thyroid and kidney functions in two cases with hypothyroidism or hyperthyroidism
| Case 1 hypothyroidism | Case 2 hyperthyroidism | |||||
|---|---|---|---|---|---|---|
| 12Y7M | 13M0M | 9Y11M | 11Y2M | 13Y6M | ||
| Before treatment | After treatment | Before onset | Before treatment | After treatment | Reference values | |
| Height (m) | 1.320 | 1.328 | 1.335 | 1.410 | 1.492 | – |
| Thyroid function | ||||||
| TSH (µIU/mL) | 1416 | 33.6 | 0.91 | < 0.003 | 1.20 | 0.50–5.00 |
| fT3 (pg/mL) | 0.78 | 3.63 | 4.20 | 68 | 4.38 | 2.30–4.30 |
| fT4 (ng/dL) | < 0.1 | 0.87 | 1.02 | 16.4 | 0.61 | 0.90–1.70 |
| Renal function | ||||||
| Cin (mL/min/1.73 m2) | 59.6 | 90.5 | ND | ND | ND | 83.5–156.7 |
| 24-h Ccr-based eGFR (mL/min/1.73 m2) | 60.0 | 94.3 | 109.2 | 123.9 | 102.8 | 83.5–156.7 |
| Cr-based eGFR (mL/min/1.73 m2) | 45.8 | 87.9 | 115.0 | 165.5 | 101.4 | 83.5–156.7 |
| cysC-based eGFR (mL/min/1.73 m2) | 117.6 | 119.2 | 107.3 | 59.5 | 114.7 | 83.5–156.7 |
| β2MG-based eGFR (mL/min/1.73 m2) | 108.5 | 115.6 | 102.3 | 62.4 | 96.8 | 83.5–156.7 |
| Urinary Cr excretion per height (g/m/day) | 0.45 | 0.45 | - | 0.36 | 0.36 | – |
Fig. 2.
Kidney function cannot be accurately measured using cystatin C-based or β2-microglobulin-based estimated glomerular filtration rate in patients with thyroid dysfunction, but evaluated using creatinine-based estimation. The cystatin C-based or β2-microglobulin-based evaluation overestimated glomerular filtration rate in case 1 with hypothyroidism (a), and underestimated glomerular filtration rate in case 2 with hyperthyroidism (b)
Although her kidney function, as shown by Cin, decreased when she was hypothyroid and increased after treatment, measurements of cysC-based eGFR and β2MG-based eGFR did not show parallel changes. In addition, 24-h urinary Cr excretion per height was 0.45 g/m/day both before and after treatment (Table 1).
Patient 2 (hyperthyroidism)
An 11-year-old girl with idiopathic nephrotic syndrome, diarrhea, general fatigue, pollakisuria, increased appetite, excessive sweating, and poor weight gain was suspected of being hyperthyroid. She had tachycardia (HR 136/min) and goiter. Her body weight was 30.0 kg (− 1.0 SD) and her body length was 141.8 cm (− 0.5 SD).
She was diagnosed with steroid resistant nephrotic syndrome at age 4 years, with histological diagnosis at age 5 years showing minimal changes in her kidneys. Treatment with cyclosporin (CyA), based on a 4-h area under the curve (AUC0-4), with a target blood concentration of 1000–1200 ng/h/mL, and mycophenolate mofetil (MMF), was successful in inducing and maintaining remission.
Blood tests at admission revealed hyperthyroidism caused by Basedow’s disease (TSH, < 0.003 µIU/mL; fT3, 65 pg/mL; fT4, 16.4 ng/dL; thyroglobulin, 371.9 ng/mL; anti-thyroglobulin antibody, 62.5 U/mL; anti-thyroid peroxidase antibody, 32.0 U/mL; TSH stimulating antibody (TSAb), 61.3%; anti-TSH receptor antibody (TRAb), 89.4 IU/L), with mild kidney hyperfunction. To determine the time of onset of hyperthyroidism, we performed tests of thyroid function in sera stored in our hospital. She was euthyroid (TSH, 0.91 µIU/mL; fT3, 4.2 pg/dL; fT4, 1.02 ng/dL) 15 months prior to diagnosis of Basedow’s disease and hyperthyroid (TSH, 0 µIU/mL; fT3, 16.20 pg/dL; fT4, 5.4 ng/dL) 7 months prior to diagnosis. Following treatment for 2 years with thiamazole and levothyroxine, her thyroid function was restored (TSH, 1.2 µIU/mL; fT3, 4.38 pg/dL; fT4, 0.61 ng/dL) and her kidney function normalized, as shown by a 24-h Ccr-based eGFR of 102.8 ml/min/1.73 m2.
