Summary
Background
Rapid population growth in Africa requires an urgent expansion and improvement of housing options. Improving housing presents a promising opportunity for malaria control by reducing indoor exposure to mosquitoes. We measured recent changes in house design in rural Uganda and evaluated their association with malaria in relation to a mass scale-up of control efforts.
Methods
This analysis was part of a cohort study designed to compare temporal changes in malaria incidence from a cohort of children and adults with temporal changes in malaria test positivity rate from health facility surveillance. All children aged 6 months to 10 years (n=384) living in 107 households in Nagongera sub-country, Tororo, Uganda, were given long-lasting insecticide-treated nets and followed between Aug 19, 2011, and June 30, 2017. Repeat rounds of indoor residual spraying of insecticide were initiated on Dec 5, 2014. Socioeconomic data were collected at two timepoints (Sept 25–Oct 9, 2013 and June 21–July 11, 2016) and houses were classified as modern (cement, wood, or metal walls, tiled or metal roof, and closed eaves) or traditional (all other homes). Associations between house design and three outcomes were evaluated before and after the introduction of indoor residual spraying: human biting rate estimated monthly in each household using US Centers for Disease Control and Prevention light traps; parasite prevalence measured routinely by microscopy every 3 months before indoor residual spraying and monthly after indoor residual spraying; and malaria incidence measured by passive surveillance.
Findings
The implementation of indoor residual spraying was associated with significant declines in human biting rate (33·5 vs 2·7 Anopheles per house per night after indoor residual spraying, p<0·0001), parasite prevalence (32·0% vs 14·0%, p<0·0001), and malaria incidence (3·0 vs 0·5 episodes per person-year at risk, p<0·0001). The prevalence of modern housing increased from 23·4% in 2013 to 45·4% in 2016 (p=0·001). Compared with traditional houses, modern houses were associated with a 48% reduction in human biting rate before indoor residual spraying (adjusted incidence rate ratio [aIRR] 0·52, 95% CI 0·36–0·73, p=0·0002), and a 73% reduction after indoor residual spraying (aIRR 0·27, 0·17–0·42, p<0·0001). Before indoor residual spraying, there was no association between house type and parasite prevalence, but after indoor residual spraying there was a 57% reduction in the odds of parasitaemia in modern houses compared with traditional houses, controlling for age, sex, and socioeconomic position (adjusted odds ratio 0·43, 95% CI 0·24–0·77, p=0·004). House type was not associated with malaria incidence before or after indoor residual spraying.
Interpretation
House design improved rapidly in rural Uganda and was associated with additional reductions in mosquito density and parasite prevalence following the introduction of indoor residual spraying. Changes to house design in endemic Africa, including closing eaves and the replacement of traditional building materials, might help further the gains achieved with more widely accepted malaria control interventions.
Funding
US National Institutes of Health, Bill & Melinda Gates Foundation, and Medical Research Council UK.
Introduction
The population of Africa is projected to expand from 1·2 billion in 2015 to over 2 billion by 2050,1 creating an urgent need to expand and improve housing options. With international focus on Sustainable Development Goal 11 to achieve safe and decent housing for all by 2030,2 large-scale investment in housing presents a promising opportunity for malaria control and elimination.3, 4 Across much of endemic Africa, modern housing designs with tiled or metal roofs and concrete or brick walls are increasingly replacing traditional mud and thatch5 and there is growing evidence that these changes might help to lower malaria risk,6, 7 primarily through the reduction of mosquito house entry by physical barriers such as door and window screening or closed eaves (the gap between the wall and roof).8
In Uganda, traditional housing is typically characterised by thatched roofs, mud walls, and open eaves, whereas more modern housing is built with metal roofs, concrete or brick walls, and closed eaves (figure 1).9 Anecdotal evidence indicates that housing is being rapidly upgraded from traditional to modern styles in many communities alongside economic and population growth, although these changes have not been documented. Previous studies have observed modern housing, compared with traditional housing, to be associated with a halving of the human biting rate and parasite prevalence in three malaria transmission settings in Jinja, Tororo, and Kanungu districts9 and an approximate halving of malaria incidence in Tororo district,10 with evidence that housing quality might partly explain the association between socioeconomic position and malaria.11
Figure 1.
