Abstract
BACKGROUND/OBJECTIVES
The objective of this study was to investigate the effects of vitamin C on inflammation, tumor development, and dysbiosis of intestinal microbiota in an azoxymethane (AOM)/dextran sulfate sodium (DSS)-induced inflammation-associated early colon cancer mouse model.
MATERIALS/METHODS
Male BALB/c mice were injected intraperitoneally with AOM [10 mg/kg body weight (b.w)] and given two 7-d cycles of 2% DSS drinking water with a 14 d inter-cycle interval. Vitamin C (60 mg/kg b.w. and 120 mg/kg b.w.) was supplemented by gavage for 5 weeks starting 2 d after the AOM injection.
RESULTS
The vitamin C treatment suppressed inflammatory morbidity, as reflected by disease activity index (DAI) in recovery phase and inhibited shortening of the colon, and reduced histological damage. In addition, vitamin C supplementation suppressed mRNA levels of pro-inflammatory mediators and cytokines, including cyclooxygenase-2, microsomal prostaglandin E synthase-2, tumor necrosis factor-α, Interleukin (IL)-1β, and IL-6, and reduced expression of the proliferation marker, proliferating cell nuclear antigen, compared to observations of AOM/DSS animals. Although the microbial composition did not differ significantly between the groups, administration of vitamin C improved the level of inflammation-related Lactococcus and JQ084893 to control levels.
CONCLUSION
Vitamin C treatment provided moderate suppression of inflammation, proliferation, and certain inflammation-related dysbiosis in a murine model of colitis associated-early colon cancer. These findings support that vitamin C supplementation can benefit colonic health. Long-term clinical studies with various doses of vitamin C are warranted.
Keywords: Vitamin C, colitis, inflammation, colonic neoplasm, microbiota
INTRODUCTION
The prevalence of colorectal cancer (CRC), which was the third major cause of cancer death in both men and women in the U.S.A in 2016 [1], has been attributed to so called westernized diet combined with unhealthy life style [2]. Chronic inflammation is one of the key risk factors of CRC. In particular, patients with inflammatory bowel disease (IBD), including ulcerative colitis (UC) or Crohn's disease (CD), are at elevated risk of developing colon cancer [3]. The infiltration of neutrophil and other immune cells that accompany inflammation damages colonic tissue and may trigger changes to the colonic mucosa that facilitate the progression of low-grade dysplasia and to high grade dysplasia, and, ultimately, to carcinoma [4].
A carcinogen, azoxymethane (AOM), and a proinflammatory agent, dextran sodium sulfate (DSS), are used to produce an inflammation-associated in vivo CRC model [5]. DSS triggers inflammation by inducing DNA damage, which is associated with the development of adenomas [6]. Because various proinflammatory mediators and cytokines are involved in the pathogenesis of CRC [5,7,8], proinflammatory cytokine suppression represents an attractive strategy for treating inflammation-associated CRC.
Gut microbiota play important roles in the digestion of otherwise inaccessible nutrients, gut epithelium repair, immune system development, fat storage, and nervous system changes [9,10]. The human gastrointestinal tract has about 10–100 trillion microorganisms [9], the composition of which is affected by diet. Perturbation of the normal gut microbial balance, a condition known as dysbiosis, can disrupt the intestinal barrier function, including its associated immune function, which can lead to auto-immunity and thus chronic inflammation [11]. Indeed, dysfunctional interactions between gut microbiota and the mucosal immune system are risk factors for IBD, CRC, and other inflammatory diseases [12].
Pyrosequencing techniques have made it possible to amplify bacterial genes and examine microbiome richness and diversity. In particular, the 16S rRNA gene has regions that are highly conserved and amenable to polymerase chain reaction (PCR) [13]. Pyrosequencing data have been used to elucidate hostmicrobiome interaction, including the conversion of healthful bacteria into disease-driving ones. Various therapies have been used to treat dysbiosis, including probiotics, prebiotics, fecal microbiota transplantation, and dietary interventions. In particular, the effects of polyphenols and water soluble vitamins on modulation of gut microbiota and their host interactions have been studied [14,15].
Vitamin C is a water soluble vitamins that is well known for its anti-oxidant activity. It is an essential nutrient for human being, due to the loss of functional gulonolactone oxidase, which biosynthesizes vitamin C from glucose [16]. Vitamin C has been reported to be protective against numerous diseases, including diabetes, cancer, vascular diseases, and eye diseases, and among others [17]. Notably, substantial epidemiologic evidence suggests that vitamin C-rich foods protect against the development of several cancers [18]. Low plasma levels of vitamin C have been associated with morbidity and mortality risk [19]. The anti-inflammatory function of vitamin C has been shown to be helpful in the treatment of in Helicobacter pylori infection, post-cardioversion inflammation, and acute inflammation of the cornea [20,21,22]. Recently, it was reported that vitamin C injection reduced oxidative stress and inflammation by regulating proinflammatory cytokines in DSS-induced acute colitis model mice [23].
Several studies have reported the effect of antioxidants on intestinal dysbiosis. Dietary polyphenols, major antioxidants interplayed with gut microbiota and exerted prebiotic-like activities on microbiota. In particular, (−)-Epigallocatechin-3-gallate (EGCG) suppressed DNA damage and lowered Firmucutes/Bacteroidetes ratio which was increased by high fat diet feeding [24]. An antioxidant blend, including, vitamin C, vitamin E, tea polyphenols, lipoic acid, and microbial antioxidants fermented by bacillus, etc restored the microbiota such as Lactobacillus and Biofidobacterim counts in early weaned piglets [25]. However, the potential effects of vitamin C on colitis-associated early-stage colon cancer and associated microbiota have not been clarified. Therefore, the aim of the present study was to investigate the ability of vitamin C to prevent colitis-associated colon cancer through regulation of inflammation and intestinal dysbiosis.
MATERIALS AND METHODS
Animals and diet
Five-week-old male BALB/c mice (Central Lab. Animal Inc., Seoul, Korea) were acclimated for a week and randomized into four groups: 1) Control (n = 9); 2) AOM/DSS-induced colon cancer (CC; n = 12); 3) AOM/DSS + 60 mg/kg body weight (b.w.) of vitamin C (V60; n = 11), 4) AOM/DSS + 120 mg/kg b.w. of vitamin C (V120; n = 11). All animals were fed an AIN-93G purified rodent pellet diet (Unifaith Inc., Seoul, Korea) ad libitum. Animals were housed individually under standard laboratory conditions (22 ± 2℃, 50 ± 5% humidity, and a 12 h/12 h light/dark cycle). Body weight and food intake were monitored twice a week throughout the experiment. At the end of the experiment, all mice were sacrificed and their whole colons were collected. Colon length was measured with an electronic digital caliper and colon tumors were counted. Colon tissues were stored at −80℃. This study protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Ewha Womans University (No. 16-001).
