Abstract
Objectives
Patients with inflammatory bowel disease have a higher risk of thrombosis, which is associated with a higher morbidity and mortality. Most data about VTE are related to hospitalized patients with active disease, but several cases happen in the outpatient setting, and are not covered by current prophylaxis recommendation. As the knowledge of VTE in outpatients is still poor, the aim of this study is to evaluate the risk, clinical data and mortality of thrombosis in patients followed in our center, comparing our findings with the current prophylaxis recommendation.
Methods
The medical electronic chart of 1093 inflammatory bowel disease patients and their image exams were actively searched for words related to thrombosis, followed by charts reviewed to collect information about the event and data regarding clinical settings and thrombosis profile.
Results
Overall, 654 Crohn’s and 439 Colitis patients were included. Thrombosis prevalence was 5.1%,and mortality rate was higher in patients who had suffered thrombosis (10.71% vs. 1.45%, OR 8.0). Half of them developed thrombosis in the outpatient setting, 52% of these had disease activity, 17% had recent hospitalization, and 10% had previous thrombosis. In 27% of cases, diagnosis was done by routine image exams, with no clinical symptoms or previous history of thrombosis. None of them had used thromboprophylaxis. However, a great majority of patients who had thrombosis during hospitalization used heparin prophylaxis.
Conclusion
Inflammatory bowel disease patients who develop thrombosis have an increased mortality risk. A significant proportion of the events happened in patients without a clear thromboprophylaxis recommendation or in those receiving heparin prophylaxis.
Introduction
Thrombosis is associated with a high mortality rate and directly impacts the quality of life of individuals1. The literature shows that patients with Crohn’s disease (CD) and ulcerative colitis (UC) have an increased risk of thrombosis, three to four folds higher than subjects without inflammatory bowel disease (IBD)2. More than 500,000 inpatients per year in the United States are diagnosed with deep venous thrombosis (DVT)3,4. Mortality rate is about 3% for DVT and more than 15% during the first 3 months after pulmonary thromboembolism (PTE)5–7. Risk factors for venous thromboembolism (VTE) include surgery, prolonged immobilization, malignancy, obesity, use of oral contraceptives, smoking, corticosteroids, pregnancy, heart failure, chronic obstructive pulmonary disease1, and also inflammatory bowel disease (IBD)8. In IBD patients, thrombosis risk increases with the extension of mucosal damage and disease activity.
The Canadian Consensus9 recommends thromboprophylaxis with heparin for hospitalized patients due to IBD flares without active bleeding or with non-severe bleeding. For outpatients, thromboprophylaxis is recommended during moderate to severe IBD flares, with a previous VTE event without a clear etiology.
Most data about VTE in IBD refer to hospitalized patients with clinical active disease. Nevertheless, literature suggests that a great proportion of thrombosis cases occur in the outpatient setting10. Thus, the aims of this study are to better characterize those events, evaluating the risk, clinical data and mortality due to thrombosis in IBD patients followed in a tertiary hospital of Sao Paulo and compare these findings with current prophylaxis recommendations.
Materials and methods
The medical electronic chart of 1093 IBD patients followed at the (Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, SP, BR) and their image exams (ultrasound, tomography or magnetic resonance) performed between January 2010 and May 2015 were actively searched for words related to VTE. Patients selected in this first search had their charts reviewed by a GI physician in order to collect information about clinical settings and thrombosis profile. If the medical report was incomplete, patients or a family member were contacted by phone to complete any missing information. IBD patients without thrombosis who had been to hospital after June/2015 were considered alive. Those who missed their follow-up appointment for any reason, had their individual’s tax registry number crossed with the national register of deceased (NGOs with data on deaths obtained from cemeteries, funeral homes and hospitals).