Traditional (left) and modern (right) houses in rural Uganda
Traditional housing is typically characterised by thatched roofs, mud walls, and open eaves, whereas modern housing is built with metal roofs, concrete or brick walls, and closed eaves.
Research in context.
Evidence before this study
We searched PubMed for titles and abstracts published in English between Jan 1, 2010, and Sept 1, 2017, with the terms “hous*” combined with “Africa”, “malaria”, “Plasmodium”, or “vector” for studies. We found two major reviews that assessed the evidence for housing improvements for malaria control in sub-Saharan Africa. First, a systematic review and meta-analysis analysed 90 studies published between 1900 and 2013. Modern houses were defined as those that had finished walls and floors, metal or tiled roofs, and closed eaves, whereas traditional homes were considered to have mud walls, thatched roofs, earth floors, open eaves, no ceiling, and no screening. Residents of modern houses had 47% lower odds of parasitaemia and 45–65% lower odds of clinical malaria than residents of traditional houses. Second, an analysis of 15 Demographic and Health Surveys and 14 Malaria Indicator Surveys found modern housing across sub-Saharan Africa to be associated with a 9–14% reduction in the odds of parasitaemia after controlling for household wealth, similar to the reduction associated with the use of insecticide-treated nets.
Added value of this study
We measured recent changes in house design in rural Uganda and evaluated their association with malaria in relation to a mass scale-up of control efforts. Our findings demonstrate a rapid increase in the prevalence of modern houses with a cement, wood, or metal wall, tiled or metal roof, and closed eaves within less than 3 years, possibly linked to broader demographic and economic development in Uganda. Modern houses were associated with up to a 73% reduction in house entry by malaria vectors and a 57% reduction in the odds of parasitaemia, compared with traditional houses. Importantly, housing improvements were associated with additional reductions in mosquito density and parasite prevalence following the scale-up of indoor residual spraying.
Implications of all the available evidence
An increasing body of observational evidence demonstrates an association between house improvements (such as metal roofs, closed eaves, and screened windows and doors) and reduced malaria transmission. However, few epidemiological studies have analysed how house designs are changing in rural Africa and how these changes might be relevant to malaria control in the era of sustainable development. Our findings suggest that rapid improvements in house design are occurring in parts of rural Africa and that these improvements might further benefit existing malaria control interventions. As endemic Africa continues to undergo unprecedented population growth, economic change, and urbanisation, there is an urgent need to establish how efforts to meet increased housing demand can be leveraged to further the gains in malaria control and elimination that have been attained with more conventional malaria control interventions, as well as to maintain elimination once achieved.
Here we build on previous evaluations of the relationship between house design and malaria in Uganda9, 11 by analysing data from a comprehensive malaria cohort study done in Nagongera sub-county, Tororo district, between Aug 19, 2011, and June 30, 2017. The study covered a period of major expansion of population-level malaria control interventions, including a universal long-lasting insecticidal net (LLIN) distribution campaign from Nov 1 to Nov 30, 2013, and four rounds of indoor residual spraying of insecticide starting on Dec 5, 2014. Although LLIN distribution was associated with little change in malaria burden,12 significant reductions in both entomological and epidemiological measures of transmission were observed following indoor residual spraying.12, 13 Here we first quantify changes in house design over time in Nagongera. Second, we evaluate the association between house design and malaria during two time periods, before and after the decline in malaria transmission associated with the introduction of indoor residual spraying. To our knowledge, this is the first study to quantify changes in housing quality in relation to malaria control in Uganda.
Methods
Study design and participants
The study was done between Aug 19, 2011, and June 30, 2017, in Nagongera sub-country, Tororo, Uganda (00°46’10·6”N, 34°01’34·1”E), an area of approximately 15 km2. Before the introduction of indoor residual spraying, malaria transmission was intense, with two annual peaks following the two rainy seasons (March to May and August to October) and an estimated annual Plasmodium falciparum entomological inoculation rate of 125 infectious bites per year in 2011–12.14 A universal LLIN distribution campaign was done in November, 2013, increasing estimated household ownership of at least one LLIN per two residents from 36% in 2012 to 62% in 2015, but having little effect on malaria burden.12 Following three rounds of indoor residual spraying with bendiocarb at about 6 month intervals, initiated on Dec 5, 2014, malaria incidence in children decreased from 3·3 episodes per person-year at risk to 0·6 episodes per person-year at risk.12 A fourth round of indoor residual spraying with Actellic was done from June 6 to July 5, 2016.