AOM/DSS-induced colitis-associated early colon cancer model
AOM/DSS-induced colon cancer is well established model that is commonly used in experimental colitis and colitis-associated CRC studies [5,26]. To induce inflammation and colon cancer, all mice in the CC, V60, and V120 groups were injected intraperitoneally with 10 mg/kg b.w. of AOM (Sigma-Aldrich, Dallas, TX). Two 7-d cycles of DSS (36–50 KDa, MP Biomedicals, Costa Mesa, CA; 2% in drinking water) were administered with 2 weeks of normal drinking water given in between the cycles. Vitamin C powder was obtained from Kwang-Dong Pharmaceutical Co., Ltd., (Seoul, Korea) and prepared fresh in distilled water on the day when it was given to mice. For mice in the V60 and V120 groups, vitamin C solution was administered daily by gavage for a total of 5 wks, starting 2 d after the AOM injection and continuing until the final day of experiment.
Disease activity index (DAI) analysis
DAI scoring of colitis signs (weight loss, fecal bleeding, and stool consistency) was conducted as described previously [27] with the scoring criteria described by Okayasu et al. [28]. DAI scores were determined at the beginning of the first DSS administration cycle and every other day thereafter until the end of the experiments. Each bi-daily DAI score was the average of scores for each of the aforementioned three signs.
DAI = (Stool consistency + Fecal bleeding + Weight loss) / 3
Histopathology
The distal colon samples (to 1 cm above the anus) were fixed in 10% neutral-buffered formalin. After 24 h, samples were embedded in paraffin for tissue sectioning. Sections (4 µm) were stained with hematoxylin and eosin (H&E) in accordance with the standard histology procedures [29].
Real-time PCR analysis
Total RNA from the middle and distal regions of the colon were extracted with TRIzol reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized by reverse transcription of 1 µg of each RNA sample with a RevertAid First Strand cDNA synthesis kit (Fermentas, Vilnius, Lithuania). Real-time quantitative PCR was performed with Power SYBR Green PCR Master Mix (Qiagen, Hilden, Germany) and target gene-specific primers. cDNA samples were analyzed by a Rotor-Gene real-time analyzer (Qiagen, Austin, TX). The PCR protocol was as follows: initiation at 95℃ for 5 min, denaturation at 95℃ for 15 s, annealing at 60℃ for 30 s, and extension at 72℃ for 10 s. The primers sequence were as follows : 5′-TGT GCA ATG GCA ATT CTG AT-3′ (forward), 5′-GGT ACT CCA GAA GAC CAG AGG A-3′ (reverse) for Interleukin (IL)-6 (IL-6); 5′-ATG GCA ACT GTT CCT GAA CTC AAC T-3′ (forward), 5′-CAG GAC AGG TAT AGA TTC TTT CCT TT-3′ (reverse) for IL-1β; 5′-TAC GCC ACA GAG AAG AAG A-3′ (forward), 5′-TGG CCT CCA GTA ACC AAT TG-3′ (reverse) for tumor necrosis factor-alpha (TNF-α); 5′-CAA CTT CAA GGG AGT CTG GA-3′ (forward), 5′-AGT CAT CTG CTA CGG GAG GA-3′ (reverse) for cyclooxygenase-2 (COX-2); 5′-ACT TCC ACT CCC TGC CCT AT-3′ (forward), 5′-GTT GCA AGC TGT CTC CTT CC-3′ (reverse) for microsomal prostaglandin E synthase-2 (mPGES-2); 5′-GAG AGC TTG GCA ATG GGA AC-3′ (forward), 5′-AGG TAC CTC AGA GCA AAC GT-3′ (reverse) for proliferating cell nuclear antigen (PCNA); 5′-TGT GAG GCA GAT GCT CAG TG-3′ (forward), 5′-AAC TTT GGC ATT GTG GAA GG-3′ (reverse) for glyceraldehyde 3-phosphate dehydrogenase (GAPDH); 5′-GAT CTG GCA CCA CAC CTT CT-3′ (forward), 5′-GGG GTG TTG AAG GTC TCA AA-3′ (reverse) for β-actin. mRNA levels were normalized to GAPDH and β-actin. The data are reported as Ct values and relative mRNA levels were quantified based on the 2−ΔΔCT model.
Genomic DNA extraction
Fecal samples were collected from each mouse 1 d before the mice were sacrificed, frozen immediately in nitrogen liquid, and then stored at −80℃. DNA was extracted from feces with a Fast DNA SPIN Kit (MP BIO, Santa Ana, CA) according to the manufacturer's instructions. Genomic DNA samples were dissolved in elution buffer (minimum volume, 50 µL) and optimal density at 260 nm was determined and DNA purity was confirmed based on the absorbance ratio at 260 nm and 280 nm.
Pyrosequencing and sequence analysis
The V3-V4 variable regions of 16S rRNA were targeted and amplified by PCR with barcode primers (27F and 518R). The following microbiome analysis steps were followed: barcode sorting, quality prescreening, trimming primer sequences, removing non-target sequences, assemble sequence for de-noise, taxonomic assignment, and chimera checking. The mechanisms were processed in Illumina Miseq software (ChunLab, Inc., Seoul, Korea). Operational taxonomic units (OTUs) were determined by XOR analysis in the CL community program (Chunlab Inc, Seoul, Korea). Microbiota were classified toxonomically based on the ExTaxon-e database with a 97% sequence similarity cutoff. The number of OTUs present in each sample was used to define species richness and diversity. In addition, the Chao 1 and Shannon estimator were used to calculate microbiota richness and diversity in Mothur and a distance matrix was created in Fast UniFrac (Chunlab Inc.: Seoul, Korea).
Statistical analysis
Data for each group are presented as mean with standard errors (SEM). Multiple comparisons between the groups were conducted by one-way analyses of variance (ANOVAs), followed by Newman-Keuls' post hoc tests. P values less than 0.05 were considered significant. All statistical analyses were performed in GraphPad PRISM (Graphpad Software, San Diego, CA).
