Version Changes
Revised. Amendments from Version 1
In response to the comments of Robert van Woesik we have removed all reference to the doldrums, the table has been rearranged so the species are ranked from most to least impacted, we have specified the aspect of the site and addressed all the grammatical and typological errors identified. In response to the comments of Misha Matz we now included sea surface temperatures and wind speeds for the year preceding the bleaching event. These data suggest it was both hotter and calmer in the week prior to the bleaching than at the same time of year in 2015. However, as Misha points out, it is not possible to prove that this bleaching was a result of hypoxia driven by calm or hot conditions. To do this we would need to have placed oxygen electrodes in the centre of colonies during the event. We have, however, added comments to the effect that wind speeds less than 3 m/s are generally regarded as the cut-off below which calm weather bleaching can occur and cited the NOAA website in support of this statement. Absolute values of wind speed are the correct metric to present – this is what affects wave and water movement. Unfortunately, we can find no literature in support of the statement that “Tissue mortality beginning in the center of the colony typically indicates anoxia..”. Therefore, we have reworded the sentence to make it clear that this statement is based on personal observation alone. We have kept the BMI index in Table 1 because this is now used to rank the taxa as requested by Robert van Woesik.
Abstract
Coral bleaching can be induced by many different stressors, however, the most common cause of mass bleaching in the field is higher than average sea surface temperatures (SST). Here, we describe an unusual bleaching event that followed very calm sea conditions combined with higher than average SST. Patterns of mortality differed from typical bleaching in four ways: 1) mortality was very rapid; 2) a different suite of species were most affected; 3) tissue mortality in Acropora spp. was often restricted to the center of the colony; 4) the event occurred early in summer. The two weeks prior to the event included 8 days where the average wind speed was less than 3 ms -1. In addition, SSTs in the weeks preceding and during the event were 1.0-1.5°C higher than the mean for the last 30 years. We hypothesize that this unusual bleaching event was caused by anoxia resulting from a lack of water movement induced by low wind speeds combined with high SST.
Keywords: climate change, coral bleaching, coral reefs, disturbance
Introduction
Coral bleaching is a generalized response that can be induced by many different stressors 1– 3. Whilst the most common cause of large scale bleaching on coral reefs is unusually high sea surface temperatures (SSTs) 4, 5, prolonged periods of calm weather have also been associated with mass bleaching events in the Caribbean 6, 7 and the Indo-Pacific 8– 10. Experimental work has also confirmed that low water flow can exacerbate thermal bleaching 11, 12.
The ecology of thermal coral bleaching in response to high SSTs is reasonably well documented. For example, colonies affected by high temperatures typically take between two to six weeks to bleach and bleached tissue can take another two to twenty weeks to die 13. In addition, species vary in their susceptibility to thermal bleaching 14, 15, resulting in a predicable hierarchy of response 16, 17. Temporal patterns are also apparent with most high temperature induced mass bleaching events generally occurring towards the end of the summer months 18, 19. Any change in this predictable bleaching ecology suggests an alternative cause (i.e., not thermal stress) for a given bleaching event.
Here, we describe an atypical bleaching event that we hypothesize was caused by an interaction of temperature with very calm sea conditions caused by an extended period of low winds. We identify a number of characteristic features of this calm weather bleaching that allow it to be distinguished from thermal bleaching in the field. Establishing the cause of specific bleaching events is vital in order to correctly attribute damage caused by climate change and other potential stressors.
Methods
The study site was on the reef crest (1 m depth) at Nata Reef, Iriomote, Japan (24.4282°N, 123.7955°E). Initial observations at the site were made between 26 and 29 May, 2016 at which point in time no bleached corals were noted. Surveys to quantify bleaching and mortality were conducted on 12 June, 2016. Twenty replicate 1m 2 quadrats were placed haphazardly on the reef crest, and the condition and species identity of all hard coral colonies with a maximum diameter greater than 5cm were recorded. Species were identified in the field following 20 and the names updated to the currently accepted names following 21 Colonies were placed in one of six bleaching categories following 22: (1) unbleached, (2) the entire colony pale, (3) 1–50% of the colony white, (4) 51–99% of the colony white, (5) 100% of colony white or fluorescent, or (6) recently dead. The data from the quadrats was pooled as the data was collected. The bleaching mortality index was calculated following 16. Data on environmental conditions leading up to the bleaching episode and for a similar time frame in 2015 were obtained from the Japan Meteorological Agency, which allows for these data to be used as long as due credit is given.
