Abstract
BACKGROUND/OBJECTIVES
Obesity and alcohol drinking are associated with metabolic syndrome. However, few studies show the relationship between alcohol drinking and metabolic syndrome according to varying degrees of obesity. This study aimed to determine the association between alcohol drinking and metabolic syndrome in obese and non-obese Korean male adults.
SUBJECTS/METHODS
This cross-sectional study included 5,867 males aged ≥ 20 years who were examined at the Soonchunhyang University health promotion center during June 2008–December 2010. The subjects were divided into non-obese (body mass index [BMI] < 25 kg/m2) and obese (BMI ≥ 25 kg/m2) groups and further divided according to weekly alcohol consumption into nondrinking (0 drinks/week), moderate drinking (≤ 14 drinks/week), and heavy drinking (> 14 drinks/week) groups. The subjects were also categorized into binge drinking and non-binge drinking groups. To obtain odds ratios (ORs) for metabolic syndrome, binary logistic regression analysis was performed.
RESULTS
The overall metabolic syndrome prevalence was 27.3% (12.8%, non-obese group; 50.4%, obese group). After adjusting for age, physical activity, and smoking, in the non-obese group, the OR for heavy drinking with binge drinking (reference: nondrinking) was 1.56 (95% confidence interval [CI] = 1.12–2.18), with a significant increase in metabolic syndrome prevalence. In the obese group, the OR for heavy drinking with binge drinking was 1.42 (95% CI = 1.07–1.88), showing a significant increase in metabolic syndrome prevalence (P < 0.05).
CONCLUSIONS
In both non-obese and obese Korean males, heavy drinking with binge drinking was associated with increased risk of metabolic syndrome. Thus, both non-obese and obese males should restrict their alcohol intake and not indulge in binge drinking.
Keywords: Body mass index, alcohol drinking, metabolic syndrome
INTRODUCTION
Excessive alcohol consumption includes heavy drinking (≥ 15 drinks/week for men; ≥ 8 drinks/week for women), binge drinking (≥ 5 drinks on an occasion for men; ≥ 4 drinks on an occasion for women), and any drinking by pregnant women or people below 21 years old [1]. Excessive drinking increases the risk of death, injury, violence, and various diseases such as high blood pressure, heart disease, stroke, liver disease, mental health problems, and cancers of the breast, throat, esophagus, liver, and colon [1,2]. Particularly, the South Korean society has a prevalent drinking culture and a generous view toward drinking. According to the World Health Organization report, South Korea had an alcohol consumption per capita of 12.3 L in 2010, the highest among Asian countries [2]. This is twice as high as the average alcohol consumption of the world. Therefore, people from South Korea have a high probability of developing various health problems due to drinking.
Previous studies have reported an association between drinking and metabolic syndrome [3,4]. Metabolic syndrome is a cluster of conditions including abdominal obesity, elevated blood pressure, hyperglycemia, elevated serum triglycerides (TGs), and low serum high-density lipoprotein (HDL) cholesterol, and it is known to increase the risk of cardiovascular disease and type 2 diabetes [5,6]. The mechanism of metabolic syndrome is not completely understood, but abdominal obesity [7,8] and insulin resistance [9] are major factors. Other known factors include genetics [10], aging [11], and various lifestyle habits [12] such as smoking, physical inactivity and alcohol consumption. The prevalence of metabolic syndrome is high worldwide; according to the 2013–2016 Korea National Health Examination, the prevalence in Korea was very high at 25.0% (men, 28.4%; women, 21.1%) [13]. This has led to increasing interest in metabolic syndrome as a major health problem that requires focused attention.
Metabolic syndrome occurs in both obese and non-obese people. However, the effects of alcohol drinking on metabolic syndrome may differ between in the non-obese people and in the obese people. This is because blood alcohol concentration is inversely correlated with body weight [14], which translates to higher blood alcohol concentration among non-obese people compared with obese people when consuming the same amount of alcohol. Several studies have investigated the relationship between alcohol drinking and metabolic syndrome [3,4,15,16], but the results were based on drinking status without obesity categories. Thus, it is necessary to determine the effects of drinking on metabolic syndrome in obese and non-obese groups.
This study aimed to determine the association between metabolic syndrome and alcohol drinking in obese and non-obese Korean male adults. Only men were studied as the prevalence of heavy drinking in women (1.8%) was much lower than in men (12.4%).
SUBJECTS AND METHODS
Design, subjects, and study period
This study has a cross-sectional design. The sample was taken from 9,465 patients aged ≥ 20 years who visited the health promotion center of Soonchunhyang University Cheonan Hospital between June 2008 and December 2010 for health examination, of which 6,293 were male. Among them, 410 patients on medical treatment for hypertension, diabetes mellitus, and/or dyslipidemia and 16 patients with missing values for baseline characteristics were excluded. Therefore, 5,867 males were included as study subjects. Written informed consent was obtained from the participants, and the study was approved by the Institutional Review Board of Soonchunhyang University Cheonan Hospital (SCHCA 2015-11-001).
Body measurement and blood tests
An automatic body measuring device (model FA-96H; Fanics Co., Busan, South Korea) was used for measuring height and weight. Body mass index (BMI) was calculated as weight (kg)/height squared (m2). Based on BMI, the subjects were categorized into non-obese (BMI < 25 kg/m2) and obese (BMI ≥ 25 kg/m2) groups. Using a tape measure, waist circumference was measured at the midpoint between the lowermost rib and uppermost iliac crest with the subject in the upright position. Blood pressure was measured in both upper arms using an automatic blood pressure measuring device after 5 min or longer of a stable state, and the higher blood pressure measured was selected. A blood sample was drawn from the antecubital vein after a 12-h fast. Serum was separated on-site and analyzed. The serum levels of glucose, TG, and HDL cholesterol were measured enzymatically using an automated analyzer (Cobas 8000 modular analyzer; Roche, Indianapolis, USA).
