Abstract
Background
A 9-valent human papillomavirus (HPV6/11/16/18/31/33/45/52/58; 9vHPV) vaccine was developed to expand coverage of the previously developed quadrivalent (HPV6/11/16/18; qHPV) vaccine.
Methods
Efficacy, immunogenicity, and safety outcomes were assessed in Latin American participants enrolled in 2 international studies of the 9vHPV vaccine, including a randomized, double-blinded, controlled with qHPV vaccine, efficacy, immunogenicity, and safety study in young women aged 16–26 years, and an immunogenicity and safety study in girls and boys aged 9–15 years. Participants (N=5312) received vaccination at Day 1, Month 2, and Month 6. Gynecological swabs were collected regularly in young women for cytological and HPV DNA testing. Serum was analyzed for HPV antibodies in all participants. Adverse events (AEs) were also monitored in all participants.
Results
The 9vHPV vaccine prevented HPV 31-, 33-, 45-, 52-, and 58-related high-grade cervical, vulvar, and vaginal dysplasia with 92.3% efficacy (95% confidence interval 54.4, 99.6). Anti-HPV6, 11, 16, and 18 geometric mean titers at Month 7 were similar in the 9vHPV and qHPV vaccination groups. Anti-HPV antibody responses following vaccination were higher among girls and boys than in young women. Most (>99%) 9vHPV vaccine recipients seroconverted for all 9 HPV types at Month 7. Antibody responses to the 9 HPV types persisted over 5 years. The most common AEs were injection-site related, mostly of mild to moderate intensity.
Conclusions
The 9vHPV vaccine is efficacious, immunogenic, and well tolerated in Latin American young women, girls, and boys. These data support 9vHPV vaccination programs in Latin America, a region with substantial cervical cancer burden.
Abbreviations: 9vHPV, 9-valent human papillomavirus; AE, adverse event; Cl, confidence interval; CIN, cervical intraepithelial neoplasia; GMT, geometric mean titer; HPV, human papillomavirus; HPV-9 cLIA, 9-valent competitive Luminex immunoassay; mMU, milli-Merck units; PCR, polymerase chain reaction; PPE, per-protocol efficacy; PPI, per-protocol immunogenicity; qHPV, quadrivalent human papillomavirus; SD, standard deviation; WHO, World Health Organization
Keywords: Human papillomavirus, Vaccine, Cervical cancer, Persistent infection, 9vHPV
1. Introduction
In Latin America (including Mexico, Central America, South America, and the Caribbean), nearly 69,000 new cases of cervical cancer and approximately 29,000 deaths related to the disease are reported annually, according to 2012 estimates [1]. This scenario makes cervical cancer the second most frequent cancer and second most frequent cause of cancer-related mortality among women in this region [1]. Nearly all cases of cervical cancer are caused by human papillomavirus (HPV). Approximately 78,000 HPV-related cancers are reported annually in Latin America, primarily comprised of cervical cancers as well as smaller numbers of vulvar, vaginal, anal, penile, and oropharyngeal cancers [2].
Despite the documented decline in cervical cancer age-standardized incidence and mortality rates in several countries in the past decades, in Latin America—with an estimated population of >320 million women—cervical cancer continues to represent an important burden; yet, large variations between and within countries are observed [3]. Age-standardized incidence rates range between 29.7 per 100,000 women in French Guyana (comparable with those of some sub-Saharan countries; period: 2003–2008) and 10.6 per 100,000 women in Cuba (period: 2004–2007), while age-standardized mortality rates range between 17.4 per 100,000 women in Belize and 6 per 100,000 women in Costa Rica (period for both countries: 2003–2007). These variations are likely related to differences in socioeconomic factors, including limitations in healthcare access and specific risk factors [3], [4]. In high-income countries, well-developed, organized screening programs with robust infrastructure, high coverage, centralized screening, high participation rates, and optimal follow-up have been highly successful in reducing cervical cancer incidence and mortality rates [5]. This has been particularly evident in some northern European countries, where robust screening programs have been in place since the 1960s and age-standardized cervical cancer incidence rates correspondingly decreased in recent years [4]. In the past decades, many Latin American countries have implemented opportunistic cytology-based screening programs, while some have also introduced HPV screening tests or visual inspection with acetic acid; however, the impact of these screening initiatives on the burden of disease has been low. This is related to the fact that they are, for the most part, opportunistic, as opposed to organized and sustainable programs (associated with a higher cost). Furthermore, large limitations exist in the systematic evaluation of the performance of these programs. Also contributing to the low impact of HPV screening initiatives are barriers that inhibit women from accessing diagnosis and treatment services. These barriers can include: limited knowledge about cervical cancer, the relevance of its early detection, and/or the role of screening programs; a lack of healthcare infrastructure and trained healthcare providers; and socio-religious/cultural factors; among others. Cytology-based screening program coverage in Latin America has not translated into reduction in cervical cancer mortality rates as cervical cancer mortality remained almost stable in most countries in the region in spite of the fact that all countries have implemented screening programs with different extents of coverage and levels of organization [3]. Projections based on current trends estimate that the number of cervical cancer-related deaths will increase by 60% from 2012 to 2030, highlighting the need for implementation or improvement of effective prevention programs [6].
The identification of HPV as a primary cause of anogenital cancers created an opportunity for disease prevention through vaccination. In clinical studies, the quadrivalent HPV (qHPV; types 6/11/16/18) vaccine and the bivalent HPV (types 16/18) vaccine prevented HPV16/18-related high-grade cervical dysplasia; the qHPV vaccine was also shown to prevent HPV16/18-related high-grade vulvar, vaginal, and anal dysplasia, plus HPV6/11-related genital warts [7]. Based on these results, HPV vaccination has been implemented as part of national vaccination programs in at least 80 countries globally [8]. In the 10 years following the introduction of the qHPV vaccine, studies assessing vaccine impact in real-world settings have demonstrated dramatic reductions (up to 90%) in the prevalence of HPV6/11/16/18-related infections among young women falling into age groups targeted by national vaccination programs, particularly in countries with high vaccine uptake [9], [10]. Corresponding decreases of up to approximately 90% in prevalence of genital warts and of up to 45% and 85% of low- and high-grade cervical lesions, respectively, have been observed in the years following vaccine availability [9]. Similarly, the introduction of the bivalent HPV vaccine was followed by marked reductions in HPV16/18-related infection and cervical disease [11].
The introduction of prophylactic HPV vaccination over the past 10 years has been recognized as a unique opportunity to reduce the burden of HPV-associated disease in Latin America. According to a report issued by the Technical Advisory Group on Vaccine-Preventable Diseases of the Pan American Health Organization, as of June 2015, HPV vaccination has been incorporated into publicly funded immunization programs in 23 countries and territories in the Americas, making HPV immunization available for an estimated 85% of a typical birth cohort of adolescent girls (N = 6.5 million) [12].
Among countries that offer the HPV vaccine through national immunization programs, on average, middle-/low-income countries have achieved higher vaccination coverage levels than high-income countries [5]. In Latin America, the reported estimated coverage among the targeted population (all ages) as of October 2014 was 71% (95% confidence interval [CI] 43.6, 100.0) [5]. As of June 2017, most national immunization programs in this region target girls between 9 and 12 years of age, and—for the most part—comprise a 0- to 6-month, 2-dose schedule (exceptions include Chile [0- to 12-month schedule], Uruguay and Paraguay [0–2–6-month schedule]) (see Supplementary Table 1). National immunization programs in Antigua, Argentina, Bermuda, Brazil, and Panama cover both girls and boys (gender-neutral vaccination).
The qHPV and bivalent HPV vaccines are designed to cover oncogenic HPV types 16 and 18, which cause ~70% of cervical cancer cases worldwide [13]; the qHPV vaccine also covers HPV types 6 and 11, which are responsible for 90% of genital warts cases [14]. Although partial and inconsistent cross-protection against other phylogenetically related oncogenic HPV types has been observed for both vaccines in some clinical studies, its extent, duration, and public health significance remain uncertain [7], [11], [15], [16], [17], [18]. Population-level assessment in real-world public health programs where high coverage has occurred showed varied levels of cross-protection [16], [17], [18]. A 9-valent HPV (9vHPV; types 6/11/16/18/31/33/45/52/58) vaccine (Gardasil 9, Merck & Co., Inc., Kenilworth, NJ, USA) was developed to provide protection against the oncogenic HPV types already covered by the existing HPV vaccines (16 and 18) and extend coverage to the 5 next most common HPV types associated with cervical cancer worldwide, including Latin America (31/33/45/52/58) [13], [19]. Globally, the 9vHPV vaccine has the potential to prevent approximately 90% of cervical, vulvar, and vaginal cancers in women, 90% of anal cancers and genital warts in women and men, and 70–85% of cervical precancers [14], [20], [21], [22]. A recent epidemiological study that assessed the proportion of cervical intraepithelial neoplasia (CIN) cases attributed to 14 HPV types at a regional level reported that approximately 88% of HPV-positive cervical cancer cases and 83% of CIN2/3 lesions in women aged 15–26 years (78% in women aged 24–45 years) were attributed to the 7 high-risk HPV types covered by the 9vHPV vaccine in Latin America [23].