This analysis was part of a cohort study designed to compare temporal changes in malaria incidence from a cohort of children and adults with temporal changes in malaria test positivity rate from health facility surveillance, for which the sample size was powered.12, 14, 15 All children aged 6 months to 10 years and their primary caregivers were enrolled from Aug 19 to Sept 17, 2011, from 100 households randomly selected from an enumeration census of all households in the sub-county. Recruitment was dynamic such that eligible children reaching age 6 months were enrolled and children reaching age 11 years were withdrawn. Households with no remaining study participants were withdrawn. From Sept 13, to Sept 26, 2013, seven additional households were enrolled to replace households where all study participants had been withdrawn. All participants were given a LLIN (PermaNet; Vestergaard Frandsen, Lausanne, Switzerland) at enrolment and followed for all their health-care needs at the study clinic for 7 days a week for up to 70 months, until June 30, 2017. LLIN use, defined as whether the participant reported sleeping under a LLIN the previous night, was more than 99% by self-report at the time of routine clinic visits. Participants received no incentives to participate other than the provision of free health care at the study clinic, clinic travel expenses, and a LLIN.
Written informed consent was obtained in the appropriate language from guardians for the participation of their child and from an adult household member for the household surveys. Approval from local leaders was obtained before beginning activities. Ethics approval was provided by the Uganda National Council for Science and Technology; Makerere University School of Medicine Research and Ethics Committee; University of California, San Francisco Committee for Human Research; Department of Biosciences Ethics Committee, Durham University; and the London School of Hygiene & Tropical Medicine Ethics Committee.
Procedures
New episodes of malaria were diagnosed by passive case detection and defined as a history of fever within the past 24 h or an elevated temperature (≥38·0°C tympanic) with a positive blood smear. Episodes of malaria were treated with artemether–lumefantrine (uncomplicated malaria) or quinine (complicated malaria). In addition, participants were invited to make a routine visit to the study clinic every 90 days, with the frequency of routine visits increasing to every 30 days starting Dec 1, 2014. At each of these visits, a thick blood smear was taken to assess for parasitaemia using active surveillance. Thick and thin blood smears were stained with 2% Giemsa and considered negative when the examination of 100 high-power fields did not reveal asexual parasites. All blood slides were read twice and discrepancies resolved by a third reviewer. The present analysis included all routine clinic visits from Aug 19, 2011, to June 30, 2017.
Descriptions of the entomological studies are provided elsewhere.14 Briefly, one US Centers for Disease Control and Prevention (CDC) light trap collection was done monthly in the main sleeping room of each house for up to 68 months between Oct 1, 2011, and June 30, 2017. Light traps were positioned with the light 1·5 m from the floor near the foot of the bed and collections made between 1900 h and 0700 h the following morning. Specimens were sorted to species level and counted.
Socioeconomic and house construction data were recorded in 2013 and 2016. Socioeconomic data (for the household wealth index) were collected first in a household survey done after 25 months of follow-up from Sept 25 to Oct 9, 2013, and second in a repeat household survey done after 57 months of follow-up from June 21 to July 11, 2016. House construction data were recorded in 2013 through independent house visits by the entomology field teams and validated by the 2013 household survey, and recorded again in the 2016 household survey. Where changes in house design were recorded between 2013 and 2016, households were asked the reason for these changes. Household surveys were administered as a structured questionnaire to one designated adult respondent from each household if they met four inclusion criteria: usually resident, present in the sampled household the night before the survey, aged at least 18 years, and agreed to provide informed written consent. Households were excluded if no adult respondent could be located on more than three occasions over 2 weeks.
Data were collected using standardised record forms entered into Microsoft Access for follow-up of study participants and using a paperless system for the household surveys.