Survey of disease history and lifestyle habits
Using self-administered questionnaires, data on physical activities, drinking, smoking habits, and history of hypertension, diabetes mellitus, dyslipidemia, and medication use were obtained. Alcohol consumption was assessed by asking for the drinking frequency (drinking days per week) in the past 30 days, as well as the type of alcoholic beverage the subject drank and the amount consumed in one day, excluding days where no alcohol was consumed. The amount of alcohol consumption per week was converted to grams according to the each type of alcoholic beverage, i.e., one 360 mL (one bottle) of sochu (traditional Korean liquor) was converted to 56 g, 355 mL (one can) of beer to 14 g, 750 mL (one bottle) of wine to 72 g, and 750 mL (one bottle) of makgeolli (traditional Korean liquor) to 36 g. Due to the lack of consensus criteria for measuring drinking level and determining the standard amount of drinking worldwide, a lifestyle guideline from the 2015 US Ministry of Agriculture and Forestry [17] was used for this study. An alcoholic drink containing 14 g of alcohol was set as the standard amount of 1 drink; a standard drink is roughly equivalent to one can of beer (355 mL), a 1/4 bottle of shochu (90 mL) and a glass of wine (150 mL). The subjects were divided into three categories according to the drinking level: nondrinking (0 drinks/week), moderate drinking (≤ 14 drinks/week), and heavy drinking (> 14 drinks/week). Having ≥ 5 drinks on the same occasion more than once in the past month was defined as binge drinking. For physical activity habits, the subjects were classified into four categories: exercise for ≥ 30 min/day for ≥ 5 days/week, 3–4 days/week, 1–2 days/week, and less than 1–3 days/month. For smoking habits, the subjects were classified into smoking (currently smoking) and nonsmoking (not smoking or had quit smoking) groups.
Diagnostic criteria for metabolic syndrome
According to newly presented diagnostic criteria from the modified Adult Treatment Panel III by the American Heart Association and National Heart, Lung, and Blood Institute in 2005, which was based on the 2001 National Cholesterol Education Program Adult Treatment Panel III [18], satisfying more than three of the following five items constitutes a diagnosis of metabolic syndrome:
1) Waist circumference: ≥ 90 cm for men
2) Systolic blood pressure (SBP): ≥ 130 mmHg or diastolic blood pressure (DBP): ≥ 85 mmHg
3) Fasting blood sugar (FBS): ≥ 100 mg/dL
4) TG: ≥ 150 mg/dL
5) HDL cholesterol: < 40 mg/dL for men
Considering racial differences, a waist circumference of ≥ 90 cm was adopted as the Korean male standard diagnostic criterion for abdominal obesity [19].
Statistical analysis