In an international study involving young women aged 16–26 years, the 9vHPV vaccine elicited non-inferior anti-HPV6/11/16/18 antibody responses compared with the qHPV vaccine and prevented HPV31/33/45/52/58-related persistent infection and disease [24], [25]. In another international study, the 9vHPV vaccine induced anti-HPV antibody responses in girls and boys 9–15 years of age that were non-inferior to responses in young women 16–26 years of age for all 9 vaccine HPV types [26]. Results from this trial supported bridging the 9vHPV vaccine efficacy results in young women 16–26 years of age to girls and boys 9–15 years of age [26]. The present subgroup analysis demonstrates the efficacy, immunogenicity, and safety of the 9vHPV vaccine in these 2 studies focused on participants from Latin America. It includes participants from Brazil, Chile, Colombia, Costa Rica, Mexico, and Peru.
2. Methods
2.1. Study design and population
Study 001 (Protocol V503-001; NCT00543543) was a phase II/III double-blind, randomized, controlled (with qHPV vaccine), dose-ranging, efficacy, immunogenicity, and safety study of the 9vHPV vaccine in women 16–26 years of age. The study design and primary results have been reported [24], [25], [27], [28], [29]. Vaccine efficacy was assessed in 14,215 participants from 18 countries, including 4744 participants (33.4%) from Latin America enrolled from 5 countries and 20 sites (Brazil: 3 sites, 765 participants; Chile: 1 site, 140 participants; Colombia: 12 sites, 2399 participants; Mexico: 2 sites, 696 participants; Peru: 2 sites, 744 participants). The participants who received either the low- or high-dose formulations of 9vHPV vaccine during the dose-selection phase of the study [29] (Colombia: n = 100; Mexico: n = 85; Peru: n = 41) were excluded from this analysis.
Study 002 (Protocol V503-002; NCT00943722) was a phase III study that evaluated the safety and immunogenicity of the 9vHPV vaccine in girls and boys 9–15 years of age with a comparison with young women 16–26 years of age. The design and primary results of this trial have been reported [26], [30]. The study enrolled 3074 participants from 17 countries, including 628 participants (20.4%) from 5 countries in Latin America (Brazil: 3 sites, 50 girls; Chile: 1 site, 40 girls; Colombia: 2 sites, 143 girls, 100 boys, 60 women; Costa Rica: 3 sites, 75 girls; Peru: 2 sites, 100 girls, 60 boys). Since a limited number of Latin American young women (n = 60) were enrolled from only 1 site in 1 country, this was not deemed an optimal comparator for the larger and more diverse group of Latin American girls and boys (n = 568; 5 countries, 11 sites). For this reason, the vaccine immunogenicity and safety in Latin American girls and boys were compared with the larger and more diverse group of Latin American young women (n = 4744; 5 countries, 20 sites) participating in Study 001. Moreover, while girls and boys were assessed for antibody persistence for 3 years, young women in Study 002 were assessed for immunogenicity only at Month 7 (1 month following completion of vaccination) and terminated the study at Month 12 [26]; thus, they cannot be a comparator for later time points. In contrast, young women from Study 001 are a suitable group for comparison of HPV antibody responses at later time points.
2.2. Vaccination and follow-up
Participants in Study 001 received a 3-dose regimen of 9vHPV vaccine or qHPV vaccine at Day 1, Month 2, and Month 6. Participants in Study 002 received a 3-dose regimen of 9vHPV vaccine at Day 1, Month 2, and Month 6.
For the efficacy evaluation in Study 001, cervical cytological samples for Pap testing, and cervical and external genital swabs for HPV polymerase chain reaction (PCR) testing were collected at Day 1, Month 7, Month 12, and every 6 months thereafter up to Month 54. Subjects with cytological abnormalities underwent colposcopy based on a protocol-specified algorithm. Tissue samples were adjudicated for a pathology diagnosis by a blinded pathology panel and tested by PCR for presence of HPV DNA as previously described [25], [28].
In Study 001, serum samples were collected at Day 1, Months 3, 7, 12, 24, 36, and 42 for assessment of HPV antibody responses. A subset of participants (including 95 participants randomly selected at 3 sites in Latin America) who received 3 doses of the 9vHPV vaccine were assessed for HPV antibody responses at Month 60 in a study extension [31]; participants in the extension then received a fourth dose of 9vHPV vaccine and were assessed for HPV antibodies at 1 week and 1 month following the fourth dose to determine whether re-exposure to the antigen via a challenge dose of the vaccine led to the development of an anamnestic response, a hallmark of immune memory. This evaluation is consistent with the guidelines set forth by the World Health Organization (WHO) requiring the assessment of induction of immune memory as part of the development of prophylactic HPV vaccines [32]. In Study 002, serum samples were collected at Day 1 and Months 7, 12, 24, and 36 [26]. Antibody responses to the 9 vaccine HPV types were assessed using a 9-valent competitive Luminex immunoassay (HPV-9 cLIA) [33].
All participants who received at least 1 study vaccination in Studies 001 and 002 and had follow-up data were included in the safety evaluation [24], [26], [34]. Briefly, beginning after each study vaccination and 15 days following, participants recorded injection-site and systemic adverse events (AEs) on a vaccination report card. Investigators were asked to assign causality to AEs based on exposure, time course, likely cause, and consistency with the vaccine's known safety profile. A serious AE was predefined as any AE that resulted in death; was deemed life-threatening; resulted in a persistent or significant disability or incapacity; resulted in or prolonged an existing inpatient hospitalization; or was a congenital anomaly, cancer, or “other important medical event”.
2.3. Statistical analysis
Vaccine efficacy was evaluated in Study 001 using the per-protocol efficacy (PPE) population. The PPE population consisted of participants who: (1) were seronegative at Day 1 and PCR-negative from Day 1 through Month 7 for the HPV type being analyzed, (2) received all 3 doses of the correct clinical material within 1 year, and (3) had no protocol violation that could interfere with the evaluation of vaccine efficacy as judged by the study director. The combined incidence of HPV31-, 33-, 45-, 52-, and 58-related persistent infections (≥6 months ±1 month visit window) and by HPV type, as well as the combined incidence of HPV31-, 33-, 45-, 52-, and 58-related cervical, vulvar, and vaginal disease are presented using incidence rates (cases per 10,000 person-years), vaccine efficacy, and the corresponding 95% CI. Vaccine efficacy was calculated as 100 × ([1−(9vHPV incidence rate / qHPV incidence rate]). The 95% CI of vaccine efficacy was calculated with the use of a binomial distribution-based exact method [35].
Immunogenicity was evaluated in the per-protocol immunogenicity (PPI) population in both studies. The PPI population included participants who: (1) were seronegative on Day 1 and [for young women aged 16–26 years] PCR-negative from Day 1 through Month 7 for the HPV type being analyzed, (2) received all 3 vaccinations within pre-specified visit intervals and had available Month 7 serology results obtained within a pre-specified interval, and (3) had no protocol violations that could interfere with the evaluation of the immune response to vaccine as judged by the study director. For each HPV type, the geometric mean titers (GMTs) and the corresponding 95% CIs were estimated using an analysis of variance model with log anti-HPV as the response and vaccination group as the fixed effect. Seroconversion rates and exact 95% CIs for a binomial proportion were also calculated. All of these immunogenicity evaluations were exploratory in nature; therefore, no statistical tests of hypotheses were performed. Non-overlapping 95% CIs were used as indicators of differences of immune response. Non-inferiority of HPV6/11/16/18 antibody response in the 9vHPV versus qHPV vaccine group in Study 001 and HPV6/11/16/18/31/33/45/52/58 antibody response in girls and boys versus young women in Study 002 was demonstrated in the overall study population [24], [26]; these analyses were pre-specified in the protocol only for the overall study population. Thus, non-inferiority analyses of immunogenicity were not conducted for the subgroups reported herein.
This regional analysis of safety data, focused on Latin American participants, provides a cross-study summary of AEs described as frequencies and percentages across study group and type of event.
3. Results
3.1. Participants
Baseline characteristics of participants from Latin America are summarized in Table 1. The majority of participants in Study 001 were sexually active; 98.7% of subjects in the 9vHPV vaccine group and 98.9% in the qHPV vaccine group had at least 1 lifetime sexual partner at study onset. The mean age at first sexual intercourse was 17.5 years in both vaccine groups. The proportion of participants from Study 001 who tested positive for HPV by PCR or serology at enrollment is shown in Table 2 and Supplementary Table 2. Study 001 participants were enrolled in the study over a period of more than 1 year and, therefore, had various durations of follow-up at the end of the study; participants from Latin America were followed-up for efficacy for a maximum of 70.4 months following Dose 1 (median 52.9 months) or 65.0 months following Dose 3 (median 47.4 months).
Table 1.