Statistical analysis
We used a wealth index previously developed for the study population.11, 16 In brief, principal component analysis was used to create a wealth index from nine variables: ownership of mobile telephones, radios, clocks, cupboards, sofas, and tables; number of people per sleeping room; access to an improved toilet; and main mode of transport to the health facility. Households were ranked by wealth scores and grouped into tertiles to give a categorical measure of socioeconomic position in 2013 and 2016. We used a definition of house type previously developed for the study area.9 Main wall material, main roof material, and eave type were used to classify homes as either modern (wood, cement, or brick walls; a metal or tiled roof and closed eaves) or traditional (all other homes) in 2013 and 2016. Cross tabulations and Pearson's χ2 test were used to explore changes in house design between 2013 and 2016.
We evaluated the relationship between house design and malaria before and after the transmission reduction associated with the implementation of indoor residual spraying. For each household risk factor, we separately analysed both 2013 and 2016 data to enable corroboration of findings over time. For each household risk factor, we modelled its association with human biting rate, the number of adult female Anopheles caught per house per collection night, which corresponds to the household vector density; parasite prevalence, the proportion of routine visits with a positive blood smear, with or without fever; and the incidence of clinical malaria, the number of new episodes of malaria per person-years of observation. Negative binomial regression was used to model the number of Anopheles caught per house per night and the number of malaria episodes per child, with the number of catch nights and person days included as offset terms. The odds of parasitaemia at the time of each routine clinic visit were modelled using logistic regression. The association between house design and human biting rate was adjusted for socioeconomic position. For the epidemiological outcomes (parasite prevalence and malaria incidence), age as a continuous variable and sex were included in the model as covariates. For all outcomes, robust SEs were used to adjust for repeat measures (clustering) at the household level. Participants missing data on socioeconomic risk factors were excluded from the analysis of those risk factors. This analysis was done separately to compare two time periods: before indoor residual spraying, from Aug 19, 2011, to Jan 31, 2015, and after indoor residual spraying, from Feb 1, 2015, to June 30, 2017. We used a difference in differences estimation to compare the average reduction in each outcome before and after indoor residual spraying in modern compared with traditional houses.
To test the hypothesis that biting rates by vectors were overall lower in localities with a higher prevalence of modern housing, local Moran's I17 was used to examine the spatial autocorrelation (clustering) of human biting rate by house type before and after indoor residual spraying at the local scale using geographical coordinates of households. Spatial autocorrelation was estimated based on a spatial weighting matrix constructed at distance lags between household pairs. The significance of the local Moran's I was calculated using a randomisation test on the Z score. Positive spatial autocorrelation occurs when, for example, a household with a specific outcome value is surrounded by neighbouring households with similar outcome value (low–low, high–high), thus forming a spatial cluster. Clusters were plotted to visualise human biting rate clustering by house type.
Data were analysed using Stata (version 13; StataCorp, College Station, TX, USA).