After categorizing the subjects into groups based on obesity and alcohol consumption, the health risk factors and components of metabolic syndrome were compared using one-way analysis of variance and the chi-square test. After adjusting for confounding variables (age, physical activity, and smoking) in both obese and non-obese groups, the odds ratios (ORs) for metabolic syndrome and its individual components according to weekly drinking level or binge drinking, as well as the ORs for metabolic syndrome according to weekly drinking level and binge drinking (moderate drinking without binge drinking, moderate drinking with binge drinking, heavy drinking without binge drinking, and heavy drinking with binge drinking), were obtained using binary logistic regression analysis. All statistical analyses were performed using Statistical Package for the Social Sciences 14.0 KO for Windows (IBM Corp., Armonk, NY, USA). P-values < 0.05 at 95% confidence intervals (CI) were considered statistically significant.
RESULTS
Characteristics of subjects
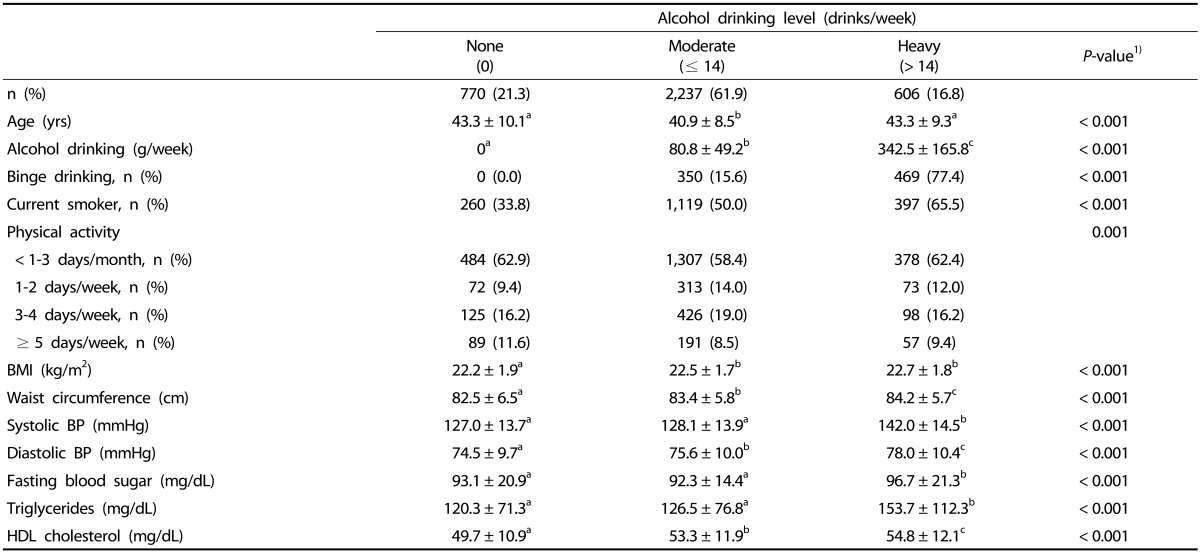
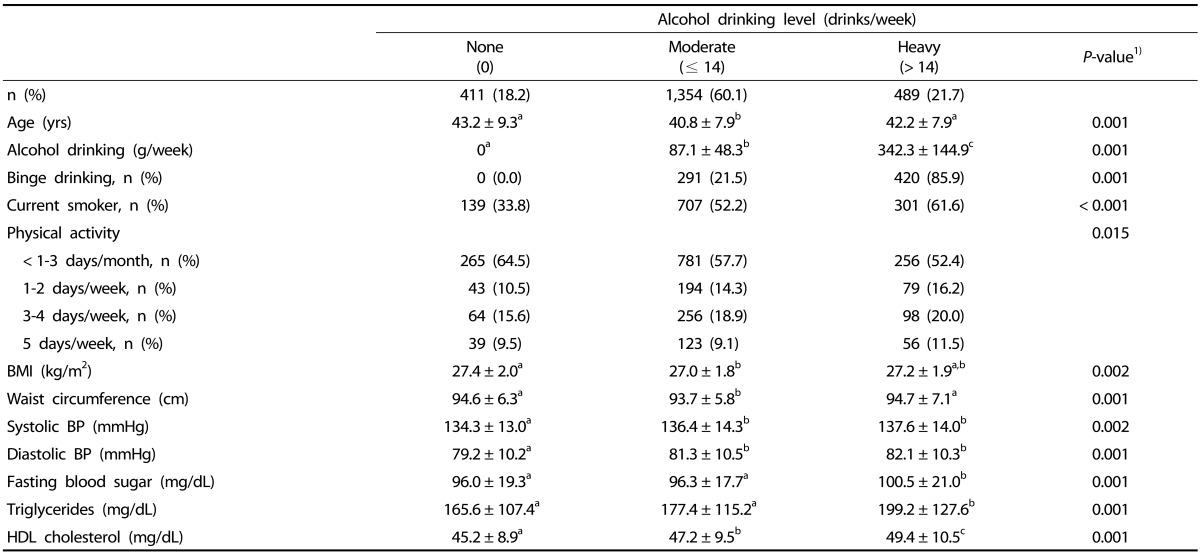
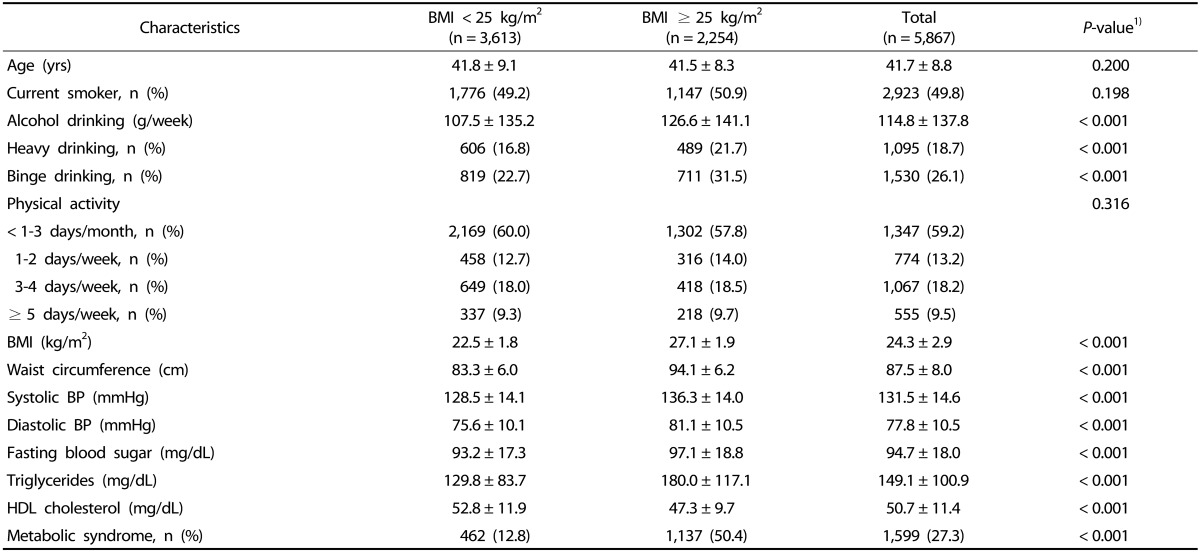
Among the 5,867 subjects, 3,613 (61.6%) and 2,254 (38.4%) were categorized to the non-obese and obese groups, respectively. The mean age ± standard deviation (SD) was 41.7 ± 8.8 years. The overall average alcohol consumption was 114.8 ± 137.8 g/week; the average in the non-obese group was 107.5 ± 135.2 g/week, and that in the obese group was 126.6 ± 141.1 g/week (P < 0.001). The percentage of heavy drinking and binge drinking was also higher in the obese group (P < 0.001). The average frequency of binge drinking was 2.2 ± 1.3 days/week in the non-obese group and 2.1 ± 1.1 days/week in the obese group, showing no significant difference (data not shown). Among male subjects with binge drinking, 96.6% reported binge drinking more than once a week (data not shown). Moreover, the percentage of binge drinking in heavy drinking male subjects was 77.4% in the non-obese group (Table 2) and 85.9% in the obese group (Table 3), showing that most of the heavy drinking Korean male subjects were also engaged in binge drinking.
Table 2. Metabolic syndrome components and lifestyle characteristics according to weekly alcohol drinking level in subjects with BMI < 25 kg/m2.
BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein.
Data are presented as means ± SD or n (%).
Superscript a,b,c indicate that values with same letters are not significantly different between groups using Turkey's multiple comparison test.
1)P-values were obtained using analysis of variance or the chi-square test.
Table 3. Metabolic syndrome components and lifestyle characteristics according to weekly alcohol drinking level in subjects with BMI ≥ 25 kg/m2.
BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein.
Data are presented as means ± SD or n (%).
Superscript a,b,c iindicate that values with same letters are not significantly different between groups using Turkey's multiple comparison test.
1)P-values were obtained by analysis of variance or the chi-square test.
The overall prevalence of metabolic syndrome was 27.3%; the prevalence in the non-obese group was 12.8%, and that in the obese group was 50.4% (P < 0.001). The mean waist circumference, SBP, DBP, FBS, and TG were higher in the obese group (P < 0.001), while the mean HDL cholesterol was higher in the non-obese group (P < 0.001) (Table 1).
Table 1. Components of metabolic syndrome and lifestyle characteristics according to BMI.
BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein.
Data are presented as means ± SD or n (%).
1) P-values were obtained using analysis of variance or the chi-square test.
Metabolic syndrome components and other characteristics according to BMI and weekly alcohol drinking level
In the non-obese group, moderate drinking male subjects had the lowest mean age (40.9 ± 8.5 years), while heavy drinking male subjects had the highest mean waist circumference, SBP, DBP, FBS, TG, and HDL cholesterol (P < 0.001) (Table 2).
In the obese group, moderate drinking male subjects had the lowest mean age (40.8 ± 7.9 years) and mean waist circumference (P < 0.05), while heavy drinking male subjects had the highest mean SBP, DBP, FBS, TG, and HDL cholesterol (P < 0.05) (Table 3).
ORs for metabolic syndrome and its individual abnormalities according to BMI and weekly alcohol drinking level
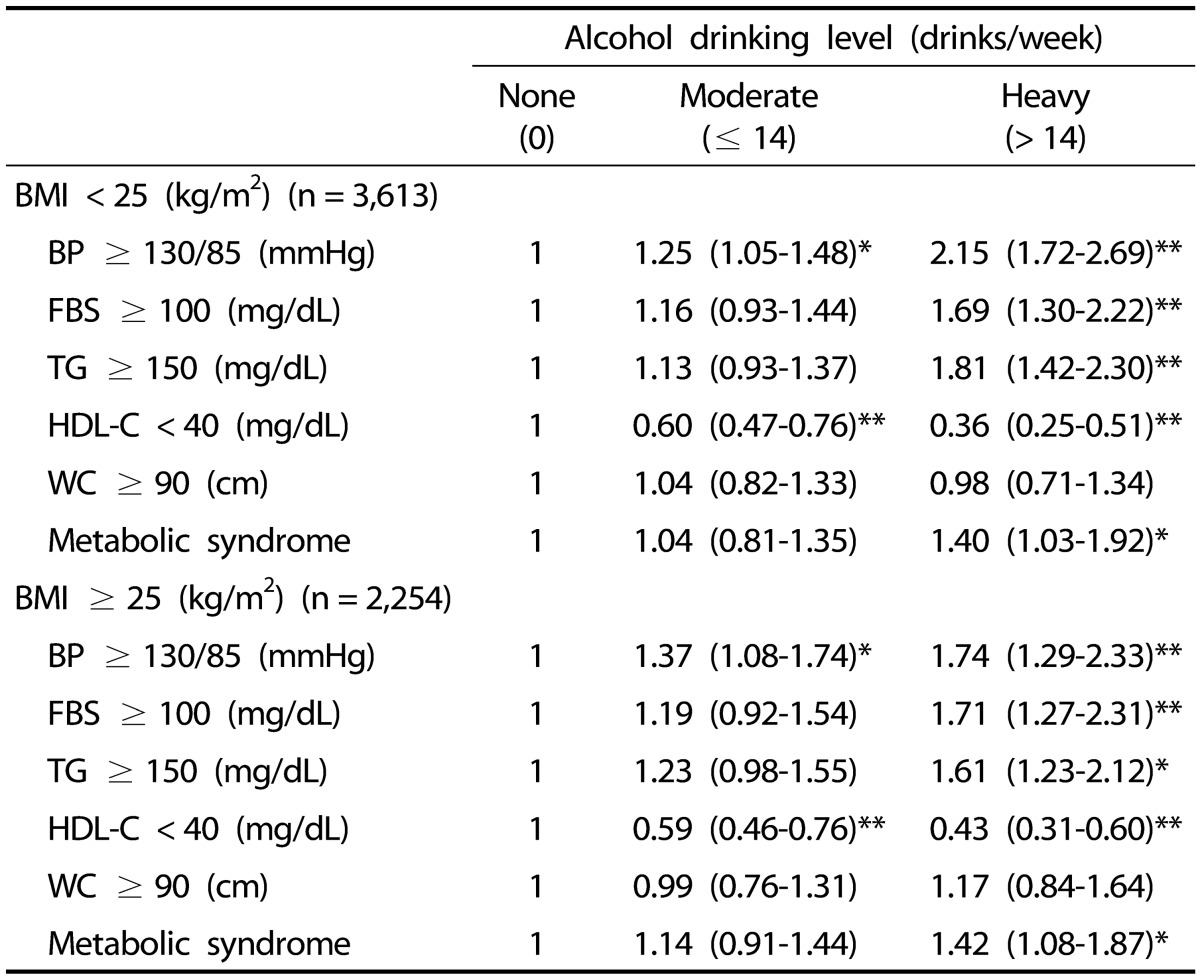
In the non-obese group (with nondrinking as reference), after adjusting for age, physical activity, and smoking, binary logistic regression analysis showed an OR of 1.40 (95% CI = 1.03–1.92) in heavy drinking male subjects, indicating a significantly increased risk of metabolic syndrome (P < 0.05) (Table 4), whereas no significant association with metabolic syndrome (OR = 1.04, 95% CI = 0.81–1.35) was shown in moderate drinking males. Among heavy drinking male subjects, higher ORs were reported for increased blood pressure, FBS, and TG, which were statistically significant (P < 0.001), while a decreased OR was reported for low HDL cholesterol (P < 0.001) (Table 4).