RESULTS
Effects of vitamin C supplementation on DAI score, colon length, and the mucosal damage
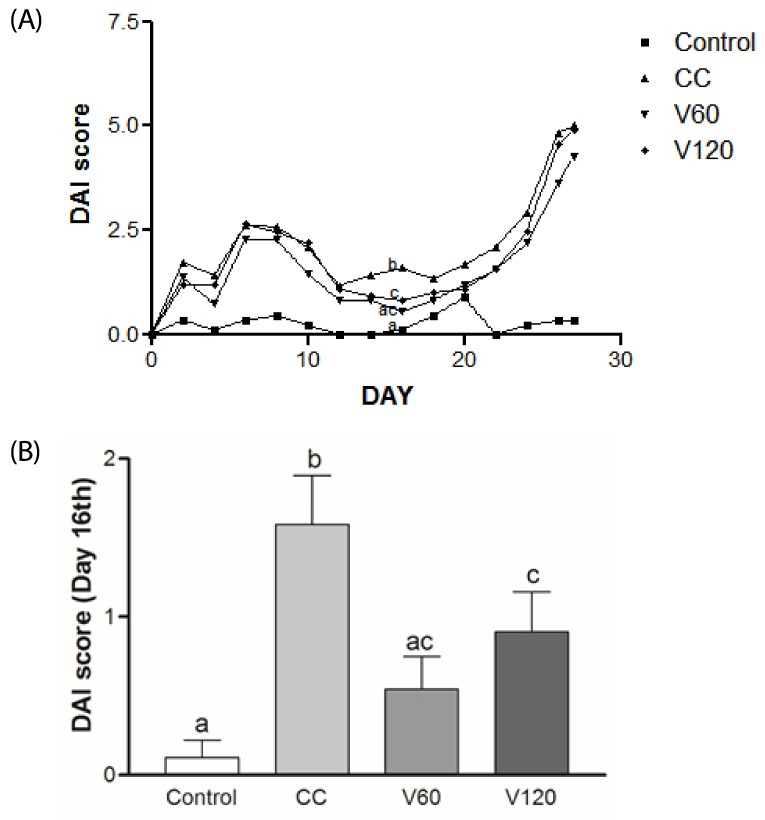
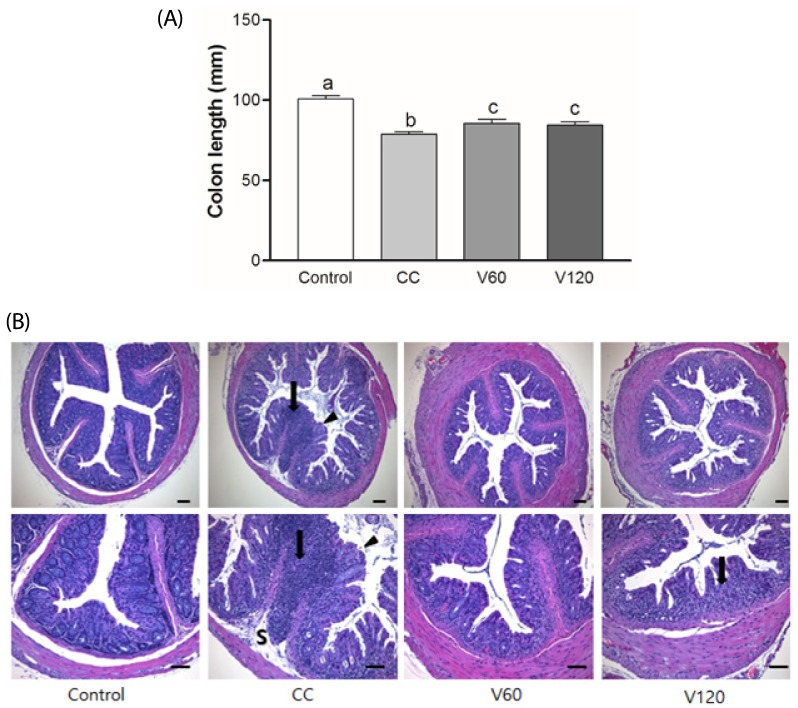
DAI score increased day by day during the first week of 2% DSS treatment and then decreased during the 2 wk inter-DSS cycle rest interval. During the second 2% DSS cycle, all of the mice exhibited signs of morbidity, including weight loss, diarrhea, and blood in the stool, resulting in higher DAI scores relative to the scores obtained from the first treatment (Fig. 1A). In particular, on day 16, during the inter-DSS cycle rest interval, the mean DAI scores for the V60 and V120 group were markedly lower than those of the CC group by 66% (P < 0.01) and 43% (P < 0.05) (Fig. 1B), indicating that vitamin C supplementation had reduced inflammation. On day 34th, the mean DAI scores for the V60 group tended to be decreased compared with the CC group, however, it was not statically significant. Notably, suppression of colitis by vitamin C occurred after cessation of DSS administration, suggesting that vitamin C supplementation may alleviate mild inflammation and promote recovery from the acute phase of colitis. Colon shortening has been documented in severe DSS-induced colitis and is considered to be indicative of inflammation severity [30]. As shown in Fig. 2A, average colon length was reduced in the CC group (7.9 ± 0.5 cm) relative to that in the Control group (10 ± 0.6 cm; P < 0.001), whereas average colon lengths observed for the V60 (8.6 ± 0.7 cm) and V120 (8.5 ± 0.7 cm) groups were longer than that of the CC group (both P < 0.05).
Fig. 1. Effects of vitamin C supplementation on DAI score.
DAI scores reflect weight loss, stool consistency, and fecal bleeding. (A) Bi-daily group DAI scores. (B) Analysis of day 16th, DAI scores among the Control, CC, V60, and V120 groups. Mean ± SEM are shown (Control, n = 9; CC, n = 12; V60 and V120, n = 11 per group). One-way ANOVAs and Newman-Keuls' post hoc tests were performed (P<0.05). CC, AOM/DSS-induced colon cancer; V60, AOM/DSS + 60 mg/kg body weight (b.w.) of vitamin C; V120, AOM/DSS + 120 mg/kg b.w. of vitamin C.
Fig. 2. Effects of vitamin C supplementation on colon length and histology in colitis-associated colon cancer.
(A) Colon length was measured and compared among four groups. Mean ± SEM are shown (Control, n = 9; CC, n = 12; V60 and V120, n = 11 per group). One-way ANOVAs and Newman-Keuls' post hoc tests were performed (P<0.05). (B) Representative histologic sections of distal colon from among four groups. Glandular epithelium destruction (arrow head), neutrophil infiltration (arrow) or submucosa extension (S) were shown. HE stain, bar = 100 µm. Magnification, upper 100x, lower 200x CC, AOM/DSS-induced colon cancer; V60, AOM/DSS + 60 mg/kg body weight (b.w.) of vitamin C; V120, AOM/DSS + 120 mg/kg b.w. of vitamin C.
In the present study, the Control group showed well-defined crypts, no neutrophil infiltration and inflammation in the submucosa. Colon tissues from DSS treated group showed severe epithelium destruction, neutrophil infiltration, and submucosal swelling (Fig. 2B). Suppression of inflammatory responses should protect the intestinal mucosa from these damaging effects. In contrast, vitamin C supplements attenuated the DSS-induced damages in colon compared to the CC group, although there was mild neutrophil infiltrations in the V120 group. Mucosal epithelial cells were less damaged and the length of colons were longer in vitamin C-supplemented group, compared to DSS-treated group.