All patients had the IBD diagnosis confirmed by endoscopic, radiological, and/or histological findings for more than 6 months before inclusion. Data were collected regarding age, sex, smoking status, risk factors for thrombosis, disease duration, extension, behavior, location and activity, pharmacological treatment, surgeries and hospitalization at the time of the event, previous or familial history of thrombosis, oral contraceptive use, and the association with central venous catheter. IBD activity was defined by the medical impression and CRP values at the time of VTE. Infliximab treatment was considered optimized if the dose of infliximab was increased to 10 mg/kg, or the interval was reduced to 6 or 4 weeks, and for adalimumab if the interval was reduced to 1 week.
For comparison purposes only, laboratory data from CD patients with thrombosis were compared with CD patients without thrombosis, whose data were extracted from a prospective study initiated in 2015. This sample is considered representative once it is obtained from the baseline population of this study. Considering a 0.05 significance and a thrombosis prevalence of 5.7%, the power of the sample size was 80.2%.
Institutional review board approval was obtained, and the requirement for informed written consent was waived.
Statistical analysis
All statistical analysis were performed with the web base statistical program SAS® Studio On Demand for Academics or R Statistics. Data distribution was analyzed using Normal-QQ-plots, histogram plots, and Shapiro test. Categorical data are summarized as the percentage of the total group. Fisher’s exact test (two-sided) was used to explore associations of parametric categorical data between the two groups. The Mann–Whitney U test (Wilcoxon rank sum test) was used to explore non-parametric data between two unpaired groups. A p value of <0.05 was considered statistically significant.
Stepwise multiple logistic regression modeling was performed in order to evaluate the association between potential risk factors and VTE. The variables tested as potential risk factors were: gender, age at time of IBD diagnosis, disease location, duration and behavior, treatment, body mass index (BMI), IBD family history, smoking status, use of oral contraception in females, steroid use, hemoglobin, platelet count, C-reactive protein (CRP), fibrinogen and factor VIII (FVIII) levels, anti-thrombin (AT), C and S deficiency. In a first step, each potential risk factor was tested separately. In a second step, all risk factors with a p value < 0.2 were entered together into the multivariate logistic regression model.
The backward stepwise method multiple logistic regression modeling was performed in order to evaluate the association between potential risk factors and VTE. The variables tested as potential risk factors were: gender, age at time of IBD diagnosis, disease location, duration and behavior, treatment, body mass index (BMI), IBD family history, smoking status, use of oral contraception in females, steroid use, hemoglobin, platelet count, C-reactive protein (CRP), fibrinogen and factor VIII (FVIII) levels, anti-thrombin (AT), C and S deficiency. In a first step, each potential risk factor was tested separately. In a second step, all risk factors with a p value < 0.2 were entered together into the multivariate logistic regression model.
Results
As shown in Table 1, 1093 IBD patients were included in this retrospective study, 654 (60%) with CD and 439 (40%) with UC. There were more females (54%) than males (46%), and age at clinical onset was 31.5 ± 14.3 years old, and the mean disease duration was 12.8 ± 7.4 years. Disease location of 640 CD patients was as follows: ileal in 214 (33.4%), colonic in 115 (18.0%), and ileocolonic in 311 (48.6%). Forty-eight CD patients (7.3%) had upper GI involvement, whereas 11 of them had the disease restricted to the upper GI. Disease behaviors (n = 637) were non-stricturing, non-penetrating in 181 (28.4%) patients, stricturing in 192 (30.1%) patients and penetrating in 264 (41.4%) patients. Of the penetrating phenotype, 96 (36.4%) patients had perianal disease. Among the 396 UC patients, 249 had pancolitis (62.9%), followed by left-sided disease in 87 (22.0%) and proctitis in 60 (15.2%).
Table 1.
