Abstract
Introduction
Research on the association between periodontitis and oral human papilloma virus (HPV) infection is inconsistent. The cross-sectional association of severe periodontitis with oral HPV infection was investigated in a sample of Hispanic adults.
Methods
Data from the 2014–2016 San Juan Overweight Adults Longitudinal Study (n = 740) was analyzed. Periodontitis assessment and self-collection of oral HPV samples followed the National Health and Nutrition Examination Survey methodology. Periodontitis was defined using the Centers of Disease Control and Prevention/American Academy of Periodontology definition. HPV typing was performed using polymerase chain reaction. Multivariate logistic regression models were used to calculate odds ratios (ORs) and 95% confidence intervals (CIs).
Results
5.7% of participants had oral HPV infection and 20.3% had severe periodontitis. Adults with severe periodontitis had higher odds of oral HPV infection than those with none/mild disease (OR=2.9, 95% CI: 1.0–8.4, p < 0.05) in multivariable analysis. Adults with clinical attachment loss≥ 7 mm and pocket depth PD≥ 6 mm had 2- to 3-fold higher odds of HPV infection.
Conclusions
Severe periodontitis was positively associated to oral HPV infection. Longitudinal evaluation of periodontal inflammation's role in acquisition and persistence of oral HPV infection is needed, as periodontitis screening could identify individuals at increased risk of HPV-related oral malignancies.
Keywords: Periodontitis, Oral HPV, Hispanics, Adults, Oral health, Puerto Rico
1. Introduction
Head and neck squamous cell carcinoma (HNSCC) includes squamous cell cancers of the nasal cavity, the paranasal sinuses, the oral cavity, the pharynx (includes the nasopharynx, oropharynx and hypopharynx) and the larynx [1]. Although high-risk (oncogenic) human papilloma virus (HPV) sub-types have been detected in HNSCC, viral infection appears to be more strongly associated to oropharyngeal cancers, particularly those in the palatine and lingual tonsils and the base of the tongue [1], [2], [3]. HPV-positive oropharyngeal cancers are increasing [2] and molecular studies have linked this increase to HPV [2]. Nonetheless, the epidemiology and natural history of oral HPV infection is still not well understood [1]. While the prevalence of HPV in oropharyngeal cancers has increased, with estimates of 72% in North America, Europe and other populations [4], prevalence of oral HPV infection in cancer-free individuals, risk factors, natural history, and transmission mechanisms remain largely unexplored. In the US, prevalence estimates of oral HPV infection were 6.9% in 2009–2010 5 and 8.1% during 2009–2012 [6]. Oral sex, male gender, older age, open-mouthed kissing, increasing number of sexual partners, HIV infection, tobacco, and marijuana and heavy alcohol use have been associated with oral HPV infection [5], [7], [8].
Although research suggests an association between chronic oral inflammation, including periodontitis, oral HPV infection, and various HNSCC [9], [10], [11], particularly oropharyngeal cancers, studies on the relationship of oral HPV infection with these chronic conditions are limited in power and ethnic/racial diversity and findings are inconsistent [1], [12]. Periodontitis has been associated to HPV-positive HNSCC, with stronger association for oropharyngeal cancer than for oral cavity and laryngeal squamous cell carcinoma [11]. These and other previous research on these associations [13], [14] suggest that chronic oral inflammation may facilitate HPV acquisition and persistence [15], and may be involved in the etiology of certain head and neck cancers by facilitating HPV infection [11], or through other pathways. Also, high-risk HPV types have been detected in gingival biopsies from patients with periodontitis, suggesting that periodontal pockets may serve as a reservoir for HPV [4], [15], [16], [17]. However, one cross-sectional (n = 102) [18] and one case-control (n = 104) [19] studies did not detect HPV-16 in any of the gingival samples evaluated.
Approximately 46% (~65 million) of adults aged ≥ 30 years in the US have periodontitis, with higher prevalence among men than women (55% vs. 37%) and among Hispanics than non-Hispanic Whites (64% vs. 41%) [20]. The prevalence of moderate and severe periodontitis varies markedly among Hispanic subgroups [21]. Although periodontitis may be a facilitator for HPV infection and certain HPV-related HNSCC, research evaluating the role of chronic oral inflammation on the natural history of oral HPV is lacking, critical knowledge to develop effective oral cancer prevention strategies are needed. In fact, periodontitis treatment has been suggested as a potential oral cancer prevention strategy [22]. Since the Hispanic population has a high burden of both oral cancer [23] and periodontitis [24], this study assessed the strength of the association between periodontitis and oral HPV infection in a Hispanic population, using validated clinical methods.
2. Material and methods
2.1. Study population
The present cross-sectional study has been described in detail elsewhere [25] and enrolled overweight/obese adults, participants of the San Juan Overweight Adults Longitudinal Study (SOALS) and aged 40–65 years at baseline, to evaluate the bi-directional, longitudinal association between periodontitis and glucose abnormalities over a three-year period. Detailed descriptions of the cohort study have been published elsewhere [26].
2.2. Participant recruitment and data collection procedures
Of 773 participants who came to the follow-up SOALS examination visit between September 2014 and May 2016, 99.7% accepted to participate in the present study. From these, 24 persons were excluded from analysis as they did not complete HPV collection (n = 4) or periodontal evaluation (n = 20) procedures, and seven because of unsatisfactory HPV results in the laboratory (samples negative for β-globin gene amplification); the remaining 740 (96% of recruited participants) were included. The study was approved by the Institutional Review Board of the University of Puerto Rico Medical Sciences Campus and written informed consent was provided by all subjects.