Role of the funding source
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
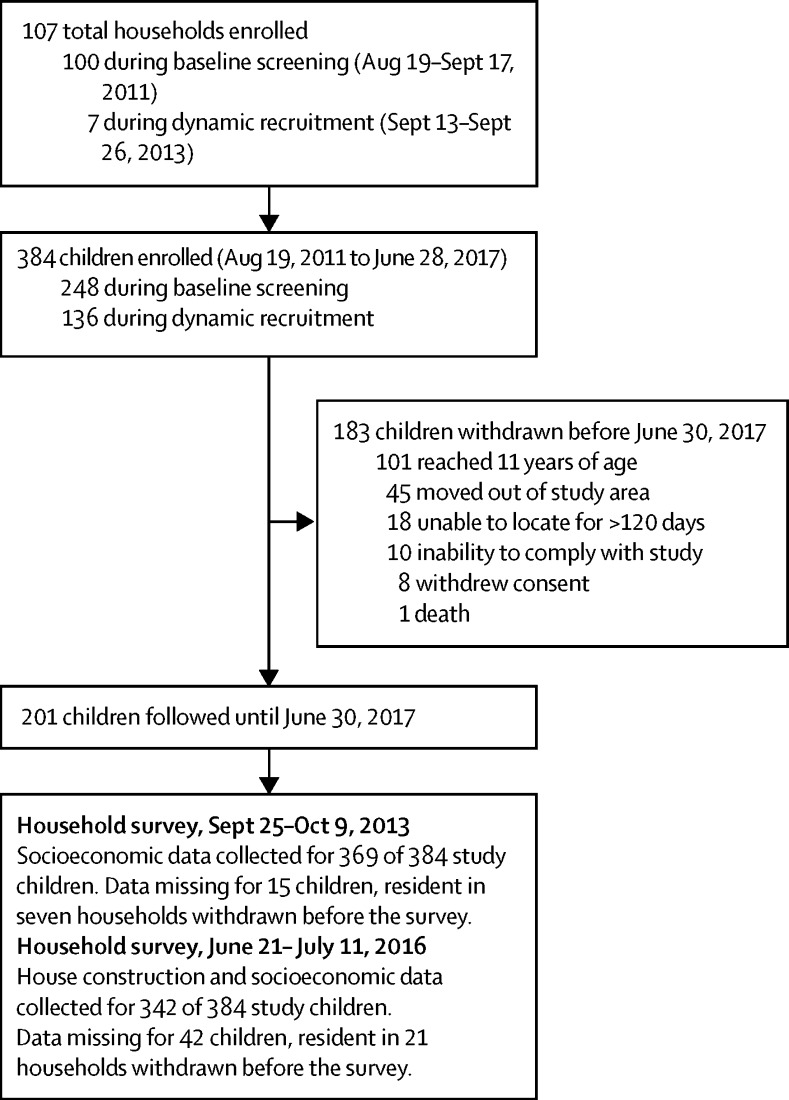
A total of 384 children resident in 107 households were enrolled between Aug 19, 2011, and June 28, 2017 (figure 2). The mean age of children during follow-up was 6·0 years (95% CI 5·7–6·2) and 181 (47%) were girls.
Figure 2.
Study profile
For the 2013 index, the first principal component explained 29·3% of overall variability in the asset variables. The weight assigned to each variable was: cupboard, 0·45; clock, 0·43; sofa, 0·41; table, 0·37; mobile, 0·30; toilet facility, 0·29; radio, 0·29; people per sleeping room, 0·19; and mode of transport to health facility, 0·10. For the 2016 index, the first principal component explained 21·5% of overall variability in the asset variables. The weight assigned to each variable was: toilet facility, 0·46; mobile telephone, 0·43; number of people per bedroom, 0·38; table, 0·33; radio, 0·31; sofa, 0·31; cupboard, 0·27; clock, 0·24; and main mode of transport to the health facility, 0·17.
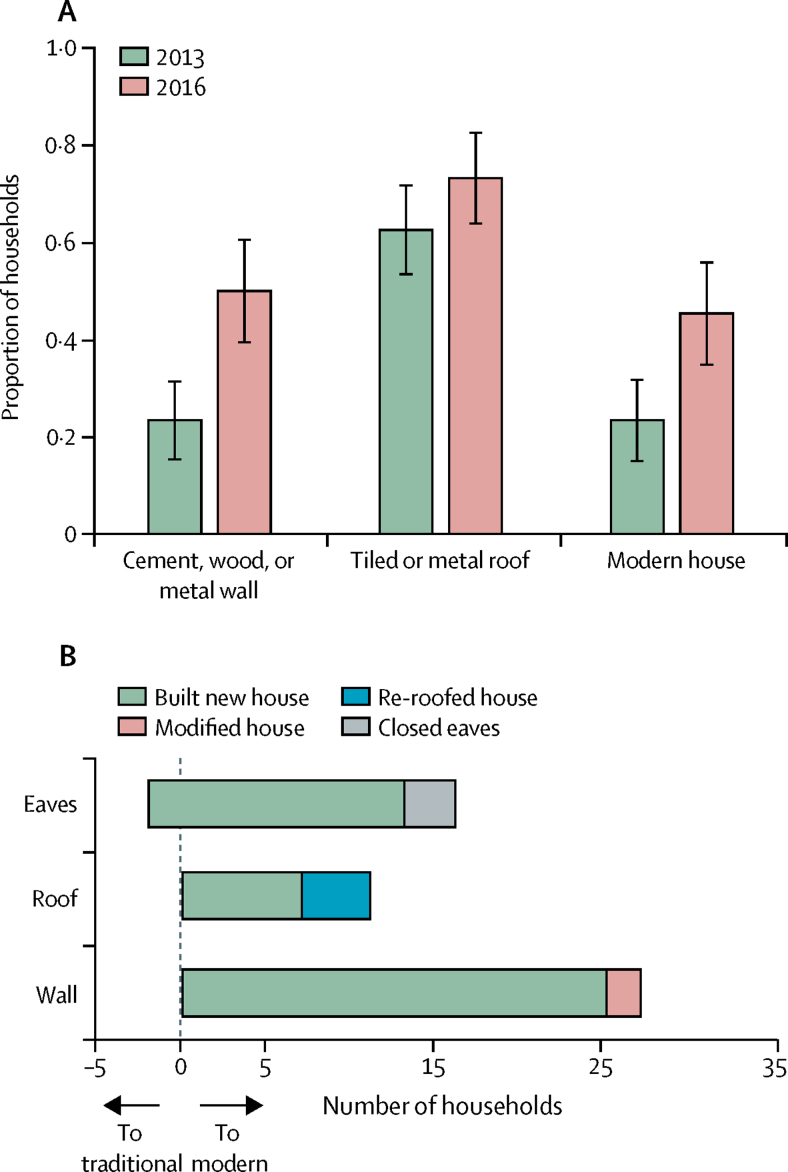
Between 2013 and 2016, there were increases in the proportion of households with cement, wood, or metal walls, tiled or metal roofs, and closed eaves, although only the increase for wall material was significant (table 1, figure 3A). Of the 11 (12·8%) households that upgraded from thatched to metal roofs, seven built new houses and four re-roofed; of the 27 (31·4%) households that upgraded from mud to brick walls, 25 built new houses and two modified existing houses; and of the 16 (18·6%) households that upgraded from open to closed eaves, 13 built