Results
Bleaching and mortality was rapid. No colonies were bleached at the time of the first surveys (26 May, 2016) yet two weeks later (12 June, 2016), 5% of colonies were dead and a further 31% were bleached ( Table 1).
Table 1. Bleaching categories of hard corals at Nata Reef on 12 June 2016.
BMI = Bleaching Mortality Index.
| taxa | unbleached | moderate | severe | dead | BMI | n |
|---|---|---|---|---|---|---|
| Acropora selago | 0 | 0 | 0 | 100 | 100 | 1 |
| Montipora aequituberculata | 0 | 0 | 0 | 100 | 100 | 3 |
| Montipora efflorescens | 0 | 27 | 27 | 45 | 73 | 11 |
| Goniastrea pectinata | 0 | 50 | 50 | 0 | 50 | 2 |
| Milleporidae | 17 | 33 | 50 | 0 | 44 | 6 |
| Dipsastraea rotumana | 0 | 100 | 0 | 0 | 33 | 1 |
| Montipora turgescens | 0 | 100 | 0 | 0 | 33 | 1 |
| Platygyra ryukyuensis | 25 | 50 | 25 | 0 | 33 | 4 |
| Platygyra verweyi | 67 | 0 | 0 | 33 | 33 | 3 |
| Dipsastrea pallida | 30 | 50 | 20 | 0 | 30 | 10 |
| Montipora crassituberculata | 46 | 32 | 18 | 4 | 26 | 28 |
| Montipora digitata | 71 | 0 | 29 | 0 | 19 | 7 |
| Acropora nasuta | 50 | 50 | 0 | 0 | 17 | 2 |
| Pocillopora damicornis | 67 | 22 | 11 | 0 | 15 | 9 |
| Pavona venosa | 57 | 43 | 0 | 0 | 14 | 7 |
| Porites annae | 60 | 40 | 0 | 0 | 13 | 5 |
| Acropora hyacinthus | 71 | 29 | 0 | 0 | 10 | 7 |
| Platygyra pini | 75 | 25 | 0 | 0 | 8 | 4 |
| Porites cylindrica | 77 | 23 | 0 | 0 | 8 | 13 |
| Acropora digitifera | 81 | 19 | 0 | 0 | 6 | 32 |
| Galaxea fascicularis | 82 | 18 | 0 | 0 | 6 | 11 |
| Favites halicora | 86 | 14 | 0 | 0 | 5 | 7 |
| Goniastrea retiformis | 86 | 14 | 0 | 0 | 5 | 14 |
| Acropora aspera | 100 | 0 | 0 | 0 | 0 | 1 |
| Acropora gemmifera | 100 | 0 | 0 | 0 | 0 | 1 |
| Astrea annuligera | 100 | 0 | 0 | 0 | 0 | 1 |
| Cyphastrea serailia | 100 | 0 | 0 | 0 | 0 | 3 |
| Favites abdita | 100 | 0 | 0 | 0 | 0 | 3 |
| Favites magnistellata | 100 | 0 | 0 | 0 | 0 | 2 |
| Montipora monasteriata | 100 | 0 | 0 | 0 | 0 | 4 |
| Pavona decussata | 100 | 0 | 0 | 0 | 0 | 2 |
| Porites lichen | 100 | 0 | 0 | 0 | 0 | 3 |
| Porites lutea | 100 | 0 | 0 | 0 | 0 | 1 |
| Porites rus | 100 | 0 | 0 | 0 | 0 | 6 |
| Psammocora contigua | 100 | 0 | 0 | 0 | 0 | 1 |
| total | 64 | 23 | 8 | 5 | 216 | 18 |
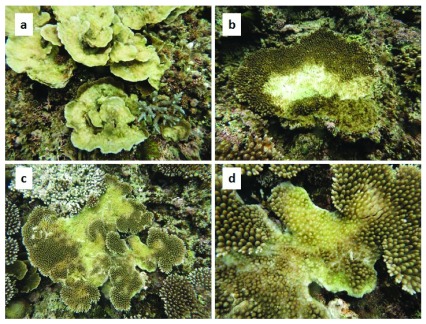
Mortality was highest in Montipora aequituberculata and M. efflorescens ( Figure 1A), and in an additional three species of the family Merulinidae, that were also badly affected ( Table 1). Bleaching and tissue mortality were generally restricted to the center of colonies in the locally abundant species Acropora digitifera and A. hyacinthus ( Figure 1B, C, D).