Baseline characteristics of Latin American participants.
|
Study 001 |
||
|---|---|---|
| Characteristics |
9vHPV vaccine women (N = 2372) aged 16–26 years |
qHPV vaccine women (N = 2372) aged 16–26 years |
| Age, years | ||
| Mean ± SD | 21.9 ± 2.4 | 21.9 ± 2.5 |
| Median | 22.0 | 22.0 |
| Range | 16–26 | 16–26 |
| Age at first sexual intercourse, years | ||
| Mean ± SD | 17.5 ± 2.1 | 17.5 ± 2.1 |
| Race, n (%) | ||
| American Indian or Alaska Native | 0 (0.0) | 0 (0.0) |
| Asian | 3 (0.1) | 1 (0.0) |
| Black or African-American | 127 (5.4) | 115 (4.8) |
| Multiracial | 1815 (76.5) | 1810 (76.3) |
| Native Hawaiian or Other Pacific Islander | 0 (0.0) | 1 (0.0) |
| White | 427 (18.0) | 445 (18.8) |
| Smoking status, n (%) | ||
| Current smoker | 441 (18.6) | 434 (18.3) |
| Former smoker | 82 (3.5) | 80 (3.4) |
| Never smoked | 1848 (77.9) | 1858 (78.3) |
| Unknown | 1 (0.0) | 0 (0.0) |
| Lifetime sexual partners, n (%)a | ||
| 1 | 818 (34.5) | 768 (32.4) |
| 2 | 716 (30.2) | 707 (29.8) |
| 3 | 488 (20.6) | 523 (22.0) |
| 4 | 318 (13.4) | 345 (14.5) |
| >4 | 1 (0.0) | 2 (0.1) |
| Non-HPV–related cervicovaginal infections or sexually transmitted diseases, n (%) | ||
| Any | 140 (5.9) | 160 (6.7) |
| Chlamydia | 140 (5.9) | 157 (6.6) |
| Gonorrhea | 3 (0.1) | 4 (0.2) |
| Contraceptive use, n (%)b | ||
| Barrier | 852 (35.9) | 867 (36.6) |
| Behavior | 359 (15.1) | 368 (15.5) |
| Hormonal | 1274 (53.7) | 1258 (53.0) |
| Day 1 composite HPV positivity, n/total n (%)c | ||
| Serologic test | 1111/2369 (46.9) | 1101/2370 (46.5) |
| PCR assay | 722/2303 (31.4) | 765/2321 (33.0) |
| Serologic test or PCR assay | 1326/2327 (57.0) | 1384/2346 (59.0) |
|
Study 002 |
||
|---|---|---|
| Characteristics |
9vHPV vaccine girls (N = 408) aged 9–15 years |
9vHPV vaccine boys (N = 160) aged 9–15 years |
| Age, years | ||
| Aged 9–12 years | 312 (76.5) | 115 (71.9) |
| Aged 13–15 years | 96 (23.5) | 45 (28.1) |
| Mean ± SD | 11.3 ± 1.8 | 11.4 ± 1.9 |
| Median | 11.0 | 11.0 |
| Range | 9–15 | 9–15 |
| Race, n (%) | ||
| American Indian or Alaska Native | 0 (0.0) | 0 (0.0) |
| Asian | 0 (0.0) | 0 (0.0) |
| Black or African-American | 15 (3.7) | 0 (0.0) |
| Multiracial | 234 (57.4) | 142 (88.8) |
| Native Hawaiian or Other Pacific Islander | 0 (0.0) | 0 (0.0) |
| White | 159 (39.0) | 18 (11.3) |
| Weight, kg | ||
| Mean ± SD | 43.0 ± 11.0 | 43.6 ± 13.2 |
| Median | 42.0 | 40.3 |
| Range | 19.5–82.8 | 15.4–79.4 |
| Body mass index, kg/m2 | ||
| Mean ± SD | 19.5 ± 3.5 | 19.5 ± 3.5 |
| Median | 19.1 | 18.8 |
| Range | 10.5–37.3 | 11.1–35.7 |
9vHPV, 9-valent human papillomavirus; HPV, human papillomavirus; mMU, milli-Merck units; PCR, polymerase chain reaction; qHPV, quadrivalent human papillomavirus; SD, standard deviation.
The percentages for the number of lifetime sexual partners were calculated on the basis of the number of participants for whom there were data on sexual history at enrollment (2372 in the 9vHPV vaccine group and 2372 in the qHPV vaccine group).
Participants may have used more than 1 contraceptive method. A participant is counted once within a category and may be counted in more than 1 category. The percentages for the numbers of participants who used contraceptives were based on the number for whom this information was available (2372 in the 9vHPV vaccine group and 2372 in the qHPV vaccine group).
Positivity by serologic test was defined as an anti-HPV titer on immunoassay of at least 30, 16, 20, 24, 10, 8, 8, 8, and 8 mMU/mL for HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58, respectively. Positivity by PCR assay was defined as positive by HPV type PCR on at least 1 of the following: labial/vulvar/perineal/perianal swab, endo-/ectocervical swab, or (if obtained) an external genital biopsy specimen or cervical biopsy specimen. The numerator in this category represents the number of HPV-positive participants and the denominator represents the total number of participants with assay results that could be evaluated.
Table 2.
Summary of Day 1 HPV status by PCR (14 HPV types) and serology (9 HPV types) in Study 001 by vaccination group.
|
9vHPV vaccine women (N = 2372) aged 16–26 years |
qHPV vaccine women (N = 2372) aged 16–26 years |
|||
|---|---|---|---|---|
| Day 1 status based on PCR | n |
Day 1 PCR positive m (%)a |
n |
Day 1 PCR positive m (%)a |
| HPV6, 11, 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, or 59b | 2297 | 1021 (44.4) | 2313 | 1058 (45.7) |
| HPV6 | 2368 | 100 (4.2) | 2370 | 110 (4.6) |
| HPV11 | 2369 | 17 (0.7) | 2370 | 23 (1.0) |
| HPV16 | 2368 | 242 (10.2) | 2368 | 262 (11.1) |
| HPV18 | 2367 | 111 (4.7) | 2370 | 84 (3.5) |
| HPV31 | 2366 | 141 (6.0) | 2369 | 159 (6.7) |
| HPV33 | 2369 | 46 (1.9) | 2370 | 39 (1.6) |
| HPV35 | 2368 | 61 (2.6) | 2369 | 53 (2.2) |
| HPV39 | 2368 | 145 (6.1) | 2369 | 144 (6.1) |
| HPV45 | 2369 | 90 (3.8) | 2370 | 86 (3.6) |
| HPV51 | 2369 | 196 (8.3) | 2369 | 194 (8.2) |
| HPV52 | 2369 | 194 (8.2) | 2368 | 200 (8.4) |
| HPV56 | 2368 | 290 (12.2) | 2368 | 282 (11.9) |
| HPV58 | 2367 | 135 (5.7) | 2367 | 118 (5.0) |
| HPV59 |
2369 |
132 (5.6) |
2369 |
127 (5.4) |
|
Day 1 status based on serology |
n |
Day 1 seropositive m (%)c |
n |
Day 1 seropositive m (%)c |
| HPV6, 11, 16, 18, 31, 33, 45, 52, or 58b | 2369 | 1111 (46.9) | 2370 | 1101 (46.5) |
| HPV6 | 2369 | 479 (20.2) | 2369 | 478 (20.2) |
| HPV11 | 2369 | 117 (4.9) | 2370 | 127 (5.4) |
| HPV16 | 2369 | 352 (14.9) | 2370 | 349 (14.7) |
| HPV18 | 2369 | 138 (5.8) | 2370 | 144 (6.1) |
| HPV31 | 2369 | 269 (11.4) | 2370 | 257 (10.8) |
| HPV33 | 2369 | 104 (4.4) | 2370 | 116 (4.9) |
| HPV45 | 2369 | 55 (2.3) | 2370 | 54 (2.3) |
| HPV52 | 2369 | 173 (7.3) | 2370 | 190 (8.0) |
| HPV58 | 2369 | 290 (12.2) | 2370 | 278 (11.7) |
N = Number of participants randomized to the respective vaccination group who received at least 1 injection.
n = Number of participants contributing to the analysis.
m = Number of participants who are positive at Day 1 with respect to the relevant method of assessment of HPV status.
9vHPV, 9-valent human papillomavirus; HPV, human papillomavirus; mMU, milli-Merck units; PCR, polymerase chain reaction; qHPV, quadrivalent human papillomavirus.
Positive by PCR is defined as positive by HPV type PCR at Day 1 on at least 1 of the following: labial/vulvar/perineal and perianal swab, endo-/ectocervical swab, or (if obtained) an external genital biopsy specimen or cervical biopsy specimen.
Non-positive participants who were missing results for 1 or more types are not counted. Percentages are calculated as 100*(m/n).
Percent seropositive represents the proportion of participants with anti-HPV serum levels ≥30, 16, 20, 24, 10, 8, 8, 8, and 8 mMU/mL at Day 1 for HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58, respectively.