2.3. Data collection procedures
2.3.1. Periodontal assessment
Periodontitis was assessed by clinical measurements of probing depth (PD) and clinical attachment loss (CAL) at six sites (disto-buccal, mid-buccal, mesio-buccal, disto-lingual, mid-lingual, and mesio-lingual buccal) for all teeth, excluding the third molars. The Centers for Disease Control/American Academy of Periodontology working definition was used to define severe periodontitis (≥2 interproximal sites with CAL≥6 mm (not on the same tooth) and ≥ 1 interproximal site with PD≥ 5 mm), moderate periodontitis (≥2 interproximal sites with CAL≥4 mm (not on the same tooth) or ≥ 2 interproximal sites with PD≥ 5 mm (not on the same tooth)), and mild periodontitis (≥2 interproximal sites with CAL≥3 mm and ≥2 interproximal sites with PD≥4 mm (not on the same tooth) or ≥ 1 site with PD≥ 5 mm). Various severity thresholds for CAL (≥ 1 site with CAL ≥ 3 mm, ≥ 4 mm, ≥ 5 mm, ≥ 6 mm, and ≥ 7 mm) and PD were also assessed (≥ 1 site with PD ≥ 4 mm, ≥ 5 mm, ≥ 6 mm, and ≥ 7 mm) [24]. The Silness and Loe Plaque Index, a measure of oral hygiene status, was determined by visual assessment of presence of bacterial plaque after passing a periodontal probe around the tooth surface of six pre-selected Ramfjörd teeth. Plaque index was coded as 0 if no plaque, 1 if dental plaque was present after passing the periodontal probe around the tooth, 2 if plaque was visible along the gingival margin, and 3 if the tooth surface was covered with abundant plaque. For analyses purposes, plaque index was categorized as no plaque to a film of plaque (0–1) vs. moderate to abundant (2–3).
2.3.2. Collection of oral HPV specimens using oral rinse
Participants were asked to self-collect oral rinse samples using the National Health and Nutrition Examination Survey (NHANES) methodology [5]. A 50-mL sterile collection cup filled with 10 mL of Scope (original mint flavor) was given to participants. If participants were chewing gum, they were asked to remove the gum prior to the sample collection. Participants were then asked to rinse/gargle with the mouthwash for 30 s, spit the mouthwash into the collection cup, while trying not to spill the liquid, and instructed to close the cup tightly. After collection, samples were transported to our laboratory at the University of Puerto Rico Comprehensive Cancer Center, where the samples were transferred to a 15-mL tube and centrifuged at 4200 rpm for 10 min. After centrifugation, the supernatant was discarded and the pellet was re-suspended with 10 mL of saline solution (PBS 1 ×). Samples were then centrifuged for a second time at 42,000 rpm for 10 min and after discarding the supernatant, the remaining pellet was re-suspended with 1 mL of saline solution (PBS 1×). The re-suspended whole pellet was then transferred to a pre-identified 2.0 mL micro-tube and stored at − 80 °C until shipment to the HPV Virology Core Laboratory, University of California, San Francisco (UCSF).
2.3.3. DNA extraction/HPV detection and typing
HPV typing was performed at UCSF using polymerase chain reaction (PCR) with modified L1 consensus primers (MY09/MY11). After thawing, the samples were processed for DNA using the Gentra Puregene Buccal Cell Kit (QIAGEN) following manufacturer's instructions. Five microliters of sample were used for PCR amplification using the standard 40 cycle protocol [27]. PCR products were typed by dot-blot hybridization using 38 type-specific probes, including oncogenic HR HPV types as defined by IARC (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 66) [28] and other non-oncogenic types (6/11, 26/69, 30, 32/42, 34, 53, 54, 57/2/27, 61, 62, 67, 68, 70, 71, 72, 73, 81, 82, 83, 84, 85, 86/87, 90/106, 97,102/89), and 2 separate mixtures: mix1 (7/13/40/43/44/55/74/91) and mix2 (3/10/28/29/77/78/94). A sample was considered HPV positive if it was positive for the consensus probes or any specific HPV type probes. Controls consisted of amplification of solution containing all the above components except for sample DNA, or DNA from cell lines with and without HPV.
2.4. Assessment of covariates
Participants completed a computer-assisted self-interview to gather information on risk factors for oral HPV infection, and provided mouthwash samples for HPV detection and typing; demographic and lifestyle variables were provided by SOALS. Information on sexual behavior and drug use was collected through the Audio CASI system (Nova Research Co., Washington, DC).
2.5. Statistical analysis
Frequency distributions were used to describe sociodemographic and lifestyle characteristics, periodontal measures, HPV infection, and HPV types. A multivariable binary logistic regression model was fitted for HPV infection and periodontitis severity, as well as for other periodontal indicators. The model was adjusted by covariates significantly associated (p < 0.05) in the bivariate analysis to both oral HPV infection and periodontitis, and other relevant variables in the literature. The statistical package Stata for Windows, version 14 (Stata Corporation, College Station, TX) was used for data management and statistical analyses.