Demographic data of IBD cohort
| CD (n = 654) | UC (n = 439) | ||
|---|---|---|---|
| Sex | n (%) | n (%) | |
| Female | 354 (54.05) | 273 (62.19) | |
| Male | 301 (45.95) | 166 (37.81) | |
| Deaths | 16 (2.44) | 5 (1.14) | |
| Location | |||
| Colonic | 116 (18.1) | Left colitis | 87 (19.8) |
| Ileal | 214 (33.4) | Distal | 60 (13.7) |
| Ileocolonic | 311 (48.5) | Pancolitis | 249 (56.7) |
| Unknown | 14(2) | Unknown | 43 (9.8) |
| Upper GI tract | 48 (7.3) | ||
| Unknown | 17 (2.6) | ||
| Behavior | |||
| Stricturing | 192 (29.3) | ||
| Inflammatory | 181 (27.6) | ||
| Penetrating | 265 (40.5) | ||
| Perianal | 97 (14.9) | ||
| VTE | 37 (5.7) | 19 (4.3) | |
VTE venous thromboembolism
The vast majority of CD patients had already taken immunomodulators (72%) and/or biologic therapy (57%), whereas UC patients had preferentially been treated with salicylate agents (76%), almost 40% with immunomodulators and a small proportion of them (20%) had received biologic therapy (Table 2). A significant higher number of CD patients had already undergone previous major abdominal surgery compared with ulcerative colitis (22.5% vs. 7.3%, p < 0.0001).
Table 2.
Medication history of IBD cohort
| Crohn n (%) | RCU n (%) | Total n (%) | |
|---|---|---|---|
| Salicylates | 210 (32.1) | 332 (75.6) | 620 (56.6) |
| Imunossupressors | 469 (71.6) | 164 (37.4) | 633 (57.9) |
| Azathioprine | 450 (68.7) | 161 (36.7) | 712 (65) |
| Methotrexate | 81 (12.4) | 11 (2.5) | 101 (9.2) |
| Anti-TNF | 374 (57.1) | 88 (20) | 462 (42.2) |
| Infliximab | 303 (46.3) | 69 (15.7) | 426 (38.9) |
| Optimized | 58 (19.1) | 13 (18.8) | 71 (16.7) |
| Adalimumab | 187 (28.5) | 39 (8.9) | 251 (22.9) |
| Optimized | 70 (37.4) | 16 (41) | 86 (34.3) |
Thrombosis was diagnosed in 56 patients with an overall prevalence of 5.1% (5.7% among patients with CD and 4.3% in those with UC), conferring a significantly higher mortality rate in the thrombosis group (6/56, 10.71 vs. 15/1037, 1.45%, odds ratio 8.0). On the other hand, there was no significant difference regarding sex, age, age at clinical onset, duration of disease or treatment between IBD with and without VTE. Patients with VTE were more likely to have undergone an abdominal surgery (28.6% vs. 15.8%, p = 0.016, OR: 2.14, 1.17–3.91) Nevertheless, after stratification, this association was observed for CD patients (37.8% vs. 21.6%, p = 0.03), but not for UC (10.5% vs. 7.2%, p = 0.64). Patients with or without VTE had the same age at clinical onset and similar disease duration at the time of VTE.
Thrombophilia screening, including AT, protein C and S, FVIII and fibrinogen, was performed in one-third of the patients who had suffered thrombosis. Results showed that the tested patients had deficiency of protein C (5/19, 26%), S (5/20, 25%) and AT (4/16, 37%). Factor VIII and fibrinogen was increased in 4 of 10 (40%) and 3 of 14 patients (21%), respectively.
Patients with VTE and CD (Table 3) had a significantly different proportion of bowel segment involved (p = 0.005), which means they had more colonic disease (37.8% vs. 16.8%), but less ileocolonic disease (37.8% vs. 49.3%) and ileal disease (24.3% vs. 34.0%), compared to CD without VTE. Nonetheless, they had the same proportion of perianal disease and behavior: stricturing (32.4% vs. 30.0%), non-stricturing, non-penetrating (27.0% vs. 28.5%), and penetrating (40.5% vs. 41.5%) (p = 0.95). Findings of bowel involvement for patients with UC were similar: pancolonic (73.7% vs. 62.3%), left colon (26.3% vs. 21.8%), and colon distal (0% vs. 16.0%) (p = 0.16).
Table 3.