3.2. Efficacy against HPV-related infection and disease
In Study 001, the efficacy of the 9vHPV vaccine with respect to HPV31-, 33-, 45-, 52-, and 58-related endpoints was 92.3% (95% CI 54.4, 99.6) for high-grade cervical, vulvar, and vaginal disease; 90.9% (95% CI 46.4, 99.6) for high-grade cervical disease; 98.0% (95% CI 88.9, 99.9) for any grade of cervical disease; 93.7% (95% CI 61.4, 99.7) for any grade of vulvar and vaginal disease (Table 3). Efficacy was 95.2% (95% CI 92.7, 97.0) for 6-month persistent infection related to HPV31/33/45/52/58. The vaccine's efficacy was robust (ranging from 92.4% to 100%) for 6-month persistent infection endpoints related to each of the 5 HPV types (Table 3). Efficacy for all these endpoints was within the ranges previously reported in the overall study population [24].
Table 3.
Summary of efficacy against HPV31-/33-/45-/52-/58-related persistent infection at ≥6 months (±first month visit window) and cervical, vulvar, and vaginal disease in the PPE population in Study 001 by vaccination group.
|
9vHPV vaccine women (N = 2372) aged 16–26 years |
qHPV vaccine women (N = 2372) aged 16–26 years |
Vaccine efficacy (95% CI) | |||
|---|---|---|---|---|---|
| Cases/n | Cases/10000 person-years | Cases/n | Cases/10000 person-years | % | |
| HPV31-/33-/45-/52-/58-related persistent infection ≥6 months (±1 month visit window)a | 22 / 2010 | 32.5 | 414 / 2029 | 673.0 | 95.2 (92.7, 97.0) |
| by HPV type | |||||
| HPV31-related persistent infection | 7 / 1696 | 12.1 | 89 / 1684 | 160.3 | 92.4 (84.3, 96.5) |
| HPV33-related persistent infection | 0 / 1888 | 0.0 | 30 / 1891 | 47.4 | 100 (89.0, 100) |
| HPV45-related persistent infection | 1 / 1879 | 1.6 | 65 / 1887 | 103.9 | 98.5 (92.0, 99.9) |
| HPV52-related persistent infection | 7 / 1721 | 12.0 | 187 / 1672 | 349.8 | 96.6 (92.9, 98.4) |
| HPV58-related persistent infection | 7 / 1668 | 12.3 | 125 / 1680 | 226.6 | 94.6 (89.0, 97.5) |
| HPV31-/33-/45-/52-/58-related cervical, vulvar, and vaginal disease (any grade) | 2 / 2032 | 2.8 | 64 / 2052 | 89.4 | 96.9 (89.2, 99.5) |
| HPV31-/33-/45-/52-/58-related high-grade cervical, vulvar, and vaginal diseaseb | 1 / 2032 | 1.4 | 13 / 2052 | 18.0 | 92.3 (54.4, 99.6) |
| By lesion type (HPV31-/33-/45-/52-/58-related) | |||||
| Cervical disease (any grade) | 1 / 2009 | 1.5 | 49 / 2018 | 71.9 | 98.0 (88.9, 99.9) |
| CIN1 | 0 / 2009 | 0.0 | 45 / 2018 | 66.0 | 100 (92.5, 100) |
| CIN2/3, AIS, and cervical cancer | 1 / 2009 | 1.5 | 11 / 2018 | 16.1 | 90.9 (46.4, 99.6) |
| Vulvar and vaginal disease (any grade) | 1 / 2028 | 1.4 | 16 / 2051 | 22.3 | 93.7 (61.4, 99.7) |
| Condyloma | 0 / 2028 | 0.0 | 3 / 2051 | 4.2 | 100 (−72.3, 100) |
| VIN1 or VaIN1 | 1 / 2028 | 1.4 | 12 / 2051 | 16.7 | 91.6 (51.2, 99.6) |
| High-grade vulvar and vaginal disease | 0 / 2028 | 0.0 | 2 / 2051 | 2.8 | 100 (−248.9, 100) |
The per-protocol efficacy population consisted of participants who were seronegative at Day 1 and PCR-negative from Day 1 through Month 7 for the vaccine HPV type being analyzed, received all 3 doses of the correct clinical material within 1 year, and had no protocol violation that could interfere with the evaluation of vaccine efficacy as judged by the study director.
Participants are counted once in each applicable endpoint category. A subject may appear in more than 1 category.
N = Number of participants randomized to the respective vaccination group who received at least 1 injection.
n = Number of participants who have at least 1 follow-up visit after Month 7.
9vHPV, 9-valent human papillomavirus; AIS, adenocarcinoma in situ; CI, confidence interval; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; PCR, polymerase chain reaction; PPE, per-protocol efficacy; VaIN, vaginal intraepithelial neoplasia; VIN, vulvar intraepithelial neoplasia; qHPV, quadrivalent human papillomavirus.
A case of persistent infection occurred if a subject, after completion of the Month 7 visit, is positive for the same HPV type by the HPV31/33/45/52/58 PCR assay to at least 1 common gene in 2 or more consecutive cervicovaginal/external genital swabs, biopsies, or definitive therapy samples obtained at 2 or more consecutive visits at least 6 months (±1-month visit windows) apart.
This endpoint includes high-grade cervical intraepithelial neoplasia, adenocarcinoma in situ, cervical cancer, high-grade vulvar intraepithelial neoplasia, high-grade vaginal intraepithelial neoplasia, vulvar cancer, and vaginal cancer. An endpoint of disease related to a given HPV type occurred if a subject developed a lesion with the relevant consensus diagnosis by the pathology panel, and PCR testing detected the relevant HPV type in an adjacent section of the same tissue block, as previously described [25], [28].
3.3. Immunogenicity
In Study 001, Month 7 GMTs for HPV types 6 and 16 among young women 16–26 years of age were similar in the 9vHPV and qHPV vaccine groups; GMT for HPV11 was lower in the 9vHPV vaccine group than in the qHPV vaccine group and GMT for HPV18 was higher in the 9vHPV vaccine group than in the qHPV vaccine group (Table 4). Similar relative results for antibody GMTs between the 9vHPV and qHPV vaccine groups for these 4 HPV types were observed in the overall study population [24]. GMTs for HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58 at Month 7 were higher in girls and boys 9–15 years of age (Study 002) than in young women 16–26 years of age (Study 001) following 9vHPV vaccination. At Month 7 (i.e. 4 weeks after the third dose) >99.3% of the PPI population in Studies 001 and 002 who received 9vHPV vaccine underwent seroconversion to the 9 HPV types (Table 4).
Table 4.
Summary of anti-HPV cLIA GMTs and seropositivity at Month 7 in the PPI population by vaccination group.
|
Study 002 |
Study 001 |
|||||||
|---|---|---|---|---|---|---|---|---|
|
9vHPV vaccine girls (N = 408) aged 9–15 years |
9vHPV vaccine boys (N = 160) aged 9–15 years |
9vHPV vaccine women (N = 2259) aged 16–26 years |
qHPV vaccine women (N = 2257) aged 16–26 years |
|||||
| Assay (cLIA) | n | GMT (95% CI), mMU/mL | N | GMT (95% CI), mMU/mL | n | GMT (95% CI), mMU/mL | n | GMT (95% CI), mMU/mL |
| Anti-HPV6 | 344 | 1964.6 (1792.1, 2153.8) | 138 | 2697.2 (2332.7, 3118.5) | 1250 | 943.4 (904.5, 983.9) | 1239 | 892.9 (856.0, 931.5) |
| Anti-HPV11 | 344 | 1453.6 (1330.4, 1588.2) | 138 | 1866.5 (1622.9, 2146.6) | 1250 | 681.5 (651.6, 712.8) | 1239 | 835.7 (798.9, 874.3) |
| Anti-HPV16 | 347 | 8190.4 (7528.0, 8911.1) | 138 | 10895.6 (9531.8, 12454.6) | 1296 | 3351.9 (3215.4, 3494.2) | 1307 | 3246.6 (3115.0, 3383.9) |
| Anti-HPV18 | 351 | 2413.5 (2189.9, 2659.9) | 138 | 3752.4 (3213.5, 4381.7) | 1494 | 887.2 (847.9, 928.4) | 1524 | 709.6 (678.4, 742.1) |
| Anti-HPV31 | 346 | 2235.5 (2033.4, 2457.7) | 138 | 2899.7 (2495.7, 3369.1) | 1414 | 722.1 (680.5, 766.2) | 1397 | 10.1 (9.5, 10.7) |
| Anti-HPV33 | 350 | 1069.8 (982.2, 1165.3) | 138 | 1518.3 (1325.0, 1739.7) | 1569 | 428.0 (409.4, 447.4) | 1570 | <4 (<4, <4) |
| Anti-HPV45 | 352 | 901.7 (812.2, 1001.2) | 139 | 1264.1 (1070.3, 1493.1) | 1560 | 290.6 (277.5, 304.3) | 1566 | <3 (<3, <3) |
| Anti-HPV52 | 352 | 1091.0 (996.6, 1194.3) | 139 | 1142.3 (989.0, 1319.3) | 1420 | 387.7 (373.1, 402.9) | 1387 | <3 (<3, <3) |
| Anti-HPV58 |
349 |
1375.8 (1260.1, 1502.0) |
139 |
1868.0 (1625.4, 2146.7) |
1386 |
476.4 (453.6, 500.2) |
1392 |
<4 (<4, 4.2) |
|
Assay (cLIA) |
n |
Seropositive (95% CI), % |
n |
Seropositive (95% CI), % |
n |
Seropositive (95% CI), % |
n |
Seropositive (95% CI), % |
| Anti-HPV6 | 344 | 99.7 (98.4, 100) | 138 | 99.3 (96.0, 100) | 1250 | 100 (99.7, 100) | 1239 | 99.8 (99.4, 100) |
| Anti-HPV11 | 344 | 100 (98.9, 100) | 138 | 100 (97.4, 100) | 1250 | 100 (99.7, 100) | 1239 | 99.9 (99.6, 100) |
| Anti-HPV16 | 347 | 100 (98.9, 100) | 138 | 100 (97.4, 100) | 1296 | 100 (99.7, 100) | 1307 | 99.9 (99.6, 100) |
| Anti-HPV18 | 351 | 100 (99.0, 100) | 138 | 100 (97.4, 100) | 1494 | 99.9 (99.6, 100) | 1524 | 99.7 (99.3, 99.9) |
| Anti-HPV31 | 346 | 100 (98.9, 100) | 138 | 100 (97.4, 100) | 1414 | 99.9 (99.5, 100) | 1397 | 51.7 (49.0, 54.3) |
| Anti-HPV33 | 350 | 100 (99.0, 100) | 138 | 100 (97.4, 100) | 1569 | 99.7 (99.3, 99.9) | 1570 | 13.0 (11.4, 14.8) |
| Anti-HPV45 | 352 | 100 (99.0, 100) | 139 | 100 (97.4, 100) | 1560 | 99.7 (99.3, 99.9) | 1566 | 11.2 (9.7, 12.9) |
| Anti-HPV52 | 352 | 100 (99.0, 100) | 139 | 100 (97.4, 100) | 1420 | 99.8 (99.4, 100) | 1387 | 3.2 (2.3, 4.2) |
| Anti-HPV58 | 349 | 100 (98.9, 100) | 139 | 100 (97.4, 100) | 1386 | 99.7 (99.3, 99.9) | 1392 | 23.1 (20.9, 25.4) |
The per-protocol immunogenicity population included all participants who were seronegative at Day 1, as well as (young women aged 16–26 years only) PCR-negative Day 1 through Month 7 for the relevant HPV type(s), received all 3 vaccinations within pre-specified visit intervals, and had Month 7 serology results obtained within a pre-specified interval, and had no protocol violations that could interfere with the evaluation of the immune response to vaccine as judged by the study director.