Effect of vitamin C supplementation on proinflammatory mediators and cytokines in middle and distal colon
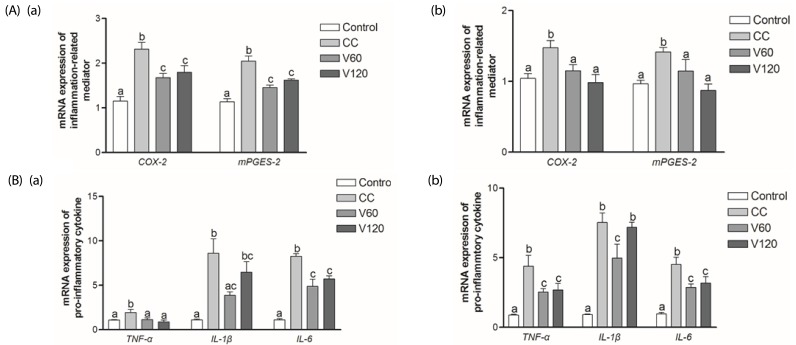
To prove the anti-inflammatory effects of vitamin C, we examined the expression of several inflammatory mediators in middle and distal portions (Fig. 3A). We found that COX-2 mRNA expressions were up-regulated significantly in the CC group, compared to the Control group, in both the middle and distal colon. Conversely, mRNA expression of COX-2 was 28% lower (P < 0.01) and 27% lower (P < 0.01) in the V60 and V120 groups, respectively, than in the CC group. The mRNA expression of mPGES-2, which encodes a downstream regulator of COX-2, in the middle colon was decreased by 29% (P < 0.001) and 21% (P < 0.001) in the V60 and the V120 groups compared with the expression levels observed for the CC group (Fig. 3Aa). In distal colon, COX-2 mRNA levels were decreased by 22% (P < 0.05) and 34% (P < 0.01) and mPGES-2 levels were decreased by 19% (P < 0.05) and 38% (P < 0.001), respectively, in the V60 and the V120 groups compared to levels observed for the CC group (Fig. 3Ab).
Fig. 3. Effect of vitamin C supplementation on proinflammatory mediators and cytokines in middle and distal colon.
(A) mRNA expressions of COX-2 and mPGES-2 (B) TNF-α, IL-1β, and IL-6 in middle colon (a) and in the distal colon (b) were analyzed using real-time PCR. β-actin and GAPDH were used as the loading control. Mean ± SEM are shown (Control, n = 9; CC, n = 12; V60 and V120, n = 11 per group). One-way ANOVAs and Newman-Keuls' post hoc tests were performed (P<0.05). CC, AOM/DSS-induced colon cancer; V60, AOM/DSS + 60 mg/kg body weight (b.w.) of vitamin C; V120, AOM/DSS + 120 mg/kg b.w. of vitamin C.
The mRNA expression levels of all three of these cytokines were up-regulated in both the middle and distal colon of the CC group, relative to the Control group (Fig. 3B). Relative to the CC group levels, mRNA expression of TNF-α was decreased by 42% (P < 0.01) and 54% (P < 0.01) in the V60 and the V120 groups in the middle colon and decreased by 42% (P < 0.05) and 39% (P < 0.01) in the V60 and the V120 groups, respectively, in the distal colon. Relative to the CC group levels, mRNA expression of interleukin (IL)-1β in the V60 group was down-regulated by 55% (P < 0.01) and 34% (P < 0.05) in the middle and the distal colon, respectively. Expressions of IL-1β mRNA in the V120 group was tended to be decreased in the V120 in both tissues, but they were not statistically significant. Relative to the CC group levels, IL-6 mRNA levels were down-regulated by 41% (P < 0.001) and 31% (P < 0.001) in the V60 and the V120 groups, respectively, in the middle colon by 37% (P < 0.01) and 29% (P < 0.05) in the V60 and the V120 groups, respectively, in the distal colon.
Effect of vitamin C supplementation on tumorigenesis and proliferation
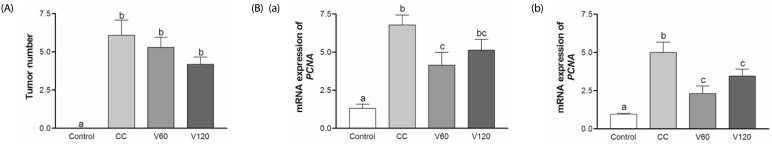
In the present study, the total number of tumors tended to be decreased by vitamin C supplementation, however not significantly (Fig. 4A). PCNA is a major marker of tumor growth. In this present study, mRNA levels of PCNA in the CC group were five-fold in the Control group in both the middle and distal colon. Vitamin C supplementation decreased the mRNA expression of PCNA by 39% (V60 group; P < 0.05) in the middle colon and by 54% (V60 group; P < 0.001) and 31% (V120 group; P < 0.05), respectively, in the distal colon (Fig. 4B).
Fig. 4. The effects of vitamin C on colon tumor number and mRNA expression of PCNA.
(A) Number of colon tumor were counted with naked eye when sacrificing the mice. (B) mRNA expressions of PCNA was analyzed in the middle colon (a) and in the distal colon (b) using real-time PCR. β-actin and GAPDH were used as the loading control. Mean ± SEM are shown (Control, n = 9; CC, n = 12; V60 and V120, n = 11 per group). One-way ANOVAs and Newman-Keuls' post hoc tests were performed (P<0.05). CC, AOM/DSS-induced colon cancer; V60, AOM/DSS + 60 mg/kg body weight (b.w.) of vitamin C; V120, AOM/DSS + 120 mg/kg b.w. of vitamin C.
Effect of vitamin C on microbial dysbiosis in colitis-associated colon cancer model
In the present study, although some microbial differences were observed between the groups, microbiota richness and alpha diversity as quantified by Goods Coverage, Chao 1, and Shannon estimators, did not reveal any consistent effect vitamin C supplementation (data not shown).