Table 4. Odds ratios and 95% confidence intervals for metabolic syndrome and its individual abnormalities according to BMI and weekly alcohol drinking level1).
BMI, body mass index; BP, blood pressure; FBS, fasting blood sugar; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; WC, waist circumference.
1) P-values were obtained by binary logistic regression analysis and adjusted for age, physical activity, and cigarette smoking.
* P < 0.05, ** P < 0.001.
Similarly, in the obese group (with nondrinking as reference), after adjusting for age, physical activity, and smoking, binary logistic regression analysis showed an OR of 1.42 (95% CI = 1.08–1.87) in heavy drinking males, indicating a significantly increased risk of metabolic syndrome (P < 0.05) (Table 4), whereas no statistically significant difference was shown with metabolic syndrome in moderate drinking male subjects. In heavy drinking male subjects, higher ORs were reported for increased blood pressure, FBS, and TG, which were statistically significant (P < 0.05), while a decreased OR was reported for low HDL cholesterol (P < 0.001) (Table 4).
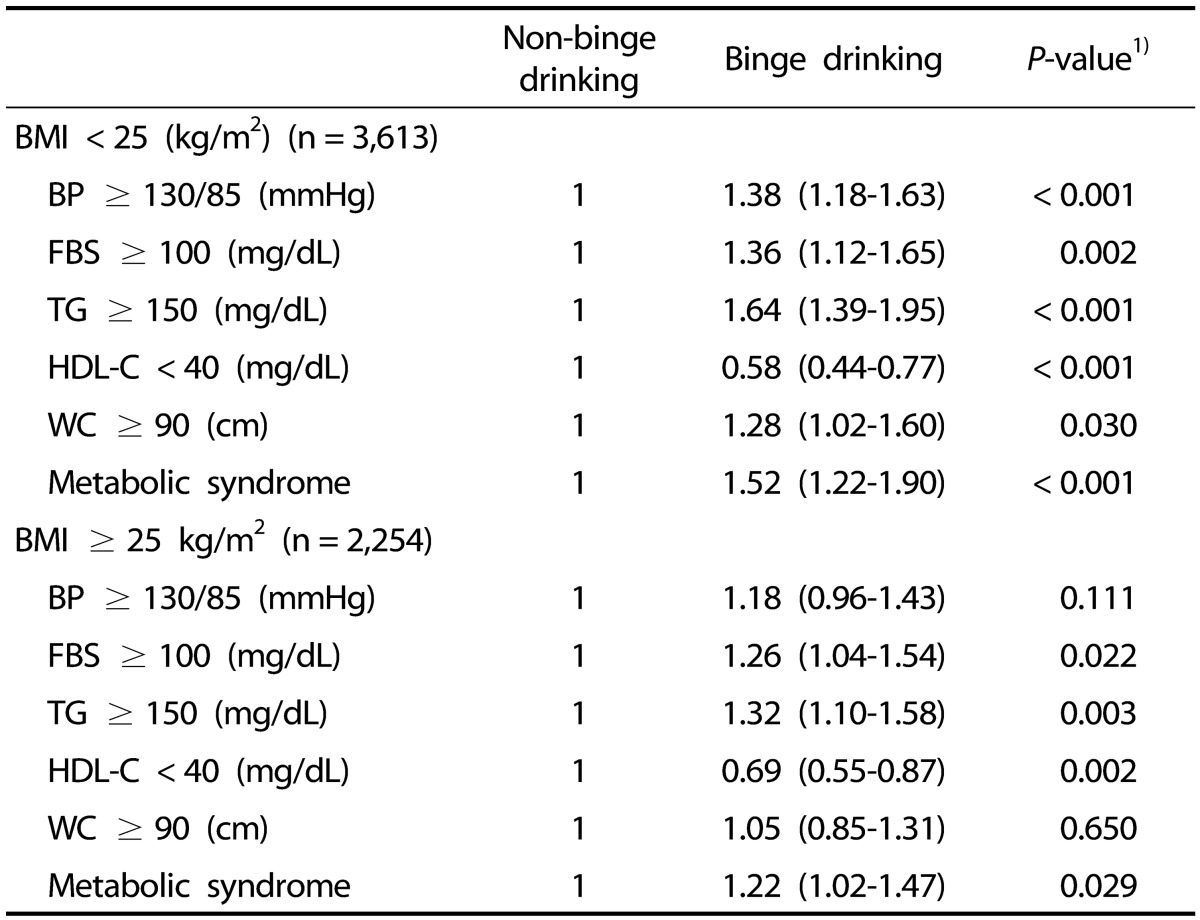
ORs for metabolic syndrome and its individual abnormalities according to BMI and binge drinking
On binary logistic regression analysis, after adjusting for age, physical activity, and smoking (with no binge drinking as reference), the non-obese group showed a higher (OR = 1.52, 95% CI = 1.22–1.90) of having metabolic syndrome with binge drinking (P < 0.001) (Table 5), and the non-obese binge drinking group had a higher OR of having increased blood pressure, FBS, TG, and waist circumference (P < 0.05) and a decreased OR of having low HDL cholesterol (P < 0.001) (Table 5). The obese binge drinking group had a higher OR of having increased FBS and TG (P < 0.05) and a decreased OR of having low HDL cholesterol (P < 0.05) (Table 5), and there was a significant association with metabolic syndrome (OR = 1.22, 95% CI = 1.02–1.47).
Table 5. Adjusted odds ratios and 95% confidence intervals for metabolic syndrome and its individual abnormalities according to BMI and binge drinking.
BMI, body mass index; BP, blood pressure; FBS, fasting blood sugar; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; WC, waist circumference.
1) P-values were obtained using binary logistic regression analysis and adjusted for age, physical activity, and cigarette smoking.