Figure 1.
( a) Dead and dying Montipora aequituberculata colonies ( b) Acropora hyacinthus colony with bleached and dying tissue in the middle of the colony ( c) a second A. hyacinthus colony ( d) close up of the colony in ( c). Images were captured using a Canon S100 digital camera in waterproof housing.
The bleaching event occurred early in June, the first month of the northern summer, following a period of low wind and higher than average sea surface temperature (SST). Eight days in the previous two weeks had average wind speeds of under 3 ms -1 ( Table 2). Winds were also mostly from the south, which is offshore at the study site and therefore likely to further reduce wave size and water motion ( Table 2). Mean daily SSTs in the month preceding the second survey were 0.0–1.5°C higher than the mean for the previous 30 years ( Table 3). Wind speeds were higher and SST lower during the same time interval in 2015 ( Table 2 & Table 3).
Table 2. Mean daily wind speeds in the 12 days prior to the first observations of bleaching on 12 June 2016 and for the same dates in 2015.
Data from Japan Meteorological Agency.
| Date | 2015 wind
speed (m/s) |
2015 wind
direction |
2016 wind
speed (m/s) |
2016 wind
direction |
|---|---|---|---|---|
| 30 May | 2.2 | SSE | 2.1 | ENE |
| 31 May | 3.1 | ENE | 1.8 | SE |
| 1 June | 2.6 | NE | 3.4 | W |
| 2 June | 4.8 | SSW | 3 | NE |
| 3 June | 4.6 | SSW | 2.1 | SW |
| 4 June | 2.3 | S | 2.1 | ENE |
| 5 June | 5.5 | SSW | 3.2 | NE |
| 6 June | 3.8 | S | 2.5 | NE |
| 7 June | 4.2 | SSW | 1.6 | SE |
| 8 June | 3.3 | SW | 1.9 | ESE |
| 9 June | 3 | SSW | 2 | ENE |
| 10 June | 4.2 | SSW | 3 | SSW |
| 11 June | 5.3 | S | 6.4 | SSW |
| 12 June | 4 | S | 8.2 | SSW |
Table 3. Sea surface temperature anomalies in the weeks preceding the bleaching event on Nata Reef and a similar time interval in 2015.
Values are the degrees in centigrade above the 30 year average for this site in each time interval. Data from the Japan Meteorological Agency.
| 10 day period
ending |
2015 SST
anomaly °C |
2016 SST
anomaly °C |
|---|---|---|
| 10 April | 0 | 0 |
| 20 April | -0.5 | 1 |
| 30 April | 0 | 1.5 |
| 10 May | 0.5 | 1.5 |
| 20 May | 0.5 | 1 |
| 30 May | 0.5 | 1 |
| 10 June | 0.5 | 1 |
Discussion
This bleaching event was different to typical thermal bleaching in a number of important ways. In particular, rapid tissue mortality, an atypical hierarchy of susceptibility, and the occurrence of the event in early summer, all distinguish this event from typical thermal bleaching. We hypothesize that unusually high SST combined with a lack of water flow due to low winds speeds resulted in anoxic stress to these colonies. This hypothesis is supported by very low wind speeds ( Table 2) combined with higher than average mean daily SST ( Table 3) in the weeks prior to the event.
In contrast to the typical thermal response, bleaching and mortality were very rapid, with a high proportion of colonies bleached and some dying within the two week period between the surveys ( Table 1). Bleaching and, in particular, mortality typically take between 4–6 weeks to present in corals following thermal stress 13. In addition, the hierarchy of susceptibility was very different to that following thermal bleaching. Here, the worst affected species included two Montipora spp. and a number of merulinids ( Table 1), when typically Acropora spp. and Pocillopora spp. are the most severely affected following thermal bleaching 5, 15, 22.