Clinical characteristics and risk factors of IBD patients with thrombosis
| CD (n = 37) | UC (n = 19) | p value | |
|---|---|---|---|
| Sex | |||
| Male | 19 (51.4) | 5 (26.3) | 0.09 |
| Female | 18 (48.6) | 14 (73.7) | |
| Oral contraceptive | 3/14 (21.4) | 0/15 (0) | |
| Previous pregnancy | 12/17 (70.6) | 10/14 (71.4) | |
| Abortion | 4/12 (33.3) | 1/10 (10) | |
| VTE | |||
| Inpatient | 18 (48.6) | 10 (50) | 0.92 |
| Prophylaxis | 14/18 (77.8) | 6/7 (85.7) | |
| Outpatient | 16/37 (45.7) | 12/19 (63) | |
| Previous VTE | 3/16 (18.7) | 0 | |
| Clinical disease activity | 8/16 (50) | 8/14 (57.1) | |
| Recent hospitalization | 10/36 (27.7) | 6/18 (33.3) | |
| Symptoms during VTE | 26/35 (74.3) | 13/19 (68.4) | |
| No | 15 (40.5) | 1(5) | |
| Prophylaxis | 14/18 (77.8) | 6/7 (85.7) | |
| Immobilization | 21/37 (56.7) | 12/19 (63.1) | 0.78 |
| Corticosteroid | 13/37 (35.1) | 10/19 (52.6) | 0.26 |
| Smoking history | 11 (19.7) | 5 (8.9) | 0.1 |
| Never | 22 (59.5) | 11 (57.9) | |
| Previous | 9 (24.3) | 4 (21.1) | |
| Active | 1 (2.7) | 1 (5.2) | |
| Unknown | 5 (13.5) | 3 (15.8) | |
| IBD surgery related | |||
| Last 3 months | 10/36 (27.8) | 6/18 (33.3) | 0.76 |
| At the same period of VTE | 8/37(19) | 3/19(16) | 0.29 |
| Catheter related | 7/37(19) | 3/19(16) | 1.0 |
| Familial VTE | 5 (8.9) | 3 (5.4) | 0.47 |
| Previous neoplasia | 2 (3.6) | 2 (3.6) | 0.12 |
| At the same time of VTE | 2/35 (5.7) | 0 | |
| Previous | 1/35 (2.8) | 1/19 (5.3) | |
When the patients with thrombosis were analyzed by type of IBD (Table 3), there was no difference between CD and UC considering previous history of surgery (40.5% vs. 26.3%, p = 0.2), previous VTE (11.8% vs. 9.1%, p = 1.0) or hospitalization (86.5% vs. 94.7%, p = 0.7). Moreover, data analysis related to the VTE period showed no difference in VTE associated with surgery (21.6% vs. 15.8%, p = 1.0), hospitalization (51.4% vs. 47.4%, p = 1.0), use of central venous access (18.9% vs. 16%, p = 1.0), VTE prophylaxis (78% vs. 86%, p = 1.0), immobilization (56.7% vs. 63.1%, p = 0.8), and corticosteroid use (35.1% vs. 52.6%, p = 0.3). Nevertheless, VTE occurred more often during active UC (94.7%) than in CD (62.2%), reaching statistical significance (p = 0.01).
The most common site of thrombosis (Table 4) was in the lower limbs (n = 24, 33%), followed by pulmonary embolism (n = 13, 18%), mesenteric vein (n = 6, 8%), portal vein (n = 5, 7%), brachiocephalic trunk (n = 4, 6%), inferior cava vein (n = 4, 6%), upper limbs (n = 3, 4%), and others. Further, nine patients (16%) had more than one site stricken by thrombosis, and they were treated with warfarin 17/29 (59%) and/or 14/29 (48%) with low-weight heparin.
Table 4.