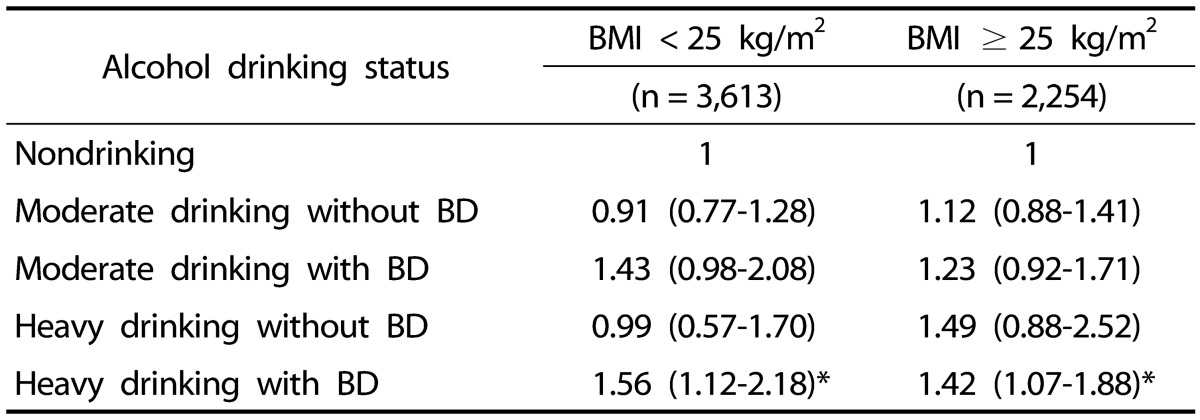
ORs for metabolic syndrome according to BMI, weekly alcohol drinking level, and binge drinking
On binary logistic regression analysis, after adjusting for age, physical activity, and smoking (with nondrinking as reference), the non-obese group had higher OR of having metabolic syndrome with the habit of both heavy drinking and binge drinking (OR = 1.56, 95% CI = 1.12–2.18). In the obese group, a a significantly higher OR of having metabolic syndrome (OR = 1.42, 95% CI = 1.07–1.88) was also observed with both heavy drinking and binge drinking (P < 0.05) (Table 6).
Table 6. Adjusted odds ratios and 95% confidence intervals for metabolic syndrome according to BMI and drinking status1).
BMI, body mass index; BD, binge drinking.
1) P-values were obtained using binary logistic regression analysis and adjusted for age, physical activity, and cigarette smoking.
* P < 0.05
DISCUSSION
In this study, the habit of heavy drinking with binge drinking was associated with increased risk of metabolic syndrome in both non-obese and obese groups, but heavy drinking without binge drinking did not increase the risk of metabolic syndrome in both groups. Previous studies [3,4,15,16] only analyzed alcohol drinking levels or the habit of binge drinking but did not consider the degree of obesity or the concurrence of heavy drinking with binge drinking. This study, however, investigated the presence of heavy drinking and binge drinking in association with BMI.
This study further found that moderate drinking (≤ 14 drinks/week) was not associated with metabolic syndrome in both obese and non-obese groups. In previous studies, moderate drinking was linked to a decreased risk of metabolic syndrome [4,20], whereas other studies showed no association [15,16]. A prospective study of adult males by Kim et al. [21] demonstrated an increased risk of metabolic syndrome with heavy (≥ 30.0 g/day) and moderate (15.0–29.9 g/day) drinking in a group with continuous drinking. However, in a study by Santos et al. [16], both heavy (≥ 30 g/day) and moderate (0.1–29 g/day) drinking were not related to metabolic syndrome. Wakabayashi reported that among Japanese men with BMI ≥ 25 kg/m2, the odds of having metabolic syndrome were low in light drinkers (< 22 g/day) and high in very heavy drinkers (≥ 44 g/day), compared with nondrinkers [22]; however, the study did not include individuals with BMI < 25 kg/m2. There is no standard for accurately measuring alcohol consumption; hence, there can be discrepancies between the amount of drinking reported by study participants and the actual amount of alcohol consumed. Moreover, due to lack of an internationally established definition of the standard drinking amount, drinking criteria were inconsistent in previous studies, leading to inconsistent results and conclusions. Various confounding variables such as age, sex, socioeconomic status, the amount of food intake in conjunction with drinking, physical activity, smoking, drinking pattern, and BMI can also affect the relationship between alcohol drinking and metabolic syndrome. Therefore, differences in confounding variables that were adjusted for in each study should be considered.
Here, heavy drinking (> 14 drinks/week), including the presence of binge drinking, was analyzed, and the results showed no increased risk of metabolic syndrome with heavy drinking in the absence of binge drinking. The increased risk only occurred with binge drinking in both obese and non-obese groups. These findings indicate that the amount of alcohol consumed on one occasion, in addition to the total amount of alcohol consumed weekly, is more specifically associated with metabolic syndrome. In a study by Fan et al. [15] the risk of metabolic syndrome increased with having ≥ 3 drinks/day or with binge drinking. A study by Lee also showed an increased risk of metabolic syndrome when both men and women engaged in binge drinking more than once a week [23]. Furthermore, in a study of male adults by Im et al. [21] the risk of metabolic syndrome increased with binge drinking in light, moderate, and heavy drinkers compared with nondrinkers, similar to the results of heavy drinking in the present study; however, differences in terms of BMI were not examined.