3. Results
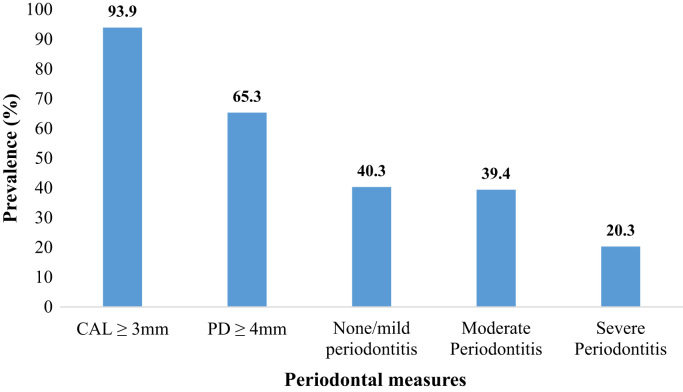
Mean age of participants was 53.5 ± 6.6 years, 72.4% were women, 54.5% had 12 years or less of education, and 18.3% had at least 11 lifetime sexual partners (Table 1). Regarding clinical variables, 93.9% of participants had at least one site with CAL≥ 3 mm whereas 65.3% had at least one site with PD≥ 4 mm. According to the CDC/APP definition, 39.4% and 20.3% had moderate and severe periodontitis, respectively (Fig. 1). Prevalence of oral HPV infection was 5.7%; with higher prevalence in men (10.3%) than women (3.9%) (p = 0.001). Not having healthcare coverage, increased number of lifetime number of sexual and oral sex partners, as well as having moderate to abundant plaque, were positively associated to oral HPV infection in bivariate analyses (p < 0.05) (Table 1).
Table 1.
Demographic, lifestyles, and clinical characteristics of the study population (n = 740).
| Study population characteristics | n (%) | Prevalence of oral HPV n (%) | P-value |
|---|---|---|---|
| Overall | – | 42 (5.7%) | |
| Sex¥ | |||
| Men | 204 (27.6) | 21 (10.3) | 0.001 |
| Women | 536 (72.4) | 21 (3.9) | |
| Age (years) ¥ | |||
| 40–49 | 237 (32.0) | 11 (4.6) | 0.40 |
| ≥ 50 | 503 (68.0) | 31 (6.2) | |
| Mean (mean±sd) | 53.5 (6.6) | ||
| Marital status | |||
| Single | 105 (14.2) | 5 (4.8) | 0.95 |
| Married/cohabitating | 332 (44.8) | 19 (5.7) | |
| Divorced/widowed | 303 (41.0) | 18 (5.9) | |
| Education¥ | |||
| ≤ 12 years | 403 (54.5) | 22 (5.5) | 0.78 |
| > 12 years | 337 (45.5) | 20 (5.9) | |
| Healthcare coverage | |||
| Yes | 685 (92.6) | 35 (5.1) | 0.02 |
| No | 55 (7.4) | 7 (12.7) | |
| Family income*¥ | |||
| < $20,000 | 396 (53.7) | 27 (6.8) | 0.16 |
| ≥ $20,000 | 342 (46.3) | 15 (4.4) | |
| Obese | |||
| No | 271 (36.6) | 16 (5.9) | 0.84 |
| Yes | 469 (63.4) | 26 (5.5) | |
| Waist Circumference | |||
| No risk | 120 (16.2) | 10 (8.3) | 0.17 |
| Risky | 83.8) | 32 (5.2) | |
| Current smoking†¥ | |||
| No | 602 (81.5) | 32 (5.3) | 0.37 |
| Yes | 137 (18.5) | 10 (7.3) | |
| Current drinking‖ | |||
| No | 398 (53.8) | 22 (5.5) | 0.85 |
| Yes | 342 (46.2) | 20 (5.9) | |
| Binge drinking‖¥ | |||
| No | 630 (84.1) | 34 (5.4) | 0.43 |
| Yes | 110 (14.9) | 8 (7.3) | |
| Marijuana use in the last 30 days (≥ 2 times) ‡¥ | |||
| No | 706 (96.1) | 38 (5.4) | 0.08† |
| Yes | 29 (3.9) | 4 (13.8) | |
| Lifetime number of sexual partners§¥ | |||
| 0–1 | 118 (16.9) | 3 (2.5) | 0.04† |
| 2–10 | 453 (64.8) | 24 (5.3) | |
| ≥ 11 | 128 (18.3) | 13 (10.2) | |
| Lifetime number of oral sexual partners*¥ | |||
| 0 | 98 (13.3) | 3 (3.1) | 0.0001† |
| 1–5 | 484 (65.6) | 20 (4.1) | |
| ≥ 6 | 156 (21.1) | 19 (12.2) | |
| Plaque¥ | |||
| No plaque to a film of plaque (0–1.49) | 635 (86.4) | 30 (4.7) | 0.004 |
| Moderate to abundant (1.50–3.0) | 100 (13.6) | 12 (12.0) |
Missing values: *(n = 2), †(n = 1), ‡(n = 5), §(n = 41). †p-value using Fisher's exact test. ‖Current drinking was defined as at least one drink in the last month and binge drinking as 5 or more drinks on a single occasion for men or 4 or more drinks on a single occasion for women. ¥Variables associated to periodontitis.
Fig. 1.
Prevalence of periodontal disease measures among the study population (n = 740).
Among the 42 individuals infected with HPV, 11.9% had high-risk HPV and 52.4% low-risk HPV infection (Table 2). The most common HPV types detected were HR type 16 (7.1%), and LR types 32/42 (19.0%), 71 (9.5) and 70 (7.1%).
Table 2.