The pattern of tissue bleaching and mortality was also unusual. In Acropora colonies the typical pattern following thermal stress is for the whole colony to bleach 13. In contrast, mortality was restricted to the center of most Acropora colonies in this event ( Figure 1a, b, c). Tissue mortality beginning in the center of the colony is suggestive of anoxia, which often occurs in aquaria with inadequate flow or oxygenation (pers obs). This pattern of mortality is also superficially similar to feeding scars caused by Acanthaster planci or Drupella spp. 23 and a naïve observer might well have attributed this mortality to either of these corallivores 24. A thorough search of the site, including underneath these and adjacent colonies, indicated that neither of these corallivores were present.
The timing of the bleaching event in early summer is also unusual. Thermal bleaching typically occurs much later in the summer. For example, recurrent seasonal bleaching on Magnetic Island, Australia, occurs in the last month of the austral summer i.e., February 18. Similarly, the 1998 mass bleaching event in Japan was first noticed in the latter part of the summer i.e., late July 25. In contrast, this calm weather event occurred early in June, the first month of the northern summer.
Doldrums-like conditions (defined by NOAA as days with average wind speeds of less than 3 ms -1) have previously been linked to mass bleaching events 6– 9. However, the capacity of calm weather to cause more localized damage outside of the typical thermal bleaching window in late summer has not previously been recognized. In addition, the potential link to anoxia, while tested in the laboratory 26, has not been made in the field. This observation is especially important in the context of the continuing increase in the scale and frequency of mass bleaching events 27 because it would generally be assumed that this small-scale phenomenon might presage a larger mass bleaching event. Determining the cause of specific bleaching events is vital in order to accurately distinguish the effects of climate change versus other causes of degradation on coral reefs.
Data availability
The pooled raw bleaching data is provided in Table 1.
Source data for Table 2 are available from the Japan Meteorological Agency, at:
Source data to generate the values in Table 3 are available from the Japan Meteorological Agency, at: http://bit.ly/2y8qlBw.
Acknowledgements
We thank the staff at the Iriomote Tropical Biosphere Research Station, University of the Ryukyus, for their assistance.
Funding Statement
This work was funded by the Australian Research Council Centre of Excellence for Coral Reef Studies (CE140100020) and VILLUM FONDEN (10114).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; referees: 2 approved]
References
- 1. Glynn PW: Coral reef bleaching in the 1980s and possible connections with global warming. Trends Ecol Evol. 1991;6(6):175–9. 10.1016/0169-5347(91)90208-F [DOI] [PubMed] [Google Scholar]
- 2. Brown BE: Coral bleaching: causes and consequences. Coral Reefs. 1997;16:S129–S38. 10.1007/s003380050249 [DOI] [Google Scholar]
- 3. Baird AH, Bhagooli R, Ralph PJ, et al. : Coral bleaching: the role of the host. Trends Ecol Evol. 2009;24(1):16–20. 10.1016/j.tree.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 4. Glynn PW: Coral reef bleaching: Ecological perspectives. Coral Reefs. 1993;12(1):1–17. 10.1007/BF00303779 [DOI] [Google Scholar]
- 5. Hughes TP, Kerry JT, Álvarez-Noriega M, et al. : Global warming and recurrent mass bleaching of corals. Nature. 2017;543(7645):373–7. 10.1038/nature21707 [DOI] [PubMed] [Google Scholar]
- 6. Jiménez C: Bleaching and mortality of reef organisms during a warming event in 1995 on the Caribbean coast of Costa Rica. Rev Biol Trop. 2001;49(Suppl 2):233–8. [PubMed] [Google Scholar]
- 7. Miller J, Muller E, Rogers C, et al. : Coral disease following massive bleaching in 2005 causes 60% decline in coral cover on reefs in the US Virgin Islands. Coral Reefs. 2009;28(4):925–37. 10.1007/s00338-009-0531-7 [DOI] [Google Scholar]