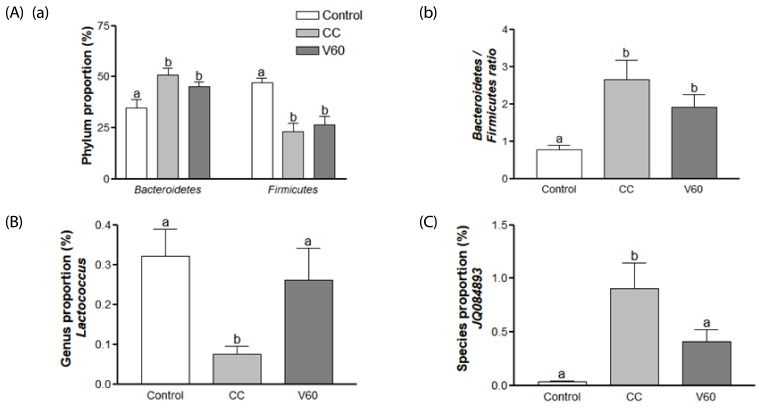
We found that proportion of Bacteroidetes was increased by about 1.5 fold while that of Firmicutes was decreased by 51% in CC group compared with the Control group (Fig. 5Aa). We did not find significant effects of vitamin C on gut microbiom composition at the phylum level (data not shown). However, it is worth noting that the V60 group tended to have decreased Bacteroidetes representation, which brought their profile closer to the Control group and further from the CC group (Fig. 5Aa). The Bacteroidetes to Firmicutes ratio was increased by about 3.5 fold (P < 0.01) in the CC group compared with the Control group and this effect was decreased partially, albeit not significantly, by vitamin C supplementation (Fig. 5Ab).
Fig. 5. Effects of vitamin C on gut microbiome taxonomy.
(A) Composition of gut microbacteria at the phylum level. (a) Changes in the proportion of Bacteroidetes and Firmicutes phyla. (b) Relative abundance of Bacteridetes/Firmicutes across the Control, CC, and V60 groups. (B) Relative abundance of genus Lactoccocus. (C) Relative abundance of JQ084893 species. Mean ± SEM are shown (n = 6 per group). One-way ANOVAs and Newman-Keuls' post hoc tests were used (P<0.05). CC, AOM/DSS-induced colon cancer; V60, AOM/DSS + 60 mg/kg body weight (b.w.) of vitamin C.
At the genus level, Lactococcus (order Lactobacillales) was decreased in the CC group by 75% (P < 0.05) and vitamin C supplementation reversed this deficit to the Control group level (Fig. 5B). At the species level, JQ084893 (genus Alistipes), were dramatically increased (P < 0.01) in the CC group and decreased 54% (P < 0.05) in the V60 group compared to the CC group (Fig. 5C).
DISCUSSION
The present study investigated the effects of vitamin C supplementation on inflammation at early colon cancer and microbiota changes in AOM/DSS mice model. Vitamin C supplementation prevented inflammation in resting period and recovered colon tissue from the subchronic inflammation in early stage of colon cancer. Expressions of inflammation related mediators and pro-inflammatory cytokines were also decreased in vitamin C supplementation groups. Tumor numbers were tended to be decreased and proliferation marker, PCNA was reduced by vitamin C supplementation. In microbiota analysis, significant changes of Lactococcus and JQ084893 levels were shown in microbial composition.
In the present study, vitamin C supplementation decreased DAI score in the resting period, especially in day of 16th.. This result indicates that the vitamin C is effective on antiinflammation in the mild inflammation. These results are consistent with those of previous study showing that a notoginseng (Panax notoginseng) treatment also suppressed colitis during a rest interval of DSS treatment [31]. In addition, prior studies has shown that vitamin C treatment could reduce histological inflammation and inflammatory cell presence in the mucosa of H.pylori-infected mice [20], alleviate mild post-cardioversion inflammation in patients [21], and attenuate oxygen-radical tissue damage and acute inflammation of the cornea in rabbits (topically administered) [22]. It was recently reported that daily intraperitoneal injection of vitamin C (100 mg/kg b.w) for 10 d suppressed oxidative stress and inflammation in DSS-induced colitis model mice [23], demonstrating similar effects were observed here in the similar model but with the vitamin C treatment being delivered via a different administration route.
Traditionally, the large intestine is divided into three parts: the portion proximal to the cecum, the middle portion, and a final distal region that culminates with the rectum. Tumors are most commonly located in middle and distal portions where the most severe DSS-induced colitis changes occur [28]. In the present studies, the COX-2 and mPGES-2 mRNA expression levels were down-regulated by vitamin C supplementation in both middle and distal part of colon. Level of inflammation-related enzymes and cytokine expressions are major indicators in colitis-associated colon cancer. COX-2 is a pro-inflammatory enzyme and the mRNA and protein levels of COX-2 are expected to be increased in the colon mucosa of mice with DSS-induced colitis [27,32]. Strong protein expression of COX-2 has also been documented in colon adenocarcinoma and adenoma cells [33]. During inflammation, COX-2 undergoes enzymatic conversion, producing prostanoids, including the pro-inflammatory agent prostaglandin E2 (PGE2) [34]. High levels of PGE2 exacerbate inflammatory responses and promote IBD and colorectal tumor growth [35,36]. COX-2 and PGE2 are regulated by various isoforms of PGE synthase, including mPGES-1, mPGES-2, and cytosolic PGE synthase (cPGES) [37]. Murakami et al. [38] described elevated mPGES-2 expression in adenocarcinoma and LPS-treated colon tissues.
Abnormal secretion of pro-inflammatory cytokines is a major cause of chronic inflammation pathologies, including acute lung injury and IBD, and may be a cofactor for carcinogenesis [7,39]. The pro-inflammatory cytokines, TNF-α, IL-1β, and IL-6 play key roles in inflammatory processes and these cytokines were elevated in patients with UC, CD, and CRC [8]. Vitamin C (20 mM) has been shown to attenuate LPS-induced TNF-α and IL-6 production by monocytes [40]. In this study, the expression of these cytokines was reduced by vitamin C supplementation in both the middle and distal part of the colon. The present findings of reduced COX-2, mPGES-2, TNF-α, IL-1β, and IL-6 mRNA levels in the middle and distal colon of vitamin C supplemented colitis groups suggest that vitamin C may protect colon tissues from inflammation in early colon cancer by regulating these proinflammatory mediators and cytokines.
PCNA overexpressions correlated with tumor stage and has been reported to be a predictor in CRC [41]. IL-6 knock-out mice have decreased mRNA expression of PCNA correlates with DSS-induced inflammation for mucosal repair by proliferating epithelial cells [32]. According to previous studies, adenocarcinomas start to develop after 4 wks of AOM/DSS treatment and become fully developed by 14–20 wks [5]. In this model, AOM is a tumor initiator and DSS is a tumor promoter [42]. The level of inflammation in the AOM/DSS model has been correlated with progression of CRC [43]. Cancer cell proliferation is a major factor of tumor growth and high proliferation in epithelial induced tumors in distal colon [44,45]. It has reported that long-term supplement of vitamin C decreased polyp area in colon cancer than the placebo group and vitamin C supplementation inhibited crypt cell proliferation in adenomatous polyps patients [46]. Jacobs et al. [47] reported that 10 or more years of long-term intake of vitamin C was associated with reduced risk of CRC mortality at any age. In another study, 750 mg/d vitamin C supplementation suppressed cell proliferation in patients with adenomatous polyps, suggesting that prolonged vitamin C supplementation may be prophylactic against the recurrence of adenomatous polyps [48]. In the present study, vitamin C reduced cell proliferation in colitis-associated colon carcinogenesis. Even if the reduced tumor numbers were not significant in the present study, the PCNA result showed its anti-cancer potential in early CRC. These findings suggest that vitamin C can ameliorate the tumor development in an early stage by reducing proliferation and inflammation.