new houses and three modified the eaves (figure 3B). Overall, the proportion of households classified as modern versus traditional increased from 23·4% in 2013 to 45·3% in 2016 (p=0·001). The prevalence of modern housing increased with socioeconomic position, from 0% in the lowest wealth tertile to 48·5% in the highest wealth tertile in 2013 (p<0·0001), and from 24·1% in the lowest wealth tertile to 60·7% in the highest wealth tertile in 2016 (p=0·02). One house that changed from modern in 2013 to traditional in 2016 was excluded from the analyses of house type as a risk factor for malaria.
Table 1.
Characteristics of study households in Nagongera, Uganda
|
Year |
p value | |||
|---|---|---|---|---|
| 2013 | 2016 | |||
| Number of households | 107 | 86 | ·· | |
| Wealth index tertile | ·· | ·· | 0·75 | |
| Poorest | 35 (35·0%) | 29 (33·7%) | ·· | |
| Middle | 32 (32·0%) | 29 (33·7%) | ·· | |
| Least poor | 33 (33·0%) | 28 (32·6%) | ·· | |
| Main floor material | ·· | ·· | 0·75 | |
| Earth, sand, or dung | 89 (83·2%) | 73 (84·9%) | ·· | |
| Bricks, tiles, or cement | 18 (16·8%) | 13 (15·1%) | ·· | |
| Main roof material | ·· | ·· | 0·12 | |
| Thatched | 40 (37·4%) | 23 (26·7%) | ·· | |
| Tiles or metal | 67 (62·6%) | 63 (73·3%) | ·· | |
| Main wall material | ·· | ·· | 0·0001 | |
| Mud | 82 (76·6%) | 43 (50·0%) | ·· | |
| Cement, wood, or metal | 25 (23·4%) | 43 (50·0%) | ·· | |
| Eaves | ·· | ·· | 0·05 | |
| Open | 51 (47·7%) | 29 (33·7%) | ·· | |
| Closed | 56 (52·3%) | 57 (66·3%) | ·· | |
| House type* | ·· | ·· | 0·001 | |
| Traditional | 82 (76·6%) | 47 (54·7%) | ·· | |
| Modern | 25 (23·4%) | 39 (45·4%) | ·· | |
Data are n (%).