Location of VTE events
| VTE location | Frequency | Percent |
|---|---|---|
| Lower limbs | 24 | 33.33 |
| Pulmonary embolism | 13 | 18.05 |
| Mesenteric vein | 6 | 8.32 |
| Portal vein | 5 | 6.94 |
| Lower cava vein | 4 | 5.55 |
| Brachiocephalic trunk | 4 | 5.55 |
| Upper limbs | 3 | 4.16 |
| Other | 13 | 18.05 |
| Total | 72 | 100 |
Forty-three percent and 63% of CD and UC patients, respectively, developed an outpatient VTE event. Fifty-three percent of them had disease activity, 29.6% had recent hospitalization, and 10.3% had previous thrombosis. In 27% of the cases, the diagnosis was made by routine image exams, with no clinical symptoms of thrombosis or previous VTE history.
In CD patients, logistic regression modeling found no significant association between VTE and the following factors: sex, age at diagnosis, BMI, disease duration, location and behavior, treatment, IBD family history, and oral contraception. CD patients who did not develop thrombosis had no C Protein deficiency, therefore we could not include in the model. S protein deficiency was withdrawn of the modeling as the interaction with steroid use (p < 0.03) compromised the convergence of the modeling. Thus, it was considered an independent variable related to VTE (OR: 13.3; IC: 2.16–79.7; p < 0.001). Smoking (OR 7.4; IC: 1.73–3.15; p = 0.006) and steroid use (OR 403; IC: 42–3870; p < 0.001) were associated with a higher risk of VTE. Regarding the treatment with steroids, the OR was high because only one patient using steroid did not develop thrombosis (Table 5).
Table 5.
Logistic regression modeling in order to identify factors associated with VTE complications in patients with CD
| Univariate logistic regression | Multivariate logistic regression | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Sex | ||||||
| Male | 1.80 | 0.87–3.72 | 0.11 | |||
| Age at diagnosis | ||||||
| <40 years old | 0.32 | 1.15–0.70 | 0.004 | |||
| Disease duration | 1.05 | 1.01–1.10 | 0.01 | |||
| Disease location | ||||||
| Colonic | 22.5 | 2.8–180.9 | 0.003 | |||
| Ileocolonic | 17.8 | 2.33–136.5 | 0.005 | |||
| Behavior | ||||||
| Stricturing | 2.3 | 0.55–3.52 | 0.48 | |||
| Fistulizing | 2.3 | 0.91–5.81 | 0.07 | |||
| Perianal | 1.19 | 0.49–2.88 | 0.69 | |||
| Treatment | ||||||
| Immunossupressors | 1.58 | 0.61–4.11 | ||||
| Biologic | 2.65 | 1.14–6.19 | 0.05 | |||
| Corticosteroid | 427 | 51.9–3518.3 | 0.001 | 403 | 42–3870 | < 0.001 |
| BMI | 0.93 | 0.86–1.02 | 0.13 | |||
| Smoking | 7.02 | 3.01–16.4 | 0.001 | 7.4 | 1.73–3.15 | 0.006 |
| IBD family history | 2.97 | 0.92–9.57 | 0.06 | |||
| Oral contraceptive | 1.53 | 0.38–6.2 | 0.54 | |||
| Hemoglobin | 0.48 | 0.38–0.63 | 0.001 | |||
| Platelets | 1.004 | 1.0006–1.007 | 0.02 | |||
| CRP | 1.004 | 0.99–1.01 | 0.16 | |||
| Fibrinogen (>400) | 0.35 | 0.04–2.95 | 0.33 | |||
| FVIII (>150) | 0.85 | 0.18–3.97 | 0.84 | |||
| AT deficiency (<79) | 2.24 | 0.43–11.7 | 0.34 | |||
| C protein deficiency (<64) | * | * | * | |||
| S protein deficiency (<55) | 13.9 | 3.05–63.7 | 0.001 | |||
5-ASA aminosalicilates, BMI body mass index, IBD inflammatory bowel disease, CRP C-reactive protein, AT anti-thrombin, *= not include in the model