- 8. Fisk DA, Done TJ: Taxonomic and bathymetric patterns of bleaching in corals, Myrimidon Reef (Queensland). Proc Fifth Inter Coral Reef Congress1985;6:149–54. Reference Source [Google Scholar]
- 9. DeCarlo TM, Cohen AL, Wong GT, et al. : Mass coral mortality under local amplification of 2 °C ocean warming. Sci Rep. 2017;7: 44586. 10.1038/srep44586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raymundo LJ, Burdick D, Lapacek VA, et al. : Anomalous temperatures and extreme tides: Guam staghorn Acropora succumb to a double threat. Mar Ecol Prog Ser. 2017;564:47–55. 10.3354/meps12005 [DOI] [Google Scholar]
- 11. Nakamura T, van Woesik R: Water-flow rates and passive diffusion partially explain differential survival of corals during the 1998 bleaching event. Mar Ecol Prog Ser. 2001;212:301–4. 10.3354/meps212301 [DOI] [Google Scholar]
- 12. Nakamura T, Yamasaki H, van Woesik R: Water flow facilitates recovery from bleaching in the coral Stylophora pistillata. Mar Ecol Prog Ser. 2003;256:287–91. 10.3354/meps256287 [DOI] [Google Scholar]
- 13. Baird AH, Marshall PA: Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar Ecol Prog Ser. 2002;237:133–41. 10.3354/meps237133 [DOI] [Google Scholar]
- 14. Jokiel PL, Coles SL: Response of Hawaiian and other Indo-Pacific reef corals to elevated temperature. Coral Reefs. 1990;8(4):155–62. 10.1007/BF00265006 [DOI] [Google Scholar]
- 15. Loya Y, Sakai K, Yamazato K, et al. : Coral bleaching: the winners and the losers. Ecol Lett. 2001;4(2):122–31. 10.1046/j.1461-0248.2001.00203.x [DOI] [Google Scholar]
- 16. McClanahan TR, Baird AH, Marshall PA, et al. : Comparing bleaching and mortality responses of hard corals between southern Kenya and the Great Barrier Reef, Australia. Mar Pollut Bull. 2004;48(3–4):327–35. 10.1016/j.marpolbul.2003.08.024 [DOI] [PubMed] [Google Scholar]
- 17. Swain TD, Vega-Perkins JB, Oestreich WK, et al. : Coral bleaching response index: a new tool to standardize and compare susceptibility to thermal bleaching. Glob Chang Biol. 2016;22(7):2475–88. 10.1111/gcb.13276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jones RJ, Berkelmans R, Oliver JK: Recurrent bleaching of corals at Magnetic Island (Australia) relative to air and seawater temperature. Mar Ecol Prog Ser. 1997;158:289–92. 10.3354/meps158289 [DOI] [Google Scholar]
- 19. Fitt WK, McFarland FK, Warner ME, et al. : Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnol Oceanogr. 2000;45(3):677–85. 10.4319/lo.2000.45.3.0677 [DOI] [Google Scholar]
- 20. Veron JE: Corals of the World. The Australian Institute of Marine Science, Townsville,2000. Reference Source [Google Scholar]
- 21. Hoeksema BW: Scleractinia. Bourne,1900. Accessed through: World Register of Marine Species. Reference Source [Google Scholar]
- 22. Marshall PA, Baird AH: Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa. Coral Reefs. 2000;19(2):155–63. 10.1007/s003380000086 [DOI] [Google Scholar]
- 23. Pratchett MS, Schenk TJ, Baine M, et al. : Selective coral mortality associated with outbreaks of Acanthaster planci L. in Bootless Bay, Papua New Guinea. Mar Environ Res. 2009;67(4–5):230–6. 10.1016/j.marenvres.2009.03.001 [DOI] [PubMed] [Google Scholar]
- 24. Baird AH, Pratchett MS, Hoey AS, et al. : Acanthaster planci is a major cause of coral mortality in Indonesia. Coral Reefs. 2013;32(3):803–12. 10.1007/s00338-013-1025-1 [DOI] [Google Scholar]
- 25. Kayanne H, Harii S, Ide Y, et al. : Recovery of coral populations after the 1998 bleaching on Shiraho Reef, in the southern Ryukyus, NW Pacific. Mar Ecol Prog Ser. 2002;239:93–103. 10.3354/meps239093 [DOI] [Google Scholar]
- 26. Ulstrup KE, Hill R, Ralph PJ: Photosynthetic impact of hypoxia on in hospite zooxanthellae in the scleractinian coral Pocillopora damicornis. Mar Ecol Prog Ser. 2005;286:125–32. 10.3354/meps286125 [DOI] [Google Scholar]
- 27. Hughes TP, Barnes ML, Bellwood DR, et al. : Coral reefs in the Anthropocene. Nature. 2017;546(7656):82–90. 10.1038/nature22901 [DOI] [PubMed] [Google Scholar]