The mechanism by which alcohol drinking is associated with metabolic syndrome remains unclear. Alcohol suppresses lipid oxidation, and the non-oxidized fat is preferentially deposited in the abdominal area [25]; furthermore, chronic alcohol consumption leads to an increased cortisol release due to stimulation of the hypothalamic-pituitary-adrenal axis [26]. This endocrine change due to alcohol drink may increase abdominal fat deposition [27]. The increased abdominal fat mass in alcohol drinkers may induce insulin resistance, leading to increased blood pressure, blood glucose, and TGs. In addition, activation of the sympathetic nervous system, impairment of baroreceptors, and stimulation of the renin-angiotensin-aldosterone system by heavy drinking may lead to hypertension [28]. A recent study showed that binge drinking induces systemic insulin resistance by impairing hypothalamic insulin action in rats [29]. Based on the aforementioned reasons, it is presumed that heavy or binge drinking increases blood pressure, blood sugar, and TGs, canceling the positive effect of increased HDL cholesterol increase [4,15,21,30,31] and ultimately increasing the risk of metabolic syndrome. This is in concert with the current study, which demonstrated that heavy drinking or binge drinking decreased the risk of low HDL cholesterol but increased the risk of high blood pressure and elevated blood sugar and TG levels.
The association between heavy drinking or binge drinking and a decreased risk of low HDL cholesterol is explained by the fact alcohol consumption increases HDL cholesterol [32,33]. However, the underlying mechanisms whereby alcohol drinking enhance HDL cholesterol levels are not yet fully clear. Alcohol may increase the hepatic production and secretion of apolipoproteins and lipoprotein particles, increase triglyceride lipase concentrations, and decrease removal of circulating HDL cholesterol [32,34]. Alcohol may reduce the concentration and activity of plasma cholesteryl ester transfer protein, which transfers plasma cholesteryl esters from HDL particles to other lipoproteins. This leads to an increase in HDL [35].
One of the strengths of this study is that through face-to-face examination, the doctors confirmed the questionnaires answered by the study subjects, which allowed more accurate examination of the association between alcohol drinking and metabolic syndrome. To confirm responses to the questions, doctors asked the questions in the questionnaire again at the time of medical examination, confirmed that it matched the answer from the self-questionnaire, and corrected any responses accordingly. Moreover, a large number of subjects (5,867 subjects) participated in the study. However, the study has several limitations. First, because of the study's cross-sectional design, a causal relationship could not be established between BMI, drinking status, and metabolic syndrome. Second, data were limited to subjects from one university hospital, and only adult male subjects were studied due to the small number of women who are heavy drinkers; hence, the findings do not represent the whole population. Third, despite the effects of alcohol on energy intake and food intake, this study did not consider diet. Lastly, the study subjects may have not reported the actual amount of alcohol consumption.
In conclusion, the concurrence of heavy drinking and binge drinking was associated with increased risk of metabolic syndrome in both non-obese and obese groups. These findings suggest specific associations of metabolic syndrome with the amount of alcohol consumed on one occasion in addition to the total amount of alcohol consumed weekly, regardless of the BMI of alcohol drinkers. Therefore, the negative metabolic effects of alcohol on the human body can be prevented by implementing guidelines that set the proper amount of alcohol that can be consumed on one occasion. A successful implementation would require proactive education of non-obese and obese people to enhance their awareness that heavy and binge drinking can increase the risk of metabolic syndrome. Future prospective studies are also needed to clarify the causal relationship between alcohol drinking and metabolic syndrome according to varying degrees of obesity.
Footnotes
This work was supported by the Soonchunhyang University Research Fund (No. 20180004).
CONFLICT OF INTEREST: The author declares no potential conflicts of interests.
References
- 1.Stahre M, Roeber J, Kanny D, Brewer RD, Zhang X. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev Chronic Dis. 2014;11:E109. doi: 10.5888/pcd11.130293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global status report on alcohol and health 2014 [Internet] Geneva: World Health Organization; 2014. [cited 2017 August 15]. Available from: http://apps.who.int/iris/bitstream/10665/112736/1/9789240692763_eng.pdf. [Google Scholar]
- 3.Sun K, Ren M, Liu D, Wang C, Yang C, Yan L. Alcohol consumption and risk of metabolic syndrome: a meta-analysis of prospective studies. Clin Nutr. 2014;33:596–602. doi: 10.1016/j.clnu.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Yoon YS, Oh SW, Baik HW, Park HS, Kim WY. Alcohol consumption and the metabolic syndrome in Korean adults: the 1998 Korean National Health and Nutrition Examination Survey. Am J Clin Nutr. 2004;80:217–224. doi: 10.1093/ajcn/80.1.217. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 6.Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 7.Cameron AJ, Boyko EJ, Sicree RA, Zimmet PZ, Söderberg S, Alberti KG, Tuomilehto J, Chitson P, Shaw JE. Central obesity as a precursor to the metabolic syndrome in the AusDiab study and Mauritius. Obesity (Silver Spring) 2008;16:2707–2716. doi: 10.1038/oby.2008.412. [DOI] [PubMed] [Google Scholar]
- 8.Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, Shofer JB, Fish BE, Knopp RH, Kahn SE. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes. 2004;53:2087–2094. doi: 10.2337/diabetes.53.8.2087. [DOI] [PubMed] [Google Scholar]