Seropositive percent represents proportion of participants with anti-HPV serum levels ≥30, 16, 20, 24, 10, 8, 8, 8, and 8 mMU/mL for HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58, respectively.
For Study 002, N=Number of participants randomized to the respective vaccination group who received at least 1 injection. For Study 001, N=Number of participants randomized to the respective vaccination group who received at least 1 injection in the immunogenicity substudy cohort (i.e., excluding participants enrolled in the dose selection phase of the study) [25], [27].
n=Number of participants contributing to the analysis.
9vHPV, 9-valent human papillomavirus; CI, confidence interval; cLIA, competitive Luminex immunoassay; GMT, geometric mean titer; HPV, human papillomavirus; mMU, milli-Merck units; PCR, polymerase chain reaction; PPI, per-protocol immunogenicity; qHPV, quadrivalent human papillomavirus.
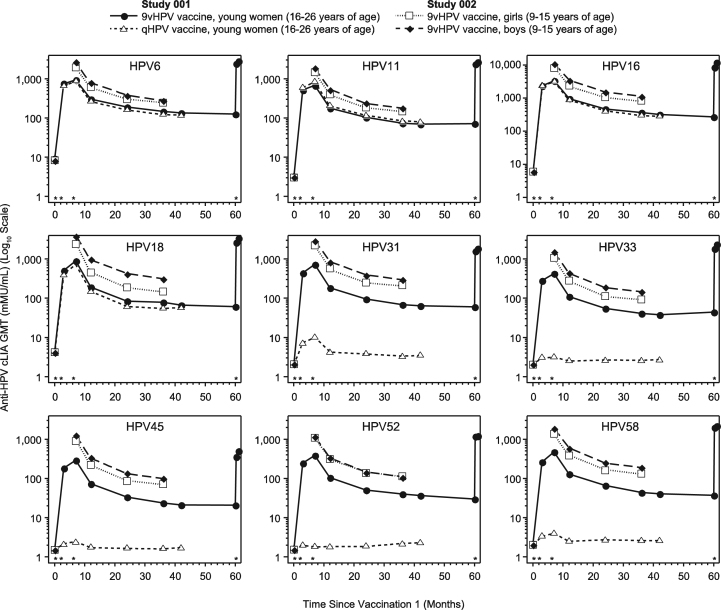
Anti-HPV cLIA GMT levels peaked at Month 7 and decreased over time to reach a plateau around Month 36 for all participants (Fig. 1). In Study 001, anti-HPV6, 11, 16, and 18 GMTs in the 9vHPV and qHPV vaccine groups followed similar kinetics from Month 7 through Month 42. No immunogenicity analysis was conducted in the qHPV vaccine group beyond Month 42 because participants in this group were offered vaccination with the 9vHPV vaccine after the base study was terminated. However, HPV antibody persistence beyond Month 42 was assessed in a subset of subjects (n=150 for the overall study; including 95 subjects from Latin America) from the 9vHPV vaccine group who were followed through Month 60 in a study extension; to assess for induction of immune memory, a challenge dose of 9vHPV vaccine was administered at Month 60, and HPV antibody levels were evaluated 1 week and 1 month later. As seen in Fig. 1, the anti-HPV GMTs persisted through Month 60 among 9vHPV vaccine recipients from Latin America; GMTs rapidly increased within 1 week after a fourth 9vHPV vaccine dose (challenge dose) for all 9 HPV types, yielding higher cLIA GMTs than those observed at Month 7. Among subjects from Latin America enrolled in the study extension, the Month 60 visit occurred between 53.1 and 66.8 months after the third dose (median: 58.9 months).
Fig. 1.
Longitudinal anti-HPV cLIA GMTs in the per-protocol immunogenicity population by vaccination group. Vaccination visits (Day 1 and Months 2, 6, and 60) are represented by asterisks above the horizontal axis of each graph. White squares represent girls 9–15 years of age who received the 9vHPV vaccine in Study 002; black diamonds represent boys 9–15 years of age who received the 9vHPV vaccine in Study 002; black circles represent young women 16–26 years of age who received the 9vHPV vaccine in Study 001; white triangles represent young women 16–26 years of age who received the qHPV vaccine in Study 001. 9vHPV, 9-valent human papillomavirus; cLIA, competitive Luminex immunoassay; GMT, geometric mean titer; HPV, human papillomavirus; qHPV, quadrivalent human papillomavirus.
From Month 7 through Month 36, GMTs remained higher in girls and boys 9–15 years of age (Study 002) than in young women 16–26 years of age (Study 001; Fig. 1). Most girls and boys (92.6% to 100%, Study 002) 9–15 years of age remained seropositive by Month 36, while most young women 16–26 years of age (77.0% to 100%, Study 001) remained seropositive by Month 60 in the 9vHPV vaccine groups.
3.4. Safety
A summary of AEs occurring from Days 1–15 following any vaccination is provided in Table 5. The 3-dose regimen of the 9vHPV vaccine appeared generally well tolerated. The most common AEs in 9vHPV vaccine recipients were injection-site related, which occurred in 86.0%, 76.9%, and 89.6% of girls, boys, and women, respectively. The most common (incidence ≥5%) injection-site–related events were pain, swelling, and erythema. Vaccine-related systemic AEs were reported, respectively, by 27.2%, 37.5%, and 31.4% of girls, boys, and women following 9vHPV vaccine administration; the most common (incidence ≥5%) were headache and pyrexia. The AE profiles of the 9vHPV and qHPV vaccines were generally similar among young women in Study 001; the frequencies of injection-site AEs were higher in the 9vHPV vaccine group (89.6%) than in the qHPV vaccine group (84.2%) and most were mild to moderate in intensity.
Table 5.