The present mouse dosages of 60 mg/kg and 120 mg/kg translate to about 300 mg/d and 600 mg/d in a 60 kg human [49], and thus are physiological doses of vitamin C that exceed recommended daily intake (100 mg/d) by less than an order of magnitude. Researchers have suggested that current recommendations are insufficient to prevent disease and have proposed to increase as much as 200 mg/d accounting for its bioavailability [50] and a daily intake of 120 mg of vitamin C has been proposed as an optimum dose for reducing chronic diseases [51]. Vitamin C levels in organs and in serum are reduced in both mice and humans when they have an infection [19,52,53]. Mice have the ability to biosynthesize vitamin C, whereas humans do not [16]. However, humans maintain comparable vitamin C levels by consuming vitamin C in food. It is important to note that supplementation with physiological doses of vitamin C in the present study can have modest anti-inflammatory benefits on colitis-associated colon cancer, at least in an experimental murine model. Further clinical and knock-out mouse studies are needed to confirm these results, including examination of long-term consequences.
Intestinal dysbiosis, a condition where a microbial ecosystem is altered [54] and is associated with the pathogenesis of inflammation-related diseases, including IBD and CRC, both of which are characterized by a loss of microbiome diversity [55,56]. Gut microbiota profiles differ between diseased and healthy people [57]. In previous studies, the diversity of overall human gut microbiome was decreased in both IBD and CRC [56,58]. Disruptive alteration of gut microbiome composition was associated with the CRC development in AOM/DSS mouse model and decreased OTUs in AOM/DSS treatment group than normal group [59]. Consistent with previous study, the Control group showed higher diversity than the CC group in the present study (data not shown). Although vitamin C supplementation did not affect OTUs and diversity, some bacteria in phylum, genus and species level showed difference compared with the CC group.
In phylum level of microbiota, the ratio of Bacteroidetes to Firmicutes was increased in the CC group compared to the Control group. Vitamin C supplementation tended to decrease Bacteroidetes and tended to be decreased the Bacteroidetes to Firmicutes ratio. Dramatic shifts in Bacteroidetes (increased) and Firmicutes (decreased) representation have been previously reported in IBD patients and DSS-induced colon mouse models [60,61]. Firmicutes, which are gram-positive bacteria, produce butyrate, a short-chain fatty acid (SCFA) that provides as an energy source for colon epithelial cells, supports restoration of the mucosal barrier function in gastrointestinal tract, and reduces CRC risk [62,63].
At the genus level, Lactococcus were recovered to control level by vitamin C supplementation. Lactococcus, which are used as probiotics, also produce SCFAs and support the integrity of the intestinal barrier, promote immune tolerance, and reduce the risk of gastrointestinal infection and IBD [64]. Orally administered Lactococcus (lactis strain) has been reported to prevent colon cancer in BALB/c mice by increasing antioxidant activity and reducing reactive oxygen species levels [65]. Hence, vitamin C supplementation may benefit colon health by increasing Lacotccous.
JQ084893 (genus Alistipes) were increased in the CC group and decreased by vitamin C supplementation at the species level. These bacteria, which are bile-tolerant, tended to be present at relatively low levels with animal-based diet [66] and in IBD, and tend to be present at high level in the healthy gut [67]. In contrast, in the present study, Alistipes were elevated in the CC group compared to the control group, and vitamin C supplementation inhibited this increase. Gut microbiota composition can vary across different populations, ages, and disease states, including IBD and CD, as well as with antibiotics use [68]. This discrepancy needs to be investigated in future studies.
In conclusion, vitamin C supplementation may alleviate acute inflammation in recovery phase and ameliorated subchronic inflammation in early colon cancer by regulating inflammatory mediators and cytokines as well as by altering the representation of inflammation-associated intestinal bacteria. Physiological doses (slightly higher than currently recommended daily levels) may give moderate protection against the progression of mild colonic inflammation and early colon cancer development.
Footnotes
This study was supported by Kwang-Dong Pharmaceutical Ltd. and the Brain Korea 21 Plus (project No: 22A20130012143).
CONFLICT OF INTEREST: The authors declare no conflicts of interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Boyle P, Langman JS. ABC of colorectal cancer: Epidemiology. BMJ. 2000;321:805–808. doi: 10.1136/bmj.321.7264.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–862. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–G17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka T. Development of an inflammation-associated colorectal cancer model and its application for research on carcinogenesis and chemoprevention. Int J Inflam. 2012;2012:658786. doi: 10.1155/2012/658786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meira LB, Bugni JM, Green SL, Lee CW, Pang B, Borenshtein D, Rickman BH, Rogers AB, Moroski-Erkul CA, McFaline JL, Schauer DB, Dedon PC, Fox JG, Samson LD. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest. 2008;118:2516–2525. doi: 10.1172/JCI35073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–1767. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szkaradkiewicz A, Marciniak R, Chudzicka-Strugala I, Wasilewska A, Drews M, Majewski P, Karpiński T, Zwoździak B. Proinflammatory cytokines and IL-10 in inflammatory bowel disease and colorectal cancer patients. Arch Immunol Ther Exp (Warsz) 2009;57:291–294. doi: 10.1007/s00005-009-0031-z. [DOI] [PubMed] [Google Scholar]
- 9.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laukens D, Brinkman BM, Raes J, De Vos M, Vandenabeele P. Heterogeneity of the gut microbiome in mice: guidelines for optimizing experimental design. FEMS Microbiol Rev. 2016;40:117–132. doi: 10.1093/femsre/fuv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamada N, Seo SU, Chen GY, Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 12.Holmes E, Li JV, Athanasiou T, Ashrafian H, Nicholson JK. Understanding the role of gut microbiome-host metabolic signal disruption in health and disease. Trends Microbiol. 2011;19:349–359. doi: 10.1016/j.tim.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Mai V, Morris JG., Jr Colonic bacterial flora: changing understandings in the molecular age. J Nutr. 2004;134:459–464. doi: 10.1093/jn/134.2.459. [DOI] [PubMed] [Google Scholar]