Modern houses were defined as those with a cement, wood, or metal wall, tiled or metal roof, and closed eaves; all other houses were defined as traditional.
Figure 3.
Changes in house design in Nagongera, Uganda, 2013–16
(A) Changes in house type among study households between 2013 and 2016. Error bars represent 95% CIs. Modern houses were defined as those with a cement, wood, or metal wall, tiled or metal roof, and closed eaves; all other houses were defined as traditional. (B) Reported reason for changes to house design between 2013 and 2016.
Monthly CDC light trap collections were completed in 99·5% of actively followed households. A total of 136 067 adult female Anopheles were caught over 6338 collection nights, yielding an overall human biting rate of 21·5 Anopheles per house per night. Of these, 121 462 (89·3%) were Anopheles gambiae sensu lato and 13 549 (10·0%) were Anopheles funestus. Human biting rate declined from 33·5 Anopheles per house per night before indoor residual spraying (3858 collection nights) to 2·7 Anopheles per house per night after indoor residual spraying (2480 collection nights; p<0·0001). After indoor residual spraying was introduced the prevalence of A gambiae sensu lato (as a proportion of all Anopheles) increased from 88·9% to 96·4% and the prevalence of A funestus declined from 10·4% to 0·9%.
Both before and after indoor residual spraying, human biting rate was consistently lower in houses with tiled or metal roofs than thatch; cement, wood, or metal walls than mud; and closed than open eaves (table 2). Overall, controlling for socioeconomic position, there was a 48% reduction in human biting rate in houses that were modern at the time of both surveys compared with houses that were traditional at the time of both surveys before indoor residual spraying (adjusted incidence rate ratio [aIRR] 0·52, 95% CI 0·36–0·73, p=0·0002), increasing to a 73% reduction in modern houses after indoor residual spraying (aIRR 0·27, 0·17–0·42, p<0·0001). There was no evidence of interaction between the indoor residual spraying time period and house type.
Table 2.
Household risk factors for human biting rate in Nagongera, Uganda, before and after the introduction of indoor residual spraying
|
Before indoor residual spraying (Oct 1, 2011, to Jan 31, 2015) |
After indoor residual spraying (Feb 1, 2015, to June 30, 2017) |
||||||
|---|---|---|---|---|---|---|---|
| HBR* (total collection nights) | IRR†(95% CI) | p value | HBR* (total collection nights) | IRR†(95% CI) | p value | ||
| 2013 household data | |||||||
| Wealth index tertile | |||||||
| Poorest | 38·3 (1275) | 1 (ref) | ·· | 3·2 (965) | 1 (ref) | ·· | |
| Middle | 32·6 (1249) | 0·88 (0·67–1·16) | 0·37 | 2·9 (828) | 0·89 (0·61–1·32) | 0·57 | |
| Least poor | 27·2 (1224) | 0·72 (0·55–0·95) | 0·02 | 1·8 (687) | 0·51 (0·34–0·75) | 0·001 | |
| Main roof material | |||||||
| Thatched | 42·3 (1431) | 1 (ref) | ·· | 3·6 (979) | ·· | ·· | |
| Tiles or metal | 28·4 (2427) | 0·62 (0·49–0·78) | <0·0001 | 2·0 (1501) | 0·57 (0·41–0·80) | 0·001 | |
| Main wall material | |||||||
| Mud | 37·8 (2984) | 1 (ref) | ·· | 3·0 (2033) | 1 (ref) | ·· | |
| Cement, wood, or metal | 18·9 (874) | 0·48 (0·37–0·61) | <0·0001 | 1·1 (447) | 0·34 (0·23–0·50) | <0·0001 | |
| Eaves | |||||||
| Open | 41·2 (1829) | 1 (ref) | ·· | 3·1 (1252) | 1 (ref) | ·· | |
| Closed | 26·7 (2029) | 0·61 (0·49–0·76) | <0·0001 | 2·2 (1228) | 0·71 (0·51–1·00) | 0·05 | |