AE summary by vaccination group.
|
Study 002 |
Study 001 |
|||
|---|---|---|---|---|
|
9vHPV vaccine girls (N = 408) aged 9–15 years |
9vHPV vaccine boys (N = 160) aged 9–15 years |
9vHPV vaccine women (N = 2364) aged 16–26 years |
qHPV vaccine women (N = 2362) aged 16–26 years |
|
| Event | n (%) | n (%) | n (%) | n (%) |
| Participants with 1 or more AEsa | 367 (90.0) | 138 (86.3) | 2217 (93.8) | 2148 (90.9) |
| Injection-site eventb | 351 (86.0) | 123 (76.9) | 2118 (89.6) | 1988 (84.2) |
| Painc | 346 (84.8) | 122 (76.3) | 2105 (89.0) | 1960 (83.0) |
| Mild | 204 (50.0) | 85 (53.1) | 1168 (49.4) | 1227 (51.9) |
| Moderate | 124 (30.4) | 37 (23.1) | 796 (33.7) | 632 (26.8) |
| Severe | 18 (4.4) | 0 (0.0) | 141 (6.0) | 101 (4.3) |
| Swelling | 160 (39.2) | 42 (26.3) | 836 (35.4) | 608 (25.7) |
| Mild: 0 to ≤2.5 cm | 106 (26.0) | 29 (18.1) | 568 (24.0) | 457 (19.3) |
| Moderate: >2.5 cm to ≤5.0 cm | 35 (8.6) | 6 (3.8) | 176 (7.4) | 111 (4.7) |
| Severe: >5.0 cm | 19 (4.7) | 7 (4.4) | 91 (3.8) | 40 (1.7) |
| Unknown | 0 (0.0) | 0 (0.0) | 1 (0.0) | 0 (0.0) |
| Erythema | 120 (29.4) | 30 (18.8) | 603 (25.5) | 488 (20.7) |
| Mild: 0 to ≤2.5 cm | 107 (26.2) | 25 (15.6) | 494 (20.9) | 419 (17.7) |
| Moderate: >2.5 cm to ≤5.0 cm | 10 (2.5) | 3 (1.9) | 78 (3.3) | 52 (2.2) |
| Severe: >5.0 cm | 3 (0.7) | 2 (1.3) | 29 (1.2) | 17 (0.7) |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pruritusc | 15 (3.7) | 0 (0.0) | 127 (5.4) | 98 (4.1) |
| Mild | 13 (3.2) | 0 (0.0) | 96 (4.1) | 75 (3.2) |
| Moderate | 2 (0.5) | 0 (0.0) | 30 (1.3) | 20 (0.8) |
| Severe | 0 (0.0) | 0 (0.0) | 1 (0.0) | 3 (0.1) |
| Systemic eventd | 201 (49.3) | 88 (55.0) | 1451 (61.4) | 1432 (60.6) |
| Any vaccine-related systemic event | 111 (27.2) | 60 (37.5) | 743 (31.4) | 692 (29.3) |
| Headache | 54 (13.2) | 25 (15.6) | 401 (17.0) | 386 (16.3) |
| Pyrexia | 40 (9.8) | 24 (15.0) | 172 (7.3) | 145 (6.1) |
| Dizziness | 11 (2.7) | 2 (1.3) | 64 (2.7) | 77 (3.3) |
| Nausea | 4 (1.0) | 3 (1.9) | 62 (2.6) | 57 (2.4) |
| Vomiting | 3 (0.7) | 4 (2.5) | 23 (1.0) | 14 (0.6) |
| Serious evente | 0 (0.0) | 2 (1.3) | 7 (0.3) | 7 (0.3) |
| Vaccine-related event | 0 (0.0) | 1 (0.6) | 1 (0.0) | 1 (0.0) |
| Death | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Discontinuation due to AEf | 0 (0.0) | 1 (0.6) | 0 (0.0) | 0 (0.0) |
| Vaccine-related event | 0 (0.0) | 1 (0.6) | 0 (0.0) | 0 (0.0) |
| Serious event | 0 (0.0) | 1 (0.6) | 0 (0.0) | 0 (0.0) |
| Serious vaccine-related event | 0 (0.0) | 1 (0.6) | 0 (0.0) | 0 (0.0) |
Every subject is counted a single time for each applicable specific AE. A subject with multiple AEs within a system organ class is counted a single time for that system organ class. The same subject may appear in different system organ classes.
N = Number of subjects who underwent randomization, received at least 1 dose of vaccine, and had at least 1 follow-up visit related to AE.
n = Number of subjects contributing to the analysis.
9vHPV, 9-valent human papillomavirus; AE, adverse event; qHPV, quadrivalent human papillomavirus.
AEs that were reported within 1–15 days after any vaccination.
Injection-site events were AEs that were reported within 1–5 days after any vaccination. A system organ class or specific AE appears on this table only if its incidence in 1 or more of the columns is greater than or equal to 2% incidence after rounding.
Intensities of pain and itching were defined: as mild if there was an awareness of the sign or symptom but it did not interfere with usual activities, as moderate if there was enough discomfort to cause interference with usual activity, and as severe if the pain or discomfort was incapacitating, rendering the participant unable to work or carry out usual activities.
Systemic events were AEs that were reported within 1–15 days after any vaccination. A system organ class or specific AE appears on this table only if its incidence in 1 or more of the columns is greater than or equal to 2% incidence after rounding.
Serious events were AEs that were reported within 1–15 days after any vaccination.
Discontinuation due to AE was reported within 1–15 days after any vaccination.
Three serious vaccine-related AEs were reported. A 10-year-old boy from Peru (Study 002) with a previous medical history of seasonal allergy and bronchial asthma experienced an asthmatic crisis 1 day after receiving Dose 1 of the 9vHPV vaccine, was hospitalized the next day for medical treatment, and fully recovered the following day; he was discontinued from the study and did not receive additional vaccination. A 26-year-old woman from Brazil (Study 001) experienced fever of 38.6 °C, body pain, headache, and malaise beginning 11 h after receiving Dose 3 of the 9vHPV vaccine; symptoms worsened during the next 12 h, despite symptomatic treatment; she was not hospitalized, and fully recovered the following day. A 23-year-old woman from Colombia (Study 001) developed headache 2 days after receiving Dose 2 of the qHPV vaccine; the headache persisted and was associated with dizziness and nausea; she was hospitalized 54 days later following an episode of intense occipital headache associated with photophobia, nausea, and chills (she had no fever); a brain computed tomography scan revealed no abnormalities; she was treated with naproxen, fully recovered, and was discharged the next day (no laboratory tests were performed).
In both studies, discontinuation from vaccination due to AEs was rare (n = 1 participant from Peru who developed a serious AE of asthmatic crisis, described above). Seven subjects from Latin America (n = 6 from Study 001, n = 1 from Study 002) died during the entire course of the studies (see the Supplement for more information); none of the deaths occurred within 15 days after vaccination and none were considered related to the study vaccine
4. Discussion
A substantial proportion of participants from Latin America were enrolled in 2 pivotal studies of the 9vHPV vaccine clinical program (33.4% [4744 participants] in Study 001, 20.4% [628 participants] in Study 002). This allowed us to conduct a robust efficacy, immunogenicity, and safety analysis on this subset of participants that may contribute to further understanding HPV-related disease prevention in the region and provide useful insight to decision-makers involved in the implementation of HPV vaccine programs.
The 9vHPV vaccine prevented HPV31-, 33-, 45-, 52-, and 58-related high-grade cervical, vulvar, and vaginal disease, as well as persistent infection related to each of these 5 HPV types, based on up to 6 years of follow-up.
Robust immune responses to the 9vHPV vaccine were observed by Month 7, with immunogenicity profiles across the covered HPV types generally consistent with those previously observed in the overall study populations [24], [26]. HPV antibody responses persisted through at least 5 years post-vaccination; administration of a challenge dose of vaccine at that time showed a robust anamnestic antibody response, consistent with the generation of immune memory [36]. The 9vHPV vaccine was generally well tolerated, with only one participant discontinuing due to an AE. No clinically meaningful differences in vaccine efficacy, immunogenicity, and safety were observed upon comparison with published global outcomes of the 9vHPV vaccine clinical program [24], [25], [26], [34].
The studies described in this manuscript have limitations. Study 001 did not use a placebo group for ethical reasons, since the bivalent HPV vaccine and the qHPV vaccine prevent pre-cancerous lesions caused by HPV16 and 18 infection. Thus, the study used the qHPV vaccine as an active comparator. Given the high efficacy of the qHPV vaccine (and the expected high efficacy of the 9vHPV vaccine), a direct comparison based on efficacy was not practical, as very few disease endpoints related to HPV6/11/16/18 were anticipated [28]. Therefore, the demonstration of similar HPV6/11/16/18 antibody responses represents a meaningful result. Of note, supportive analyses conducted in the global study showed comparable incidence of infection and disease related to HPV6/11/16/18 types between the 2 vaccine groups, which further support that the 2 vaccines offer similar protection against HPV6/11/16/18 [24], [25].
Another limitation is that Studies 001 and 002 were limited in duration. Long-term follow-up studies of the qHPV vaccine have shown persistence of protection for at least 10 years post-vaccination, suggesting that the 9vHPV vaccine could also offer long-term protection [37]. In Study 001, the 9vHPV vaccine demonstrated efficacy against HPV6/11/16/18/31/33/45/52/58-related persistent infection, disease, cytological abnormalities, and procedures for up to 6 years [25], and a 10-year, long-term, follow-up extension of Study 001 is under way to assess duration of protection (Protocol V503-021; NCT02653118) [38]. Similarly, a long-term follow-up extension of Study 002 (Protocol V503-002; NCT00943722) aimed to assess antibody persistence and effectiveness through 10 years post-Dose 3 is ongoing.