Prevalence of HPV types detected among participants having oral HPV infection (n = 42).
| HPV type | Number positive | Percent |
|---|---|---|
| Any HR-HPV type | 5 | 11.9 |
| 16 | 3 | 7.1 |
| 33 | 1 | 2.4 |
| 52 | 1 | 2.4 |
| Any LR-HPV type | 22 | 52.4 |
| 32/42 | 8 | 19.0 |
| 61 | 1 | 2.4 |
| 70 | 3 | 7.1 |
| 71 | 4 | 9.5 |
| 72 | 2 | 4.8 |
| 82 + | 1 | 2.4 |
| 84 | 1 | 2.4 |
| 62 & 83 | 1 | 2.4 |
| Mix1 | 1 | 2.4 |
| Unknown HPV type | 15 | 35.7 |
Adults having at least one site with CAL≥ 6 mm (8.1%) and CAL≥ 7 mm (10.0%) had significantly higher prevalence of oral HPV infection than their counterparts (3.8% and 4.1%, respectively). Persons having at least one site with PD≥ 4 mm (7.5%) also had higher prevalence of HPV compared to their counterparts (2.3%). Other categories of PD evaluated were also associated with oral HPV infection. Prevalence of infection was significantly higher among those with severe periodontitis (11.3%) than among with none (2.6%) or mild/moderate (5.3%) disease (Table 3).
Table 3.
Prevalence of oral HPV infection of study participants by selected periodontal disease measures and logistic regression models of the association between periodontitis indicators and oral HPV infection.
| Variable | Prevalence of oral HPV | Crude OR (95% CI) | Adjusted OR (95% CI)† |
|---|---|---|---|
| ≥ 1 site with CAL≥ 3 mm | |||
| No | 1 (2.2) | 1.0 | 1.0 |
| Yes | 41 (5.9) | 2.8 (0.4–20.5) | 1.7 (0.2–13.5) |
| ≥ 1 site with CAL≥ 4 mm | |||
| No | 7 (4.0) | 1.0 | 1.0 |
| Yes | 35 (6.2) | 1.6 (0.7–3.6) | 1.0 (0.4–2.4) |
| ≥ 1 site with CAL≥ 5 mm | |||
| No | 13 (4.3) | 1.0 | 1.0 |
| Yes | 29 (6.7) | 1.6 (0.8–3.1) | 1.0 (0.5–2.2) |
| ≥ 1 site with CAL≥ 6 mm | |||
| No | 16 (3.8) | 1.0 | 1.0 |
| Yes | 26 (8.1) | 2.2 (1.2–4.2)† | 1.6 (0.7–3.1) |
| ≥ 1 site with CAL≥ 7 mm | |||
| No | 22 (4.1) | 1.0 | 1.0 |
| Yes | 20 (10.0) | 2.6 (1.4–4.9)† | 2.1 (1.0–4.1)¥ |
| ≥ 1 site with PD≥ 3 mm | |||
| No | 2 (4.1) | 1.0 | 1.0 |
| Yes | 40 (5.8) | 1.4 (0.3–6.2)† | 0.9 (0.2–3.9) |
| ≥ 1 site with PD≥ 4 mm | |||
| No | 6 (2.3) | 1.0 | 1.0 |
| Yes | 36 (7. 5) | 3.4 (1.4–8.1)† | 2.3 (0.9–5.8)¥ |
| ≥ 1 site with PD≥ 5 mm | |||
| No | 16 (3.5) | 1.0 | 1.0 |
| Yes | 26 (9.2) | 2.8 (1.5–5.3)† | 1.9 (1.0–3.9)¥ |
| ≥ 1 site with PD≥ 6 mm | |||
| No | 20 (3.5) | 1.0 | 1.0 |
| Yes | 22 (12.9) | 4.1 (2.2–7.6)† | 3.0 (1.5–6.0)† |
| ≥ 1 site with PD≥ 7 mm | |||
| No | 31 (4.7) | 1.0 | 1.0 |
| Yes | 11 (12.9) | 3.0 (1.4–6.2)† | 2.4 (1.1–5.2)† |
| Periodontitis severity | |||
| None | 6 (2.6) | 1.0 | 1.0 |
| Mild/Moderate | 19 (5.3) | 2.1 (0.8–5.4) | 1.5 (0.6–4.1) |
| Severe | 17 (11.33) | 4.8 (1.9–12.6)† | 2.9 (1.0–8.4)† |
| Periodontitis severity | |||
| Non-Severe | 25 (4.2) | 1.0 | 1.0 |
| Severe | 17 (11.3) | 2.9 (1.5–5.5)† | 2.1 (1.0–4.3)† |
Adjusted by sex, age, healthcare coverage, plaque, current smoking, and lifetime number of oral sex partners.
p < 0.05.
p < 0.10.
Multivariate models were adjusted by age, sex, health care coverage, plaque index, number of lifetime oral sexual partners, and current smoking. Although oral HPV infection was not significantly associated with having ≥ 1 site with CAL≥ 3 mm (OR = 1.7, 95% CI = 0.2–13.5) in multivariable analysis, there was a marginally significant association with CAL≥ 7 mm (OR = 2.1, 95% CI = 1.0–4.1, p < 0.10) (Table 3). PD≥ 6 mm (OR=3.0, 95% CI = 1.5–6.0) and PD≥ 7 mm (OR=2.4, 95% CI = 1.1–5.2) remained strongly associated with oral HPV infection in multivariable models.