Table 1 and Fig. 2b show changes in kidney function of this patient. Although Cin could not be examined, her 24-h Ccr-based eGFR, Cr-based eGFR, cysC-based eGFR, and β2MG-based eGFR 15 months before diagnosis were 109.2, 115.0, 107.3, and 102.3 ml/min/1.73 m2, respectively, indicating normal kidney function. On admission, however, these eGFRs were 123.9, 165.5, 59.5, and 62.4 ml/min/1.73 m2, respectively, indicating mildly overactive kidney function and the differences in these four parameters. After 2 years of treatment, these eGFRs were 102.8, 101.4, 114.7, and 96.8 ml/min/1.73 m2, respectively, indicative of normalized kidney function. Cr excretion per height over 24-h urine was 0.36 g/m/day before and after treatment (Table 1).
Discussion
eGFRs based on Cr, cysC, and β2MG are surrogate measurements of Cin. Factors that can influence serum Cr, cysC, and β2MG concentrations can therefore overestimate or underestimate kidney function.
This report describes two patients with thyroid dysfunction who experienced parallel changes in kidney function. Similar findings have been observed in other patients [8, 11, 12]. To our knowledge, however, no study has utilized Cin to evaluate kidney function in pediatric patients with hyperthyroidism, although one report describes the use of Cin to evaluate kidney function in pediatric patients with hypothyroidism [13].
Patient 1 with hypothyroidism showed reduced kidney function (about 60 ml/min/1.73 m2), as revealed by Cin and 24-h Ccr-based eGFR. Although effective renal plasma flow (ERPF) and filtration fraction (FF) were not measured in this patient, ERPF was reported unaffected despite a reduction in GFR in patients with hypothyroidism [8], suggesting that a decrease in GFR may parallel a decrease in FF. In addition, Cin and 24-h Ccr-based eGFR showed that kidney function in this patient recovered as her thyroid function improved.
Patient 2 with hyperthyroidism showed an increase in kidney function during the acute phase, with her kidney function becoming normalized by treatment with antithyroid drug, which normalized her thyroid function. Because Cin could not be performed, kidney function in this patient could be evaluated only by 24-h Ccr-based eGFR. Similar to this patient, other patients with hyperthyroidism have increased kidney function, with their kidney function becoming significantly decreased and normalized following treatment with antithyroid drugs [9].
This study had several limitations, including the small number of patients. In addition, Cin, regarded as the gold standard to assess kidney function, could not be measured in the patient with hyperthyroidism, unfortunately because of a retrospective case study.
The other tests of kidney function, cysC-based eGFR and β2MG-based eGFR, overestimated GFR in the hypothyroid patient and underestimated GFR in the hyperthyroid patient. Our findings suggest that hyperthyroidism and hypothyroidism influence the production and serum concentrations of cysC [10] and β2MG [14] through a metabolic rate-mediated mechanism. In both our patients, 24-h urinary Cr secretion was identical before and after treatment, suggesting that Cr production is not altered in patients with thyroid dysfunction. Therefore, Cr-based eGFR may accurately estimate GFR, in agreement with Cin or 24-h Ccr-based eGFR.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent of the patients involved was provided about the publication of this article.