- 14.Dueñas M, Muñoz-González I, Cueva C, Jiménez-Girón A, Sánchez-Patán F, Santos-Buelga C, Moreno-Arribas MV, Bartolomé B. A survey of modulation of gut microbiota by dietary polyphenols. Biomed Res Int. 2015;2015:850902. doi: 10.1155/2015/850902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biesalski HK. Nutrition meets the microbiome: micronutrients and the microbiota. Ann N Y Acad Sci. 2016;1372:53–64. doi: 10.1111/nyas.13145. [DOI] [PubMed] [Google Scholar]
- 16.Nishikimi M, Yagi K. Molecular basis for the deficiency in humans of gulonolactone oxidase, a key enzyme for ascorbic acid biosynthesis. Am J Clin Nutr. 1991;54:1203S–1208S. doi: 10.1093/ajcn/54.6.1203s. [DOI] [PubMed] [Google Scholar]
- 17.Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care. 2002;5:66–74. doi: 10.1046/j.1523-5408.2002.00005.x. [DOI] [PubMed] [Google Scholar]
- 18.Block G. Vitamin C and cancer prevention: the epidemiologic evidence. Am J Clin Nutr. 1991;53:270S–282S. doi: 10.1093/ajcn/53.1.270S. [DOI] [PubMed] [Google Scholar]
- 19.Deicher R, Ziai F, Bieglmayer C, Schillinger M, Horl WH. Low total vitamin C plasma level is a risk factor for cardiovascular morbidity and mortality in hemodialysis patients. J Am Soc Nephrol. 2005;16:1811–1818. doi: 10.1681/ASN.2004100850. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Willen R, Wadstrom T. Astaxanthin-rich algal meal and vitamin C inhibit Helicobacter pylori infection in BALB/cA mice. Antimicrob Agents Chemother. 2000;44:2452–2457. doi: 10.1128/aac.44.9.2452-2457.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korantzopoulos P, Kolettis TM, Kountouris E, Dimitroula V, Karanikis P, Pappa E, Siogas K, Goudevenos JA. Oral vitamin C administration reduces early recurrence rates after electrical cardioversion of persistent atrial fibrillation and attenuates associated inflammation. Int J Cardiol. 2005;102:321–326. doi: 10.1016/j.ijcard.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 22.Kasetsuwan N, Wu FM, Hsieh F, Sanchez D, McDonnell PJ. Effect of topical ascorbic acid on free radical tissue damage and inflammatory cell influx in the cornea after excimer laser corneal surgery. Arch Ophthalmol. 1999;117:649–652. doi: 10.1001/archopht.117.5.649. [DOI] [PubMed] [Google Scholar]
- 23.Yan H, Wang H, Zhang X, Li X, Yu J. Ascorbic acid ameliorates oxidative stress and inflammation in dextran sulfate sodium-induced ulcerative colitis in mice. Int J Clin Exp Med. 2015;8:20245–20253. [PMC free article] [PubMed] [Google Scholar]
- 24.Remely M, Ferk F, Sterneder S, Setayesh T, Roth S, Kepcija T, Noorizadeh R, Rebhan I, Greunz M, Beckmann J, Wagner KH, Knasmüller S, Haslberger AG. EGCG prevents high fat diet-induced changes in gut microbiota, decreases of DNA strand breaks, and changes in expression and DNA methylation of Dnmt1 and MLH1 in C57BL/6J male mice. Oxid Med Cell Longev. 2017;2017:3079148. doi: 10.1155/2017/3079148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu J, Xu C, Chen X, Cai X, Yang S, Sheng Y, Wang T. Regulation of an antioxidant blend on intestinal redox status and major microbiota in early weaned piglets. Nutrition. 2014;30:584–589. doi: 10.1016/j.nut.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Yu C, Wen XD, Zhang Z, Zhang CF, Wu XH, Martin A, Du W, He TC, Wang CZ, Yuan CS. American ginseng attenuates azoxymethane/dextran sodium sulfate-induced colon carcinogenesis in mice. J Ginseng Res. 2015;39:14–21. doi: 10.1016/j.jgr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim KM, Kim YS, Lim JY, Min SJ, Shin JH, Ko HC, Kim SJ, Lim Y, Kim Y. Sasa quelpaertensis leaf extract suppresses dextran sulfate sodium-induced colitis in mice by inhibiting the proinflammatory mediators and mitogen-activated protein kinase phosphorylation. Nutr Res. 2014;34:894–905. doi: 10.1016/j.nutres.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 29.Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Araki A, Kanai T, Ishikura T, Makita S, Uraushihara K, Iiyama R, Totsuka T, Takeda K, Akira S, Watanabe M. MyD88-deficient mice develop severe intestinal inflammation in dextran sodium sulfate colitis. J Gastroenterol. 2005;40:16–23. doi: 10.1007/s00535-004-1492-9. [DOI] [PubMed] [Google Scholar]
- 31.Wen XD, Wang CZ, Yu C, Zhao L, Zhang Z, Matin A, Wang Y, Li P, Xiao SY, Du W, He TC, Yuan CS. Panax notoginseng attenuates experimental colitis in the azoxymethane/dextran sulfate sodium mouse model. Phytother Res. 2014;28:892–898. doi: 10.1002/ptr.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sánchez-Fidalgo S, Cárdeno A, Sánchez-Hidalgo M, Aparicio-Soto M, de la Lastra CA. Dietary extra virgin olive oil polyphenols supplementation modulates DSS-induced chronic colitis in mice. J Nutr Biochem. 2013;24:1401–1413. doi: 10.1016/j.jnutbio.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang D, Dubois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29:781–788. doi: 10.1038/onc.2009.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheibanie AF, Yen JH, Khayrullina T, Emig F, Zhang M, Tuma R, Ganea D. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23--〉IL-17 axis. J Immunol. 2007;178:8138–8147. doi: 10.4049/jimmunol.178.12.8138. [DOI] [PubMed] [Google Scholar]
- 36.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gudis K, Tatsuguchi A, Wada K, Futagami S, Nagata K, Hiratsuka T, Shinji Y, Miyake K, Tsukui T, Fukuda Y, Sakamoto C. Microsomal prostaglandin E synthase (mPGES)-1, mPGES-2 and cytosolic PGES expression in human gastritis and gastric ulcer tissue. Lab Invest. 2005;85:225–236. doi: 10.1038/labinvest.3700200. [DOI] [PubMed] [Google Scholar]
- 38.Murakami M, Nakashima K, Kamei D, Masuda S, Ishikawa Y, Ishii T, Ohmiya Y, Watanabe K, Kudo I. Cellular prostaglandin E2 production by membrane-bound prostaglandin E synthase-2 via both cyclooxygenases-1 and -2. J Biol Chem. 2003;278:37937–37947. doi: 10.1074/jbc.M305108200. [DOI] [PubMed] [Google Scholar]