In Latin America, the 9vHPV vaccine could prevent the majority of HPV-related anogenital cancer cases (including approximately 88% of cervical cancers) [39] and CIN2/3 cases (approximately 83% and 78% in women 15–26 and 24–45 years of age, respectively) [23]. Compared with the qHPV vaccine, the 5 additional HPV types included in the 9vHPV vaccine (31/33/45/52/58) accounted for approximately 33% and 18% of CIN2/3 lesions among Latin American women 15–26 years of age and 24–45 years of age, respectively [23]. This scenario, together with the demonstrated solid safety and efficacy profile of the 9vHPV vaccine, suggest that the inclusion of the 9vHPV vaccine in effectively implemented national immunization programs within Latin America will have a significant impact on the HPV-associated burden of disease, both in countries with HPV vaccination programs and those in which these programs are not yet available. Efficiently implemented, sustainable vaccination programs including the 9vHPV vaccine have the potential to substantially decrease the number of invasive procedures related to the treatment of cervical dysplasia (in countries with cervical cancer screening programs), thereby potentially reducing costs and anxiety associated with such procedures. Notably, a significant impact of HPV vaccination is expected in middle-/low-income countries deficient in organized screening programs, in which current bivalent and qHPV vaccines are estimated to potentially reduce cancer risk by 40–50% at 70% vaccine uptake [23].
There has been increasing interest in vaccinating males in addition to females, both to prevent HPV-related infection and disease among males and to maximize herd effects of vaccination [40], [41], and gender-neutral vaccination programs have been implemented in some Latin American countries (e.g. Antigua, Argentina, Bermuda, Brazil, and Panama). In clinical trials, the qHPV vaccine prevented HPV-related infection, genital warts, and anal dysplasia in males [42], [43], and the 9vHPV vaccine could confer additional benefits in males by protecting against HPV31/33/45/52/58, which are responsible for approximately 8% HPV-related anal cancers and 9% HPV-related penile cancers [20], [44]. The current results indicate that the 9vHPV vaccine was generally well tolerated and highly immunogenic among Latin American boys.
The WHO recommends the inclusion of HPV vaccines in national immunization programs where appropriate and feasible, noting that HPV vaccination should be 1 component of a comprehensive, integrated strategy to prevent cervical cancer and HPV-related disease, in addition to education, training, and screening efforts [45]. Despite these recommendations and the promising public health benefits of widespread HPV vaccination, implementation of vaccination programs can present a challenge and requires careful planning, effective communication, and a health education strategy [46].
Abundant and consistent data including evidence from active surveillance and large epidemiological studies, support the safety of HPV vaccination [12]. Indeed, the WHO Global Advisory Committee on Vaccine Safety has stated that systematic investigations of safety concerns raised about HPV vaccines have not to date revealed any safety issue that would alter its recommendations for the use of the vaccine [47]. However, incorrect perceptions regarding safety of the vaccine can occur among a proportion of the public, media, and even healthcare professionals, and clusters of anxiety-related reactions to immunization have negatively impacted immunization programs in some regions, including the Latin American region [12], [48]. Healthcare professionals and authorities must be aware of this possibility and be prepared to respond in a deliberate, informed, and carefully balanced manner (i.e. neither dismissing nor overreacting), to avoid fueling potential outbreaks [12]. In addition, in contrast with vaccinating young children against acute infectious diseases (where the benefit is taken for granted), vaccinating adolescents and young women to prevent diseases that develop much later in life presents a unique challenge and requires health education efforts and raising the awareness about HPV-related cancers [49]. Preparation, communication, and enhancing of vaccine infrastructure can ensure implementation of high coverage and sustainable vaccination programs [46], [49].
5. Conclusions
The 9vHPV vaccine has shown to be efficacious, immunogenic, and generally well-tolerated in participants from Latin America from two global clinical trials. These data support widespread vaccination programs in Latin America, particularly taking into account 10 years of real-world experience following introduction of the qHPV vaccine, which significantly reduced HPV-associated infection and disease in countries with a national vaccination program [9], [10]. The integration and systematic implementation of primary and secondary preventative interventions should be assessed for the potential to further reduce cervical cancer in this region. Expansion of the target population for vaccination may also be useful in accelerating the public health impact of vaccination programs.
Acknowledgments
Medical writing assistance was provided by Erin M. Bekes, PhD, of Complete Medical Communications, Hackensack, NJ. This assistance was funded by Merck & Co., Inc., Kenilworth, NJ, USA.
Acknowledgments
Role of the funding source
This work was supported by Merck & Co., Inc., Kenilworth, NJ, USA, which manufactures the qHPV and 9vHPV vaccines. Authors and others employed by the sponsor, together with external investigators, directly contributed to study design, data collection, analysis and interpretation, and writing of the report, and preparation of the manuscript for submission. All authors approved the final version of the manuscript. The decision to submit the manuscript was made by the lead author in conjunction with the sponsor and co-authors. The sponsor did not have potential to prevent submission of the manuscript. The opinions expressed in the manuscript represent the collective views of the authors and do not necessarily reflect the official position of the sponsor.
Conflicts of Interest
Ángela María Ruiz-Sternberg has received grants from Merck & Co., Inc., Kenilworth, NJ, USA, through Rosario University, to conduct clinical trials of HPV vaccines.
Edson D Moreira Jr has received research grants, financial compensation for consultation and advisory board work with Merck & Co., Inc., Kenilworth, NJ, USA, and his institution has received financial support for other HPV vaccine-related studies from Merck & Co., Inc., Kenilworth, NJ, USA.
Jaime A Restrepo has nothing to disclose.
Eduardo Lazcano-Ponce has nothing to disclose.
Robinson Cabello has nothing to disclose.
Arnaldo Silva has nothing to disclose.
Rosires Andrade has received grants from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, as a Principal Investigator during the conduct of the study.
Francisco Revollo has received personal fees from Merck & Co., Inc., Kenilworth, NJ, USA, during and outside the conduct of the study.
Santos Uscanga has nothing to disclose.
Alejandro Victoria has nothing to disclose.
Ana María Guevara has nothing to disclose.
Joaquín Luna has received grants from Unidad de Investigaciones, Fundación Universitaria Sanitas during the conduct of the study.
Manuel Plata has nothing to disclose.
Claudia Nossa Dominguez has nothing to disclose.
Edison Fedrizzi has received grants, personal fees, and non-financial support from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, as a Principal Investigator for the V503-001 trial.
Eugenio Suarez has received grants and personal fees from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, during the conduct of the study.
Julio C Reina has nothing to disclose.
Misoo C Ellison, Erin Moeller, Michael Ritter, Christine Shields, Miguel Cashat, Gonzalo Perez, and Alain Luxembourg are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, who may own stock and/or hold stock options in the Company.
Primary investigators In Latin America
Study V503-001:
Brazil: Rosires P. Andrade, Edison Fedrizzi, Edson D. Moreira; Chile: Eugenio Suarez; Colombia: Nestor Balcazar, Claudia C. Cruz, Ana M. Guevara, Joaquin Luna, Ivette Maldonado, Claudia J. Nossa, Manuel A. Plata, Jaime A. Restrepo, Francisco Revollo, Angela M. Ruiz-Sternberg, Lina M. Trujillo, Alejandro Victoria; Mexico: Eduardo Lazcano, Margarita Santiago, Santos Uscanga; Peru: Robinson L. Cabello, Arnaldo Silva.
Study V503-002:
Brazil: Adriana B. Campaner, Luciano S. Hammes, Edson D. Moreira; Chile: Ximena Cerda; Colombia: Julio C. Reina, Jaime A. Restrepo; Costa Rica: Adriano Arguedas, Gerardo Broutin, Kenneth Loaiciga; Peru: Theobaldo Herrera, Lenka Kolevic.
Footnotes
NCT number: NCT00543543; NCT00943722.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.pvr.2017.12.004.
Appendix A. Supplementary material
Supplementary material
References
- 1.International Agency for Research on Cancer GLOBOCAN 2012: estimated cancer incidence. Mortal. Preval. Worldw. 2012 〈http://globocan.iarc.fr/Pages/fact_sheets_population.aspx〉 (accessed 12 June 2017) [Google Scholar]
- 2.de Martel C., Plummer M., Vignat J., Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer. 2017;141:664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murillo R., Herrero R., Sierra M.S., Forman D. Cervical cancer in Central and South America: burden of disease and status of disease control. Cancer Epidemiol. 2016;44 Suppl 1:S121–S130. doi: 10.1016/j.canep.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Vaccarella S., Lortet-Tieulent J., Plummer M. Worldwide trends in cervical cancer incidence: impact of screening against changes in disease risk factors. Eur. J. Cancer. 2013;49:3262–3273. doi: 10.1016/j.ejca.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Bruni L., Diaz M., Barrionuevo-Rosas L. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob. Health. 2016;4:e453–e463. doi: 10.1016/S2214-109X(16)30099-7. [DOI] [PubMed] [Google Scholar]
- 6.Pan American Health Organization (PAHO), World Health Organization (WHO), Cervical Cancer in the Americas. 〈http://www.paho.org/hq/index.php?Option=com_topics&view=rdmore&cid=3673&Itemid=40936&lang=en〉 (accessed 11 June 2017).