The patients with CD and VTE who died were younger at disease diagnosis (median 25 vs. 34 years old, p = 0.001), had more stricturing disease (p = 0.04), previous smoking history (p = 0.02) and were using more steroids (p = 0.048). The mean disease duration was 16 years. Death was associated with VTE in 3 out of 5 patients: 2 died of adenocarcinoma associated with a long-term and refractory disease,1 died due to a rupture of aortic abdominal aneurysm not related to the disease or to VTE. Other causes of deaths were 1 died of digestive hemorrhage secondary to esophagus varices associated with a non cirrhotic portal thrombosis, 1 died of pulmonary embolism and 1 of multiple organ failure in a refractory Crohn’s disease after a long period of hospitalization. The latter patient was receiving infliximab and azathioprine. In addition, two of them had thrombophilia—protein S deficiency and factor V mutation—and the majority had three or more thrombosis risk factors, as illustrated in Table 6. The other IBD deaths were mainly related to cancer complications: 2 patients with diffuse large B-cell non-Hodgkin’s lymphoma, 2 with metastatic colorectal cancer, 1 with metastatic cholangiocarcinoma associated with primary sclerosing cholangitis, 1 with low differentiated carcinoma infiltrating lung tissue. Four patients died from septic shock (2 were taking azathioprine and 2, infliximab), 2 of myocardial infarction, 1 of disseminated tuberculosis (taking infliximab and methotrexate). In 2 patients, we could not determine the cause of the death.
Table 6.
Clinical characteristics of CD patients who died
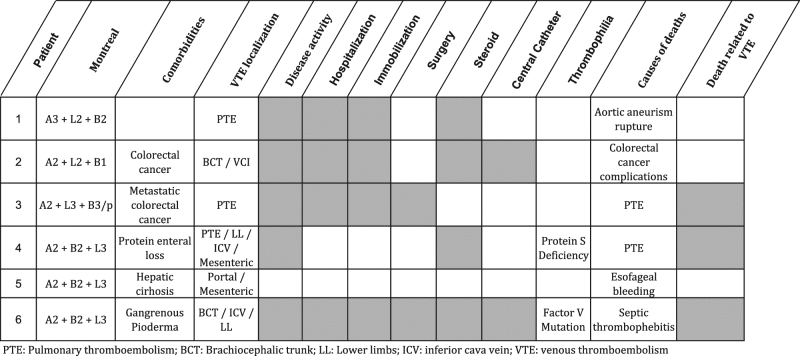
|
PTE pulmonary thromboembolism, BCT brachiocephalic trunk, LL lower limbs, ICV inferior cava vein, VTE venous thromboembolism
Discussion
The prevalence of thrombosis in our center is in line with other studies, affecting 5.1% of patients with IBD enrolled in this cohort. Thirty patients (53.6%) had VTE as an outpatient complication, half of them had no evidence of clinical disease activity, whereas one-third of them had no clinical symptoms of thrombosis, and the diagnosis was made by routine exams.
The Third European Evidence-based Consensus11 advocates that VTE prophylaxis should be considered for all patients with Crohn’s disease, both hospitalized and outpatients with severe disease. For UC, they recommended it for all patients admitted to the hospital, but they also considered it following hospital discharge or surgery, and for outpatients with active disease, but without a clear statement to whom it may be indicated. The Canadian Consensus12 recommends thromboprophylaxis with heparin for all patients hospitalized due to IBD flares without active bleeding or with non-severe bleeding and for outpatients, during moderate to severe IBD flares, with a previous VTE related to exacerbation of the disease itself. Nevertheless, there is significant variation in reported practices for VTE prophylaxis in IBD patients among gastroenterologists13.
In this study, 10.3% of patients had previous thrombosis, and half of outpatients had clinical activity, demonstrated by medical impression and CRP values. Thus, a significant proportion of patients would not be suitable for the actual outpatient VTE prophylaxis recommendation. Likewise, VTE prophylaxis, besides its proven efficacy, could not be enough to prevent all VTE in hospitalized patients with active disease because most of these events happened while they were receiving heparin thromboprophylaxis. One possible explanation could be the evidence in the literature reporting that IBD patients may develop some degree of anti-thrombin deficiency14, which might impact the heparin response. The measurement of anti-thrombin was available only in 16 patients, and it was deficient in four of them (37%).