- 9.Ferrannini E, Haffner SM, Mitchell BD, Stern MP. Hyperinsulinaemia: the key feature of a cardiovascular and metabolic syndrome. Diabetologia. 1991;34:416–422. doi: 10.1007/BF00403180. [DOI] [PubMed] [Google Scholar]
- 10.Junyent M, Arnett DK, Tsai MY, Kabagambe EK, Straka RJ, Province M, An P, Lai CQ, Parnell LD, Shen J, Lee YC, Borecki I, Ordovás JM. Genetic variants at the PDZ-interacting domain of the scavenger receptor class B type I interact with diet to influence the risk of metabolic syndrome in obese men and women. J Nutr. 2009;139:842–848. doi: 10.3945/jn.108.101196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Razzouk L, Muntner P. Ethnic, gender, and age-related differences in patients with the metabolic syndrome. Curr Hypertens Rep. 2009;11:127–132. doi: 10.1007/s11906-009-0023-8. [DOI] [PubMed] [Google Scholar]
- 12.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korean Statistical Information Service. Statistical database for metabolic syndrome [Internet] Daejeon: Statistics Korea; [cited 2018 January 11]. Available from: http://kosis.kr/statisticsList/statisticsListIndex.do?menuId=M_01_01&vwcd=MT_ZTITLE&parmTabId=M_01_01. [Google Scholar]
- 14.Posey D, Mozayani A. The estimation of blood alcohol concentration: Widmark revisited. Forensic Sci Med Pathol. 2007;3:33–39. doi: 10.1385/FSMP:3:1:33. [DOI] [PubMed] [Google Scholar]
- 15.Fan AZ, Russell M, Naimi T, Li Y, Liao Y, Jiles R, Mokdad AH. Patterns of alcohol consumption and the metabolic syndrome. J Clin Endocrinol Metab. 2008;93:3833–3838. doi: 10.1210/jc.2007-2788. [DOI] [PubMed] [Google Scholar]
- 16.Santos AC, Ebrahim S, Barros H. Alcohol intake, smoking, sleeping hours, physical activity and the metabolic syndrome. Prev Med. 2007;44:328–334. doi: 10.1016/j.ypmed.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015–2020 Dietary guidelines for Americans. 8th ed [Internet] Washington, D.C.: U.S. Department of Agriculture; 2015. [cited 2016 February 3]. Available from: https://health.gov/dietaryguidelines/2015/guidelines/ [Google Scholar]
- 18.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 19.Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ, Kim DY, Kwon HS, Kim SR, Lee CB, Oh SJ, Park CY, Yoo HJ. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007;75:72–80. doi: 10.1016/j.diabres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Freiberg MS, Cabral HJ, Heeren TC, Vasan RS, Ellison RC. Alcohol consumption and the prevalence of the metabolic syndrome in the U.S.: a cross-sectional analysis of data from the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2954–2959. doi: 10.2337/diacare.27.12.2954. [DOI] [PubMed] [Google Scholar]
- 21.Kim BJ, Kim BS, Kang JH. Alcohol consumption and incidence of metabolic syndrome in Korean men. A 3-year follow-up study. Circ J. 2012;76:2363–2371. doi: 10.1253/circj.cj-12-0315. [DOI] [PubMed] [Google Scholar]
- 22.Wakabayashi I. Relationship between alcohol consumption and metabolic syndrome in Japanese men with overweight or obesity. Obes Res Clin Pract. 2011;5:e79–e156. doi: 10.1016/j.orcp.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Lee K. Gender-specific relationships between alcohol drinking patterns and metabolic syndrome: the Korea National Health and Nutrition Examination Survey 2008. Public Health Nutr. 2012;15:1917–1924. doi: 10.1017/S136898001100365X. [DOI] [PubMed] [Google Scholar]
- 24.Im HJ, Park SM, Choi JH, Choi EJ. Binge drinking and its relation to metabolic syndrome in Korean adult men. Korean J Fam Med. 2014;35:173–181. doi: 10.4082/kjfm.2014.35.4.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suter PM, Tremblay A. Is alcohol consumption a risk factor for weight gain and obesity? Crit Rev Clin Lab Sci. 2005;42:197–227. doi: 10.1080/10408360590913542. [DOI] [PubMed] [Google Scholar]
- 26.Stokes PE. Adrenocortical activation in alcoholics during chronic drinking. Ann N Y Acad Sci. 1973;215:77–83. doi: 10.1111/j.1749-6632.1973.tb28251.x. [DOI] [PubMed] [Google Scholar]
- 27.Björntorp P. Obesity. Lancet. 1997;350:423–426. doi: 10.1016/S0140-6736(97)04503-0. [DOI] [PubMed] [Google Scholar]
- 28.Klatsky AL. Alcohol and hypertension. Clin Chim Acta. 1996;246:91–105. doi: 10.1016/0009-8981(96)06230-4. [DOI] [PubMed] [Google Scholar]
- 29.Lindtner C, Scherer T, Zielinski E, Filatova N, Fasshauer M, Tonks NK, Puchowicz M, Buettner C. Binge drinking induces whole-body insulin resistance by impairing hypothalamic insulin action. Sci Transl Med. 2013;5:170ra14. doi: 10.1126/scitranslmed.3005123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shelmet JJ, Reichard GA, Skutches CL, Hoeldtke RD, Owen OE, Boden G. Ethanol causes acute inhibition of carbohydrate, fat, and protein oxidation and insulin resistance. J Clin Invest. 1988;81:1137–1145. doi: 10.1172/JCI113428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castelli WP, Doyle JT, Gordon T, Hames CG, Hjortland MC, Hulley SB, Kagan A, Zukel WJ. Alcohol and blood lipids. The cooperative lipoprotein phenotyping study. Lancet. 1977;2:153–155. doi: 10.1016/s0140-6736(77)90176-3. [DOI] [PubMed] [Google Scholar]
- 32.Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319:1523–1528. doi: 10.1136/bmj.319.7224.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaziano JM, Buring JE, Breslow JL, Goldhaber SZ, Rosner B, VanDenburgh M, Willett W, Hennekens CH. Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. N Engl J Med. 1993;329:1829–1834. doi: 10.1056/NEJM199312163292501. [DOI] [PubMed] [Google Scholar]
- 34.Savolainen MJ, Kesaniemi YA. Effects of alcohol on lipoproteins in relation to coronary heart disease. Curr Opin Lipidol. 1995;6:243–250. doi: 10.1097/00041433-199508000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Hannuksela M, Marcel YL, Kesaniemi YA, Savolainen MJ. Reduction in the concentration and activity of plasma cholesteryl ester transfer protein by alcohol. J Lipid Res. 1992;33:737–744. [PubMed] [Google Scholar]