Adults with moderate periodontitis had similar odds of oral HPV infection as those with no or mild periodontitis. Relative to adults with no or mild periodontitis, those with severe periodontitis had significantly higher odds (OR = 4.8, 95% CI: 1.9–12.6) of oral HPV infection than their counterparts; this association remained statistically significant (OR = 2.9, 95% CI: 1.0–8.4) after adjustment for covariates. Meanwhile, severe periodontitis (yes/no) was associated with oral HPV infection in crude (OR = 2.9, 95% CI = 1.5–5.5) and covariate adjusted analysis (OR = 2.1, 95% CI = 1.0–4.3, p = 0.04). Similarly, in a sub-analysis excluding those with none/mild periodontitis, adults with severe periodontitis had higher odds of oral HPV infection compared to those with moderate periodontitis in either crude (OR = 3.0, 95% CI: 1.4–6.4) and covariate-adjusted analysis (OR = 2.6, 95% CI: 1.1–6.1).
4. Discussion
To our knowledge, this is the first study to evaluate the association between oral HPV infection and periodontitis in Hispanic adults by using validated methods for oral HPV detection and typing, and periodontitis assessments. Overall, 5.7% of the study population had oral HPV infection; the prevalence in men more than double that of women (10.3% vs. 3.9%). HPV-16 was the most common HR type detected (7.1%) among those HPV-positive. These estimates are lower than the estimates in Puerto Rico for adult drug users (12.5%) [29] and for men from an STI clinic (20%) [30]. The prevalence estimate of oral HPV infection found in this study is comparable to the pooled prevalence of oral HPV infection among cancer-free individuals (4.5%); however, that study showed similar HPV prevalence by gender but higher prevalence (28%) for HPV-16 [31]. Our results are also consistent with the findings of a population-based study in Peru that found an overall prevalence of oral infection of 6.8% among individuals aged 10–85 years, with a higher prevalence among men than women [32]. Meanwhile, the multi-centric HIV Infection in Men study reported a lower prevalence of oral HPV infection of 4%, with HPV-16 accounting for 14% of all infections [33]. Similarly, the 2009–2010 NHANES documented that the prevalence of oral HPV infection in the US was 6.9%, and no significant differences were found by gender or race/ethnicity [5]. Nonetheless, more recent US data showed a prevalence of infection of 8.1%, and lower odds of infection with low-risk HPV types among non-Hispanic Whites compared to Hispanics [6]. Lower odds of both low-risk and high-risk HPV infections were also seen for women in the NHANES studies.
According to the CDC/APP definition, 48.2% and 20.3% of participants in our study had mild/moderate and severe periodontitis, respectively, figures higher than estimates for the US general population (37.1% and 8.9% of adults aged ≥ 30 years, respectively) [20]. Our estimates are also comparable to data for Hispanics aged 18–74 years in the 2008–2011 Hispanic Community Health Study/Study of Latinos [21], [24]. In that study, periodontitis prevalence was 32.3% for moderate and 9.8% for severe disease, with Puerto Ricans having the highest prevalence of severe periodontitis as compared to other Hispanic sub-groups [24].
Our study also showed a positive association between severe periodontitis and oral HPV infection. Although the US study based on the 2009–2010 and the 2011–2012 NHANES data did not find an association between periodontitis and oral HPV infection (OR = 1.04, 95% CI: 0.63–1.73), periodontitis severity was not assessed in this study [12]. While no HPV-16 was detected in gingival samples from patients with periodontitis in two small studies in India [18] and Brazil [19], another study in India found HR-HPV in 50% of samples from periodontal pockets [17]. Meanwhile, another study in Finland detected HR-HPV types in 26% of their gingival biopsies suggesting that periodontitis may be a risk factor for oral HPV infection [15]. The observations that both oral HPV infection and periodontitis indicators were higher among men than women in this population [25] are consistent with studies in other populations [20], [24] and with the observed higher risk of oropharyngeal cancer, specifically HPV-related and HPV-positive oropharyngeal tumors, in men as compared to women [23], [34]. These findings support the potential role of HPV infection and chronic local inflammation in oral cancer risk development.
PD was a stronger predictor of oral HPV infection compared to CAL; even though all PD categories evaluated were associated with HPV infection, only the most severe category of CAL (≥ 7 mm) remained marginally associated with periodontitis in multivariable analysis. The periodontal pockets may be a reservoir of HPV infection, and hence more associated with HPV compared to CAL, which is a composite measure based on probing depth and gingival recession.
Despite the lack of consistency from previous studies, various mechanisms could explain the biological plausibility of the association between periodontitis and HPV [19]. Periodontal pathogens cause an inflammatory response that results in periodontitis, that subsequently leads to permanent pathological changes in the periodontium, including formation of periodontal pockets, alveolar bone loss, and tooth loss [35], [36]. Periodontal pockets, rather than being the sites for carcinogenesis, seem to be a source of inflammatory cytokines, bacteria, and viruses in saliva [14]. The inflamed periodontium and subsequent pathological changes provide the perfect environment for HPV infection incidence and persistence [11], while the periodontal pocket may serve as reservoir for HPV [15]. HPV infects basal cells of the epithelium [37], which become exposed within the periodontal pocket [38] and also gaining access through microabrasions [11]. Periodontal pathogens also activate cytokines. As the inflamed periodontium continuously releases inflammatory cytokines, these promote inflammation that could lead to tissue injury and damage, which are proportional to the severity of periodontitis [10], [13]. Thus, periodontal mucosal damage mediated by inflammation may facilitate the acquisition and persistence of oral HPV infection, and modulate proliferation of HPV and of oncogene expression (E6, E7) in HPV infected epithelial cells, similar to the HPV-driven cervical carcinogenic pathway [10], [39], [40] This rationale is consistent with studies showing that inflammatory cytokines (e.g., IL-1, IL-6, and TNF-α) modulate HPV proliferation and expression of its oncogenes in cervical epithelial cells [10]. Basal cell proliferation is also increased with chronic inflammation, leading to higher HPV viral load in saliva as well as higher risk of transmission [38]. Thus, it is possible that periodontitis and oral HPV infection are associated through direct effects of the oral microbiota or through the stimulation of chronic inflammation; two mechanisms that could possibly be related [41]. Despite the lack of sufficient scientific evidence in this area, it is important to recognize that the relationship between periodontitis and oral HPV infection may be bidirectional, as recent evidence suggests that viral agents, including HPV, may contribute to periodontitis development [17], [42]. When HPV infects basal cells, it causes epithelial cell proliferation [43], disturbance on the odontogenic tissues [44], and inflammation, which in turn could result in progression to periodontitis [35].