References
- 1.Uemura O, Nagai T, Ishikura K, Ito S, Hataya H, Gotoh Y. Creatinine-based equation to estimate the glomerular filtration rate in Japanese children and adolescents with chronic kidney disease. Clin Exp Nephrol. 2014;18(4):626–33. doi: 10.1007/s10157-013-0856-y. [DOI] [PubMed] [Google Scholar]
- 2.Nagai T, Uemura O, Ishikura K, Ito S, Hataya H, Gotoh Y. Creatinine-based equations to estimate glomerular filtration rate in Japanese children aged between 2 and 11 years old with chronic kidney disease. Clin Exp Nephrol. 2013;17(6):877–81. doi: 10.1007/s10157-013-0799-3. [DOI] [PubMed] [Google Scholar]
- 3.Uemura O, Nagai T, Ishikura K, Ito S, Hataya H, Gotoh Y. Cystatin C-based equation for estimating glomerular filtration rate in Japanese children and adolescents. Clin Exp Nephrol. 2014;18(5):718–25. doi: 10.1007/s10157-013-0910-9. [DOI] [PubMed] [Google Scholar]
- 4.Ikezumi Y, Uemura O, Nagai T, Ishikura K, Ito S, Hataya H. Beta-2 microglobulin-based equation for estimating glomerular filtration rates in Japanese children and adolescents. Clin Exp Nephrol. 2015;19(3):450–457. doi: 10.1007/s10157-014-1015-9. [DOI] [PubMed] [Google Scholar]
- 5.Cimerman N, Brguljan PM, Krasovec M, Suskovic S, Kos J. Serum cystatin C, a potent inhibitor of cysteine proteinases, is elevated in asthmatic patients. Clin Chim Acta. 2000;300(1–2):83–95. doi: 10.1016/S0009-8981(00)00298-9. [DOI] [PubMed] [Google Scholar]
- 6.Bokenkamp A, Grabensee A, Stoffel-Wagner B, Hasan C, Henne T, Offner G. The beta2-microglobulin/cystatin C ratio–a potential marker of post-transplant lymphoproliferative disease. Clin Nephrol. 2002;58(6):417–22. [PubMed] [Google Scholar]
- 7.Yilmaz B, Koklu S, Yuksel O, Arslan S. Serum beta 2-microglobulin as a biomarker in inflammatory bowel disease. World J Gastroenterol. 2014;20(31):10916–10920. doi: 10.3748/wjg.v20.i31.10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karanikas G, Schutz M, Szabo M, Becherer A, Wiesner K, Dudczak R. Isotopic renal function studies in severe hypothyroidism and after thyroid hormone replacement therapy. Am J Nephrol. 2004;24(1):41–5. doi: 10.1159/000075628. [DOI] [PubMed] [Google Scholar]
- 9.Kimmel M, Braun N, Alscher MD. Influence of thyroid function on different kidney function tests. Kidney Blood Press Res. 2012;35(1):9–17. doi: 10.1159/000329354. [DOI] [PubMed] [Google Scholar]
- 10.Fricker M, Wiesli P, Brandle M, Schwegler B, Schmid C. Impact of thyroid dysfunction on serum cystatin C. Kidney Int. 2003;63(5):1944–1947. doi: 10.1046/j.1523-1755.2003.00925.x. [DOI] [PubMed] [Google Scholar]
- 11.Al-Fifi S, Girardin C, Sharma A, Rodd C. Moderate renal failure in association with prolonged acquired hypothyroidism in children. Acta Paediatr. 1999;88(7):715–717. doi: 10.1111/j.1651-2227.1999.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 12.del-Rio Camacho G, Tapia Ceballos L, Picazo Angelin B, Ruiz Moreno JA, Hortas Nieto ML, Romero Gonzalez J. Renal failure and acquired hypothyroidism. Pediatr Nephrol. 2003;18(3):290–292. doi: 10.1007/s00467-002-1033-9. [DOI] [PubMed] [Google Scholar]
- 13.Elgadi A, Verbovszki P, Marcus C, Berg UB. Long-term effects of primary hypothyroidism on renal function in children. J Pediatr. 2008;152(6):860–864. doi: 10.1016/j.jpeds.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 14.Roiter I, Da Rin G, De Menis E, Foscolo GC, Legovini P, Conte N. Increased serum beta 2-microglobulin concentrations in hyperthyroid states. J Clin Pathol. 1991;44(1):73–4. doi: 10.1136/jcp.44.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uemura O, Nagai T, Yamakawa S, Kaneko T, Hibi Y, Yamasaki Y. Assessment of kidney function in children by enzymatic determination of 2- or 24-h creatinine clearance: comparison with inulin clearance. Clin Exp Nephrol. 2016;20(3):462–468. doi: 10.1007/s10157-015-1166-3. [DOI] [PubMed] [Google Scholar]