- 39.Meduri GU, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107:1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 40.Härtel C, Strunk T, Bucsky P, Schultz C. Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine. 2004;27:101–106. doi: 10.1016/j.cyto.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Jung IK, Choi HJ, Kim SS, Hong SH. Prognostic significance of proliferating cell nuclear antigen (PCNA) expression in patients with colorectal carcinoma. J Korean Cancer Assoc. 1995;27:550–558. [Google Scholar]
- 42.Suzuki R, Kohno H, Sugie S, Tanaka T. Dose-dependent promoting effect of dextran sodium sulfate on mouse colon carcinogenesis initiated with azoxymethane. Histol Histopathol. 2005;20:483–492. doi: 10.14670/HH-20.483. [DOI] [PubMed] [Google Scholar]
- 43.De Robertis M, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L, Signori E, Fazio VM. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog. 2011;10:9. doi: 10.4103/1477-3163.78279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onodera H, Maetani S, Kawamoto K, Kan S, Kondo S, Imamura M. Pathologic significance of tumor progression in locally recurrent rectal cancer: different nature from primary cancer. Dis Colon Rectum. 2000;43:775–781. doi: 10.1007/BF02238013. [DOI] [PubMed] [Google Scholar]
- 45.Jackson PE, O'Connor PJ, Cooper DP, Margison GP, Povey AC. Associations between tissue-specific DNA alkylation, DNA repair and cell proliferation in the colon and colon tumour yield in mice treated with 1,2-dimethylhydrazine. Carcinogenesis. 2003;24:527–533. doi: 10.1093/carcin/24.3.527. [DOI] [PubMed] [Google Scholar]
- 46.Bussey HJ, DeCosse JJ, Deschner EE, Eyers AA, Lesser ML, Morson BC, Ritchie SM, Thomson JP, Wadsworth J. A randomized trial of ascorbic acid in polyposis coli. Cancer. 1982;50:1434–1439. doi: 10.1002/1097-0142(19821001)50:7<1434::aid-cncr2820500733>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 47.Jacobs EJ, Connell CJ, Patel AV, Chao A, Rodriguez C, Seymour J, McCullough ML, Calle EE, Thun MJ. Vitamin C and vitamin E supplement use and colorectal cancer mortality in a large American Cancer Society cohort. Cancer Epidemiol Biomarkers Prev. 2001;10:17–23. [PubMed] [Google Scholar]
- 48.Cahill RJ, O'Sullivan KR, Mathias PM, Beattie S, Hamilton H, O'Morain C. Effects of vitamin antioxidant supplementation on cell kinetics of patients with adenomatous polyps. Gut. 1993;34:963–967. doi: 10.1136/gut.34.7.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 50.Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A. 1996;93:3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr. 1999;69:1086–1107. doi: 10.1093/ajcn/69.6.1086. [DOI] [PubMed] [Google Scholar]
- 52.Jenab M, Riboli E, Ferrari P, Sabate J, Slimani N, Norat T, Friesen M, Tjønneland A, Olsen A, Overvad K, Boutron-Ruault MC, Clavel-Chapelon F, Touvier M, Boeing H, Schulz M, Linseisen J, Nagel G, Trichopoulou A, Naska A, Oikonomou E, Krogh V, Panico S, Masala G, Sacerdote C, Tumino R, Peeters PH, Numans ME, Bueno-de-Mesquita HB, Büchner FL, Lund E, Pera G, Sanchez CN, Sánchez MJ, Arriola L, Barricarte A, Quirós JR, Hallmans G, Stenling R, Berglund G, Bingham S, Khaw KT, Key T, Allen N, Carneiro F, Mahlke U, Del Giudice G, Palli D, Kaaks R, Gonzalez CA. Plasma and dietary vitamin C levels and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) Carcinogenesis. 2006;27:2250–2257. doi: 10.1093/carcin/bgl096. [DOI] [PubMed] [Google Scholar]
- 53.Strnadová E, Prokopic J. Changes in ascorbic acid content in various organs and serum of mice experimentally infected with Taenia crassiceps (Zeder, 1800) cysticerci. Folia Parasitol (Praha) 1985;32:185–188. [PubMed] [Google Scholar]
- 54.Mosca A, Leclerc M, Hugot JP. Gut microbiota diversity and human diseases: should we reintroduce key predators in our ecosystem? Front Microbiol. 2016;7:455. doi: 10.3389/fmicb.2016.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 56.Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Fölsch UR, Timmis KN, Schreiber S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685–693. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O'Sullivan O, Fitzgerald GF, Deane J, O'Connor M, Harnedy N, O'Connor K, O'Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP, O'Toole PW. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 58.Ahn J, Sinha R, Pei Z, Dominianni C, Wu J, Shi J, Goedert JJ, Hayes RB, Yang L. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013;105:1907–1911. doi: 10.1093/jnci/djt300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zackular JP, Baxter NT, Iverson KD, Sadler WD, Petrosino JF, Chen GY, Schloss PD. The gut microbiome modulates colon tumorigenesis. MBio. 2013;4:e00692–e00613. doi: 10.1128/mBio.00692-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berry D, Kuzyk O, Rauch I, Heider S, Schwab C, Hainzl E, Decker T, Müller M, Strobl B, Schleper C, Urich T, Wagner M, Kenner L, Loy A. Intestinal microbiota signatures associated with inflammation history in mice experiencing recurring colitis. Front Microbiol. 2015;6:1408. doi: 10.3389/fmicb.2015.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yeom Y, Kim BS, Kim SJ, Kim Y. Sasa quelpaertensis leaf extract regulates microbial dysbiosis by modulating the composition and diversity of the microbiota in dextran sulfate sodium-induced colitis mice. BMC Complement Altern Med. 2016;16:481. doi: 10.1186/s12906-016-1456-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen HM, Yu YN, Wang JL, Lin YW, Kong X, Yang CQ, Yang L, Liu ZJ, Yuan YZ, Liu F, Wu JX, Zhong L, Fang DC, Zou W, Fang JY. Decreased dietary fiber intake and structural alteration of gut microbiota in patients with advanced colorectal adenoma. Am J Clin Nutr. 2013;97:1044–1052. doi: 10.3945/ajcn.112.046607. [DOI] [PubMed] [Google Scholar]
- 63.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. 2013;6:39–51. doi: 10.1177/1756283X12459294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Moreno de LeBlanc A, LeBlanc JG, Perdigón G, Miyoshi A, Langella P, Azevedo V, Sesma F. Oral administration of a catalase-producing Lactococcus lactis can prevent a chemically induced colon cancer in mice. J Med Microbiol. 2008;57:100–105. doi: 10.1099/jmm.0.47403-0. [DOI] [PubMed] [Google Scholar]
- 66.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Willing BP, Dicksved J, Halfvarson J, Andersson AF, Lucio M, Zheng Z, Järnerot G, Tysk C, Jansson JK, Engstrand L. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology. 2010;139:1844–1854.e1. doi: 10.1053/j.gastro.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 68.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]