Our study has several strengths. The sample size achieved was larger than in most previous reports. Our study population limited to overweight and obese Puerto Ricans provided a unique, high-risk study population for periodontitis. Both periodontitis and oral HPV testing were assessed using standard validated methods. Measures of risk factors for oral HPV infection were collected using ACASI methodology, a useful tool for collecting sensitive risk behavioral data. However, the possibility of residual confounding cannot be excluded. There are several limitations that should be noted. Since a non-probability sampling was used to select and recruit the parent study population, we cannot directly generalize our findings. While this would limit our ability to generalize the distribution of periodontitis and oral HPV infection, this would not impact the validity of the associations as there is no reason to expect that this would be different in this sample. Given the cross-sectional analyses, we cannot establish whether periodontal disease was a risk factor or consequence of oral HPV infection. Finally, no markers of HPV transcriptional activity [45] were measured, limiting our ability to determine HPV infectivity and its relationship with periodontitis.
Despite these limitations, we conclude that severe periodontitis may contribute to oral HPV infection and persistence. The identification of the role of periodontitis on oral HPV infection could provide key information for the development of preventive public health strategies, as periodontitis screening could identify individuals at increased risk of oral HPV infection and potential HPV-related malignant lesions. Further longitudinal studies with larger samples are needed to examine the role of chronic periodontal inflammation in facilitating acquisition and persistence of oral HPV infection, and vice versa. This information is essential to develop preventive public health strategies, as periodontitis screening could identify individuals at increased risk of HPV-related HSNCC. In addition, new strategies to improve dentists’ ability to counsel patients on the importance of HPV vaccination as a preventive measure for HPV-related HSNCC, as research suggests that this vaccine may also protect against oral HPV infection [46], [47].
Acknowledgements
This study was funded by the National Institute of Dental and Craniofacial Research (NIDCR Grant # 5R21DE024850-02) of the National Institutes of Health (NIH) and by the National Institute on Minority Health and Health Disparities of the NIH under Award Number 2U54MD007587. The content is solely the responsibility of the authors and does not necessarily represent the official vies of the National Institutes of Health. The authors declare that there are no conflicts of interest in this study. We would like to acknowledge the support of the following research staff in data collection and data management procedures: Amarilis Asencio, Reynaldo P é rez, Christian Rivera, Marietta Personeni, Frances García, Izamar Rivera, Mariem Navarro, Yasmarie Santana, Shalimar Delgado, María Ortiz, Cynthia Ruiz, Natalia Ortiz, Shalane Morales, Diana Medina, Damarys Santiago, Ana Rosario, Vicmar Quiles, Grace Vélez, Yadiris Santaella, Griseila Cruz, Tatiana Castillo, José Vergara, and Francisco Muñoz from the University of Puerto Rico. In addition, we acknowledge the support of María Da Costa from UCSF San Francisco for HPV typing of the samples.
Acknowledgments
Funding Statement
This study was funded by the National Institute of Dental and Craniofacial Research (NIDCR Grant # 5R21DE024850-02) of the National Institutes of Health (NIH) and by the National Institute on Minority Health and Health Disparities of the NIH under Award Number 2U54MD007587. The content is solely the responsibility of the authors and does not necessarily represent the official vies of the National Institutes of Health.