The associated factors related to thrombosis were smoking (with an adjusted OR of 7.4), S protein deficiency (with an adjusted OR of 13.3) and steroid use (with an adjusted OR of 403). To date, the physiopathology of thrombosis in IBD has not been fully elucidated. Previous studies suggest that the hypercoagulable state in IBD patients is explained by elevated levels of pro-coagulant factors and reduced levels of endogenous anticoagulants (e.g., C and S protein, anti-thrombin, among others). According to the laboratory profile in our study, VTE patients showed S, C protein and anti-thrombin deficiency besides higher platelet and CRP levels. Therefore, we can infer that CD patients with thrombosis might have a higher inflammation status, confirmed by higher use of steroids in this subgroup. Smoking status is a well-known VTE risk factor.
In our patients, a significantly higher proportion of deaths occurred in patients with Crohn’s disease who had suffered VTE (15.8% vs. 1.4%, p = 0.001), which is in agreement with the high mortality rate among IBD patients following an acute VTE15–17. After regression modeling, the factors associated to death were age at diagnosis (with an adjusted OR 0.34), use of biologics (with an adjusted OR of 0.2) and VTE episodes (with an adjusted OR of 11.4). The early age could be related with a more aggressive disease, and the protective factor of biologic drugs could be associated with a better control of the systemic inflammation. Of note, studies on long-term mortality following VTE are sparse. This information reinforces the importance of being aware of the individual risk factors to make it possible to better stratify the patients to introduce an adequate VTE prophylaxis even in case of outpatients.
As all retrospective studies, the study has limitations regarding data collection. We cannot infer causality from the results and, as it was performed in a tertiary hospital, it might have suffered selection bias. Furthermore, because it was a retrospective study, we did not evaluate the endoscopic activity, which may be a subgroup of increased risk regarding outpatients.
Conclusion
This study shows that IBD patients have a higher risk of thrombosis, which was associated with a higher mortality risk. A significant proportion of these events occurred in patients without a clear recommendation for thromboprophylaxis by most guidelines or in those receiving heparin prophylaxis. Taken together, these data suggest that new approaches are needed to reduce the risk of VTE, thus diminishing morbidity and mortality rates in this specific population.
Study Highlights
What is current knowledge
Inflammatory bowel disease (IBD) patients have an increased risk of venous thromboembolism (VTE).
VTE is associated with high morbidity and mortality.
Prophylaxis is required for hospitalized IBD patients.
What is new here
VTE occurred in patients without prophylaxis recommendation.
Current prophylaxis is insufficient to avoid VTE.
Patients may develop VTE despite the use of heparin prophylaxis.
Competing interests
Guarantor of the article: André Zonetti de Arruda Leite.
Specific author contributions: A.R.A.: contributed to the study design, implemented the study protocol, collected and interpreted the data, wrote the first draft of the manuscript, contributed to subsequent revisions, and contributed to its intellectual content; L.L.B.: oversaw the implementation of the study protocol and contributed to its intellectual content; M.F.C.A., A.S.C., A.O.M.C.D., A.M.S.: contributed to the implementation of the study protocol and collected the data; A.Z.A.L.: conceived and designed the study, oversaw the study implementation and data collection, interpreted the data, contributed to the writing of the first draft of the manuscript and its subsequent revisions and contributed to its intellectual content. A.R.A. and A.Z.A.L. are the guarantors of this work and, as such, had full access to all data in the study and take full responsibility for the integrity of data and the accuracy of data analysis. The final draft submitted has been approved by each author of this study.
Financial support: Grant #2015/06196-0, São Paulo Research Foundation (FAPESP).