- 7.Schiller J.T., Castellsagué X., Garland S.M. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(Suppl 5):F123–F138. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wigle J., Fontenot H.B., Zimet G.D. Global Delivery of Human Papillomavirus Vaccines. Pediatr. Clin. North Am. 2016;63:81–95. doi: 10.1016/j.pcl.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Garland S.M., Kjaer S.K., Munoz N. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clin. Infect. Dis. 2016;63:519–527. doi: 10.1093/cid/ciw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markowitz L.E., Liu G., Hariri S. Prevalence of HPV After Introduction of the Vaccination Program in the United States. Pediatrics. 2016;137:e20151968. doi: 10.1542/peds.2015-1968. [DOI] [PubMed] [Google Scholar]
- 11.Drolet M., Bénard É., Boily M.C. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect. Dis. 2015;15:565–580. doi: 10.1016/S1473-3099(14)71073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Technical Advisory Group on Vaccine-preventable Diseases of the Pan American Health Organization, Final Report of the XXIII Meeting of the Technical Advisory Group (TAG), 2015.
- 13.de Sanjose S., Quint W.G., Alemany L. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 14.Garland S.M., Steben M., Sings H.L. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J. Infect. Dis. 2009;199:805–814. doi: 10.1086/597071. [DOI] [PubMed] [Google Scholar]
- 15.Malagón T., Drolet M., Boily M.C. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect. Dis. 2012;12:781–789. doi: 10.1016/S1473-3099(12)70187-1. [DOI] [PubMed] [Google Scholar]
- 16.Mesher D., Soldan K., Lehtinen M. Population-level effects of human papillomavirus vaccination programs on infections with nonvaccine genotypes. Emerg. Infect. Dis. 2016;22:1732–1740. doi: 10.3201/eid2210.160675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanton C., Mesher D., Beddows S. Human papillomavirus (HPV) in young women in Britain: population-based evidence of the effectiveness of the bivalent immunisation programme and burden of quadrivalent and 9-valent vaccine types. Papillomavirus Res. 2017;3:36–41. doi: 10.1016/j.pvr.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woestenberg P.J., King A.J., van der Sande M.A. No evidence for cross-protection of the HPV-16/18 vaccine against HPV-6/11 positivity in female STI clinic visitors. J. Infect. 2017;74:393–400. doi: 10.1016/j.jinf.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Parkin D.M., Almonte M., Bruni L. Burden and trends of type-specific human papillomavirus infections and related diseases in the Latin America and Caribbean region. Vaccine. 2008;26(Suppl 11):L1–L15. doi: 10.1016/j.vaccine.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 20.Alemany L., Saunier M., Alvarado-Cabrero I. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int. J. Cancer. 2015;136:98–107. doi: 10.1002/ijc.28963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joura E.A., Ault A., Bosch F.X. Attribution of 12 high-risk human papillomavirus genotypes to infection and cervical disease. Cancer Epidemiol. Biomark. Prev. 2014;23:1997–2008. doi: 10.1158/1055-9965.EPI-14-0410. [DOI] [PubMed] [Google Scholar]
- 22.Serrano B., de Sanjosé S., Tous S. Human papillomavirus genotype attribution for HPVs 6, 11, 16, 18, 31, 33, 45, 52 and 58 in female anogenital lesions. Eur. J. Cancer. 2015;51:1732–1741. doi: 10.1016/j.ejca.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Castellsague X., Ault K.A., Bosch X. Human papillomavirus detection in cervical neoplasia attributed to 12 high-risk human papillomavirus genotypes by region. Papillomavirus Res. 2016;2:61–69. doi: 10.1016/j.pvr.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joura E.A., Giuliano A.R., Iversen O.E. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N. Engl. J. Med. 2015;372:711–723. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 25.Huh W., Joura E., Giuliano A.R. Efficacy, immunogenicity, and safety of a nine-valent human papillomavirus vaccine in women aged 16–26 years: final analyses of a randomised, double-blind trial. Lancet. 2017;390:2143–2159. doi: 10.1016/S0140-6736(17)31821-4. [DOI] [PubMed] [Google Scholar]
- 26.Van Damme P., Olsson S.E., Block S. Immunogenicity and safety of a 9-valent HPV vaccine. Pediatrics. 2015;136:e28–e39. doi: 10.1542/peds.2014-3745. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y.H., Gesser R., Luxembourg A. A seamless phase IIB/III adaptive outcome trial: design rationale and implementation challenges. Clin. Trials. 2015;12:84–90. doi: 10.1177/1740774514552110. [DOI] [PubMed] [Google Scholar]
- 28.Luxembourg A., Bautista O., Moeller E. Design of a large outcome trial for a multivalent human papillomavirus L1 virus-like particle vaccine. Contemp. Clin. Trials. 2015;42:18–25. doi: 10.1016/j.cct.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Luxembourg A., Brown D., Bouchard C. Phase II studies to select the formulation of a multivalent HPV L1 virus-like particle (VLP) vaccine. Hum. Vaccin. Immunother. 2015;11:1313–1322. doi: 10.1080/21645515.2015.1012010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luxembourg A., Moreira E.D., Jr., Samakoses R. Phase III, randomized controlled trial in girls 9-15 years old to evaluate lot consistency of a novel nine-valent human papillomavirus L1 virus-like particle vaccine. Hum. Vaccin. Immunother. 2015;11:1306–1312. doi: 10.1080/21645515.2015.1009819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guevara A., Cabello R., Woelber L. Antibody persistence and evidence of immune memory at 5 years following administration of the 9-valent HPV vaccine. Vaccine. 2017;35:5050–5057. doi: 10.1016/j.vaccine.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization (WHO), Guidelines to assure the quality, safety and efficacy of recombinant HPV virus-like particle vaccines. WHO/BS/06.2050, 2006.
- 33.Roberts C., Green T., Hess E. Development of a human papillomavirus competitive luminex immunoassay for 9 HPV types. Hum. Vaccin. Immunother. 2014;10:2168–2174. doi: 10.4161/hv.29205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreira E.D., Jr., Block S.L., Ferris D. Safety profile of the 9-valent HPV vaccine: a combined analysis of 7 Phase III clinical trials. Pediatrics. 2016;138:e20154387. doi: 10.1542/peds.2015-4387. [DOI] [PubMed] [Google Scholar]
- 35.Chan I.S.F., Bohidar N.R. Exact power and sample size for vaccine efficacy studies. Comm. Stat. Theory Methods. 1998;27:1305–1322. [Google Scholar]
- 36.Zinkernagel R.M. On differences between immunity and immunological memory. Curr. Opin. Immunol. 2002;14:523–536. doi: 10.1016/s0952-7915(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 37.Kjaer S.K., Nygård M., Dillner J. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 nordic countries. Clin. Infect. Dis. 2017;66:339–345. doi: 10.1093/cid/cix797. [DOI] [PubMed] [Google Scholar]
- 38.Luxembourg A., Kjaer S.K., Nygard M. Design of a long-term follow-up effectiveness, immunogenicity and safety study of women who received the 9-valent human papillomavirus vaccine. Contemp. Clin. Trials. 2017;52:54–61. doi: 10.1016/j.cct.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Serrano B., Alemany L., Tous S. Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease. Infect. Agent Cancer. 2012;7:38. doi: 10.1186/1750-9378-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehtinen M., Apter D. Gender-neutrality, herd effect and resilient immune response for sustainable impact of HPV vaccination. Curr. Opin. Obstet. Gynecol. 2015;27:326–332. doi: 10.1097/GCO.0000000000000208. [DOI] [PubMed] [Google Scholar]
- 41.Schmeler K.M., Sturgis E.M. Expanding the benefits of HPV vaccination to boys and men. Lancet. 2016;387:1798–1799. doi: 10.1016/S0140-6736(16)30314-2. [DOI] [PubMed] [Google Scholar]
- 42.Giuliano A.R., Palefsky J.M., Goldstone S. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N. Engl. J. Med. 2011;364:401–411. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palefsky J.M., Giuliano A.R., Goldstone S. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N. Engl. J. Med. 2011;365:1576–1585. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 44.Alemany L., Cubilla A., Halec G. Role of human papillomavirus in penile carcinomas worldwide. Eur. Urol. 2016;69:953–961. doi: 10.1016/j.eururo.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization (WHO) Human papillomavirus vaccines: WHO position paper. Wkly. Epidemiol. Rec. 2017;92:241–268. [Google Scholar]
- 46.World Health Organization (WHO), Guide to Introducing HPV Vaccine into National Immunization Programmes. 〈http://apps.who.int/iris/bitstream/10665/253123/1/9789241549769-eng.pdf?Ua=1〉 (accessed 15 March 2017).
- 47.Global Advisory Committee on Vaccine Safety, World Health Organization (WHO), Statement on Safety of HPV Vaccines. 〈http://www.who.int/vaccine_safety/committee/GACVS_HPV_statement_17Dec2015.pdf?Ua=1〉, 17 December 2015 (accessed 20 June 2017).
- 48.World Health Organization (WHO) Global advisory committee on vaccine safety, 2-3 December 2015. Wkly. Epidemiol. Rec. 2016;91:21–32. [PubMed] [Google Scholar]
- 49.World Health Organization (WHO), WHO HPV Vaccine Communication: Special considerations for a unique vaccine. 〈http://apps.who.int/iris/bitstream/10665/250279/1/WHO-IVB-16.02-eng.pdf?Ua=1〉 (accessed 15 March 2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material