References
- 1.Cleveland J.L., Junger M.L., Saraiya M., Markowitz L.E., Dunne E.F., Epstein J.B. The connection between human papillomavirus and oropharyngeal squamous cell carcinomas in the United States: implications for dentistry. J. Am. Dent. Assoc. 2011:915–924. doi: 10.14219/jada.archive.2011.0298. 〈http://www.ncbi.nlm.nih.gov/pubmed/21804058〉 (Accessed 3 January 2017) [DOI] [PubMed] [Google Scholar]
- 2.Gillison M.L., Chaturvedi A.K., Anderson W.F., Fakhry C. Epidemiology of human papillomavirus–positive head and neck squamous cell carcinoma. J. Clin. Oncol. 2015:3235–3242. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woods R., O’Regan E.M., Kennedy S., Martin C., O’Leary J.J., Timon C. Role of human papillomavirus in oropharyngeal squamous cell carcinoma: a review. World J. Clin. Cases. 2014:172–193. doi: 10.12998/wjcc.v2.i6.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehanna H., Beech T., Nicholson T. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer–systematic review and meta- analysis of trends by time and region. Head Neck. 2013:747–755. doi: 10.1002/hed.22015. [DOI] [PubMed] [Google Scholar]
- 5.Gillison M.L., Broutian T., Pickard R.K.L. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA. 2012:693. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orosco R.K., Kedarisetty S., Hecht A.S., Chang D.C., Coffey C.S., Weissbrod P.A. Predictors of high-risk and low-risk oral HPV infection in the United States. Laryngoscope. 2016:1365–1372. doi: 10.1002/lary.25822. [DOI] [PubMed] [Google Scholar]
- 7.van Aar F., Mooij S.H., van der Sande M.A.B. Twelve-month incidence and clearance of oral HPV infection in HIV-negative and HIV-infected men who have sex with men: the H2M cohort study. BMC Infect. Dis. 2014:668. doi: 10.1186/s12879-014-0668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Souza G., Wentz A., Kluz N. Sex differences in risk factors and natural history of oral human papillomavirus infection. J. Infect. Dis. 2016:1893–1896. doi: 10.1093/infdis/jiw063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han Y.W., Houcken W., Loos B.G., Schenkein H.A., Tezal M. Periodontal disease, atherosclerosis, adverse pregnancy outcomes, and head-and-neck cancer. Adv. Dent. Res. 2014;26(1):47–55. doi: 10.1177/0022034514528334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tezal M. Interaction between chronic inflammation and oral HPV infection in the etiology of head and neck cancers. Int J. Otolaryngol. 2012:1–9. doi: 10.1155/2012/575242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tezal M., Scannapieco F.A., Wactawski-Wende J. Local inflammation and human papillomavirus status of head and neck cancers. Arch. Otolaryngol. Head Neck Surg. 2012:669–675. doi: 10.1001/archoto.2012.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiener R.C., Sambamoorthi U., Jurevic R.J. Association of periodontitis and human papillomavirus in oral rinse specimens: results from the national health and nutrition survey 2009–2012. J. Am. Dent. Assoc. 2015:382–389. doi: 10.1016/j.adaj.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tezal M., Sullivan M.A., Hyland A. Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol. Biomark. Prev. 2009:2406–2412. doi: 10.1158/1055-9965.EPI-09-0334. [DOI] [PubMed] [Google Scholar]
- 14.Tezal M., Sullivan Nasca M., Stoler D.L. Chronic periodontitis-human papillomavirus synergy in base of tongue cancers. Arch. Otolaryngol. Head Neck Surg. 2009:391–396. doi: 10.1001/archoto.2009.6. [DOI] [PubMed] [Google Scholar]
- 15.Hormia M., Willberg J., Ruokonen H., Syrjänen S. Marginal periodontium as a potential reservoir of human papillomavirus in oral mucosa. J. Periodontol. 2005:358–363. doi: 10.1902/jop.2005.76.3.358. [DOI] [PubMed] [Google Scholar]
- 16.Fuster-Rossello L., Ribotta E., Cuffini C., Fuster-Juan M. Human papilloma virus in oral mucosa and its association with periodontal status of gynecologically infected women. Acta Odontol. Latinoam. 2014:82–88. doi: 10.1590/S1852-48342014000200007. [DOI] [PubMed] [Google Scholar]
- 17.Dayakar M.M., Shipilova A., Gupta D. Periodontal pocket as a potential reservoir of high risk human papilloma virus: a pilot study. J. Indian Soc. Periodontol. 2016:136–140. doi: 10.4103/0972-124X.170815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacob A., House V., Nagar G., Janam P., Mohan J., Vijayamma B. Prevalence of human papilloma virus in marginal periodontium and its association with periodontitis: a cross sectional study. J. Indian Soc. Periodontol. 2014:447–450. doi: 10.4103/0972-124X.138682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horewicz V.V., Feres M., Rapp G.E., Yasuda V., Cury P.R. Human papillomavirus-16 prevalence in gingival tissue and its association with periodontal destruction: a case-control study. J. Periodontol. 2010:562–568. doi: 10.1902/jop.2009.090571. [DOI] [PubMed] [Google Scholar]
- 20.Eke P.I., Dye B.A., Wei L. Update on prevalence of periodontitis in adults in the United States: nhanes 2009 to 2012. J. Periodontol. 2015:611–622. doi: 10.1902/jop.2015.140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanders A.E., Campbell S.M., Mauriello S.M. Heterogeneity in periodontitis prevalence in the hispanic community health study/study of Latinos. Ann. Epidemiol. 2014:455–462. doi: 10.1016/j.annepidem.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gholizadeh P., Eslami H., Yousefi M., Asgharzadeh M., Aghazadeh M., Kafil H.S. Role of oral microbiome on oral cancers, a review. Biomed. Pharmacother. 2016:552–558. doi: 10.1016/j.biopha.2016.09.082. [DOI] [PubMed] [Google Scholar]