Potential competing interests None.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Heit JA. The epidemiology of venous thromboembolism in the community: implications for prevention and management. J. Thromb. Thrombolysis. 2006;21:23–29. doi: 10.1007/s11239-006-5572-y. [DOI] [PubMed] [Google Scholar]
- 2.Scoville EA, et al. Venous thromboembolism in patients with inflammatory bowel diseases: a case–control study of risk factors. Inflamm. Bowel Dis. 2014;20:631–636. doi: 10.1097/MIB.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LaMori, J. C., Shoheiber, O., Mody, S. H. & Bookhart, B. K. Inpatient resource use and cost burden of deep vein thrombosis and pulmonary embolism in the United States. Clin Ther.37, 62–70 (2015). [DOI] [PubMed]
- 4.Yusuf H. R., Tsai J., Atrash H. K., Boulet S. & Grosse S. D. Venous thromboembolism in adult hospitalizations—United States, 2007–2009. MMWR. 22, 401–4 (2012). [PubMed]
- 5.Irving PM, Pasi KJ, Rampton DS. Thrombosis and inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2005;3:617–628. doi: 10.1016/S1542-3565(05)00154-0. [DOI] [PubMed] [Google Scholar]
- 6.Kohoutova D, Moravkova P, Kruzliak P, Bures J. Thromboembolic complications in inflammatory bowel disease. J. Thromb. Thrombolysis. 2015;39:489–498. doi: 10.1007/s11239-014-1129-7. [DOI] [PubMed] [Google Scholar]
- 7.Fabio F, Lykoudis P, Gordon P. Thromboembolism in inflammatory bowel disease: an insidious association requiring a high degree of vigilance. Semin. Thromb. Hemost. 2011;37:220–225. doi: 10.1055/s-0031-1273086. [DOI] [PubMed] [Google Scholar]
- 8.Novacek G, et al. Inflammatory bowel disease is a risk factor for recurrent venous thromboembolism. Gastroenterolgy. 2010;139:779–787.e1. doi: 10.1053/j.gastro.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen GC, et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. 2014;146:835–848.e6. doi: 10.1053/j.gastro.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 10.Grainge, M. J., West, J. & Card, T. R. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet375, 657–663 (2010). [DOI] [PubMed]
- 11.Gionchetti P, et al. Third European evidence-based consensus on the diagnosis and management of crohn’s disease 2016: part 2: surgical management and special situations. J. Crohn’s Colitis. 2017;11:135–149. doi: 10.1093/ecco-jcc/jjw169. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen, G. C. et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology146, 835–848 (2014). [DOI] [PubMed]
- 13.Sam JJ, Bernstein CN, Razik R, Thanabalan R, Nguyen GC. Physicians’ perceptions of risks and practices in venous thromboembolism prophylaxis in inflammatory bowel disease. Dig. Dis. Sci. 2013;58:46–52. doi: 10.1007/s10620-012-2435-6. [DOI] [PubMed] [Google Scholar]
- 14.Higaki S, et al. Clinical significance of measuring blood coagulation factor XIIIA regularly and continuously in patients with Crohn’s disease. J. Gastroenterol. Hepatol. 2006;21:1407–1411. doi: 10.1111/j.1440-1746.2006.04319.x. [DOI] [PubMed] [Google Scholar]
- 15.Fumery M, et al. Thromboembolic events and cardiovascular mortality in inflammatory bowel diseases: a meta-analysis of observational studies. J. Crohn’s Colitis. 2014;8:469–479. doi: 10.1016/j.crohns.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen GC, Sam J. Rising prevalence of venous thromboembolism and its impact on mortality among hospitalized inflammatory bowel disease patients. Am. J. Gastroenterol. 2008;103:2272–2280. doi: 10.1111/j.1572-0241.2008.02052.x. [DOI] [PubMed] [Google Scholar]
- 17.Bollen L, et al. Thromboembolism as an important complication of inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2016;28:1–7. doi: 10.1097/MEG.0000000000000495. [DOI] [PubMed] [Google Scholar]