- 23.Suárez E., Calo W.A., Hernández E.Y., Diaz E.C., Figueroa N.R., Ortiz A.P. Age-standardized incidence and mortality rates of oral and pharyngeal cancer in Puerto Rico and among Non-Hispanics Whites, Non-Hispanic Blacks, and Hispanics in the USA. BMC Cancer. 2009:129. doi: 10.1186/1471-2407-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiménez M.C., Sanders A.E., Mauriello S.M., Kaste L.M., Beck J.D. Prevalence of periodontitis according to Hispanic or Latino background among study participants of the Hispanic community health study/study of Latinos. J. Am. Dent. Assoc. 2014:805–816. doi: 10.14219/jada.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortiz A.P., González D., Ramos J., Muñoz C., Reyes J., Peréz C.M. Association of marijuana use with oral HPV infection and periodontitis among Hispanic adults: implication for oral cancer prevention. J. Periodontol. 2017 doi: 10.1002/JPER.17-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pérez C.M., Muñoz F., Andriankaja O.M. Cross-sectional associations of impaired glucose metabolism measures with bleeding on probing and periodontitis. J. Clin. Periodontol. 2017:142–149. doi: 10.1111/jcpe.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palefsky J.M., Holly E.A., Ralston M.L., Da Costa M M Da, Greenblatt R.M. Prevalence and risk factors for anal human papillomavirus infection in human immunodeficiency virus (HIV)-positive and high-risk HIV-negative women. J. Infect. Dis. 2001:383–391. doi: 10.1086/318071. [DOI] [PubMed] [Google Scholar]
- 28.Bouvard V., Baan R., Straif K. Special report: policy A review of human carcinogens—Part B: biological agents. Lancet Oncol. 2009:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 29.Ortiz A.P., Reyes J.C., Palefsky J. Oral human papillomavirus infection among drug users in Puerto Rico. P R Health Sci. J. 2014:190–196. 〈http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=medl&NEWS=N&AN=25563037〉 [PubMed] [Google Scholar]
- 30.Colon-López V., Quiñones-Avila V., Del Toro-Mejías L.M. Oral HPV infection in a clinic-based sample of Hispanic men. BMC Oral Health. 2014;14:7. doi: 10.1186/1472-6831-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreimer A.R., Bhatia R.K., Messeguer A.L., González P., Herrero R., Giuliano A.R. Oral human papillomavirus in healthy individuals: a systematic review of the literature. Sex. Transm. Dis. 2010:386–391. doi: 10.1097/OLQ.0b013e3181c94a3b. [DOI] [PubMed] [Google Scholar]
- 32.Rosen B.J., Walter L., Gilman R.H., Cabrerra L., Gravitt P.E., Marks M.A. Prevalence and correlates of oral human papillomavirus infection among healthy males and females in Lima, Peru. Sex. Transm. Infect. 2016:149–154. doi: 10.1136/sextrans-2014-051942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreimer A.R., Villa A., Nyitray A.G. The epidemiology of oral HPV infection among a multinational sample of healthy men. Cancer Epidemiol. Biomark. Prev. 2011:172–182. doi: 10.1158/1055-9965.EPI-10-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaturvedi A.K., D’Souza G., Gillison M.L., Katki H.A. Burden of HPV-positive oropharynx cancers among ever and never smokers in the U.S. population. Oral. Oncol. 2016:61–67. doi: 10.1016/j.oraloncology.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Page R.C., Eke P.I. Case definitions for use in population-based surveillence of periodontitis. J. Periodontol. 2007:1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 36.Kim J., Amar S. Periodontal disease and systemic conditions: a bidirectional relationship. Odontology. 2006:10–21. doi: 10.1007/s10266-006-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanley M.A. Epithelial cell responses to infection with human papillomavirus. Clin. Microbiol. Rev. 2012:215–222. doi: 10.1128/CMR.05028-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hübbers C.U., Akgül B. HPV and cancer of the oral cavity. Virulence. 2015:244–248. doi: 10.1080/21505594.2014.999570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chow L.T., Broker T.R., Steinberg B.M. The natural history of human papillomavirus infections of the mucosal epithelia. APMIS. 2010:422–449. doi: 10.1111/j.1600-0463.2010.02625.x. [DOI] [PubMed] [Google Scholar]
- 40.Koutsky L.A., Holmes K.K., Critchlow C.W. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N. Engl. J. Med. 1992:1272–1278. doi: 10.1056/NEJM199210293271804. [DOI] [PubMed] [Google Scholar]
- 41.Scannapieco F.A., Wang B., Shiau H.J. Oral bacteria and respiratory infection: effects on respiratory pathogen adhesion and epithelial cell proinflammatory cytokine production. Ann. Periodontol. 2001:78–86. doi: 10.1902/annals.2001.6.1.78. [DOI] [PubMed] [Google Scholar]
- 42.Rodrigues P.M. de S., Teixeira A.L., Kustner E.C., Medeiros R. Are herpes virus associated to aggressive periodontitis? A review of literature. J. Oral. Maxillofac. Pathol. 2015:348–355. doi: 10.4103/0973-029X.174621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tommasino M. The human papillomavirus family and its role in carcinogenesis. Semin. Biol. 2014:13–21. doi: 10.1016/j.semcancer.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 44.de Paula A.M., Carvalhais J.N., Domingues M.G., Barreto D.C., Mesquita R.A. Cell proliferation markers in the odontogenic keratocyst: effect of inflammation. J. Oral. Pathol. Med. 2000:477–482. doi: 10.1034/j.1600-0714.2000.291001.x. 〈http://www.ncbi.nlm.nih.gov/pubmed/11048963〉 (Accessed 8 January 2017) [DOI] [PubMed] [Google Scholar]
- 45.Halec G., Schmitt M., Dondog B. Biological activity of probable/possible high-risk human papillomavirus types in cervical cancer. Int J. Cancer. 2013:63–71. doi: 10.1002/ijc.27605. [DOI] [PubMed] [Google Scholar]
- 46.M. Gillison, T. Broutian, B. Graubard, et al. Impact of HPV vaccination on oral HPV infections among young adults in the U.S. in: Meeting Library. Accesed 5 June 2017.
- 47.Herrero R., Quint W., Hildesheim A. Reduced prevalence of oral human papillomavirus (HPV) 4 Years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. Ramqvist T. PLOS One. 2013:e68329. doi: 10.1371/journal.pone.0068329. [DOI] [PMC free article] [PubMed] [Google Scholar]