| 2016 household data | |||||||
| Wealth index tertile | |||||||
| Poorest | 41·2 (1085) | 1 (ref) | ·· | 3·0 (841) | 1 (ref) | ·· | |
| Middle | 30·2 (1128) | 0·76 (0·57–1·03) | 0·07 | 2·5 (796) | 0·84 (0·55–1·27) | 0·40 | |
| Least poor | 29·5 (1070) | 0·75 (0·55–1·01) | 0·05 | 2·4 (787) | 0·79 (0·52–1·19) | 0·26 | |
| Main roof material | |||||||
| Thatched | 44·0 (870) | 1 (ref) | ·· | 3·8 (659) | 1 (ref) | ·· | |
| Tiles or metal | 29·8 (2413) | 0·68 (0·52–0·89) | 0·005 | 2·2 (1765) | 0·58 (0·40–0·84) | 0·004 | |
| Main wall material | |||||||
| Mud | 39·7 (1616) | 1 (ref) | ·· | 3·7 (1217) | 1 (ref) | ·· | |
| Cement, wood, or metal | 27·6 (1667) | 0·70 (0·55–0·88) | 0·003 | 1·6 (1207) | 0·44 (0·33– 0·60) | <0·0001 | |
| Eaves | |||||||
| Open | 42·3 (1084) | 1 (ref) | ·· | 3·5 (824) | 1 (ref) | ·· | |
| Closed | 29·3 (2199) | 0·70 (0·55–0·91) | 0·007 | 2·2 (1600) | 0·64 (0·45–0·90) | 0·01 | |
| All household data | |||||||
| House typeठ| |||||||
| Traditional (2013 and 2016) | 38·8 (1712) | 1 (ref) | ·· | 3·5 (1295) | 1 (ref) | ·· | |
| Traditional (2013) and modern (2016) | 33·5 (958) | 0·90 (0·69–1·19) | 0·48 | 2·0 (693) | 0·55 (0·39–0·79) | 0·001 | |
| Modern (2013 and 2016) | 18·7 (573) | 0·52 (0·36–0·73) | 0·0002 | 1·0 (407) | 0·27 (0·17–0·42) | <0·0001 | |
Human biting rate (HBR) is the number of adult female Anopheles collected per house per night (total adult female Anopheles caught/total nights of collection).
IRR=incidence rate ratio.
Modern houses were defined as those with a cement, wood, or metal wall, tiled or metal roof, and closed eaves; all other houses were defined as traditional; IRR for house type was adjusted for household socioeconomic position.
Before indoor residual spraying, IRR adjusted for 2013 household socioeconomic position and after indoor residual spraying, IRR adjusted for 2016 household socioeconomic position.
8641 (92·4%) of 9352 scheduled routine clinic visits were completed among actively followed children. A total of 8641 blood smears were taken, of which 1908 (22·1%) had malaria parasites. All children contributed at least one smear. Before indoor residual spraying, 1243 (32·0%) of 3885 total blood smears were positive; after indoor residual spraying, 665 (14·0%) of 4756 total blood smears were positive (p<0·0001). Before indoor residual spraying, there was a strong association between socioeconomic position and parasitaemia, with a 43–49% reduction in odds of parasitaemia in the highest compared with the lowest wealth tertile, controlling for age and sex (2013 data: adjusted odds ratio [aOR] 0·51, 95% CI 0·35–0·75, p=0·001; 2016 data: aOR 0·57, 0·40–0·81, p=0·002; table 3). After indoor residual spraying, there was a similar strength of association with a 48–50% reduction in odds of parasitaemia in the highest compared with the lowest wealth tertile, controlling for age and sex (2013 data: aOR 0·52, 95% CI 0·30–0·92, p=0·03; 2016 data: aOR 0·50, 0·25–0·99, p=0·05). Before indoor residual spraying, there was no evidence of an association between house type and parasitaemia, but after indoor residual spraying there was a 57% reduction in the odds of parasitaemia in houses classified as modern in both surveys compared with houses classified as traditional in both surveys, controlling for age, sex, and socioeconomic position (aOR 0·43, 95% CI 0·24–0·77, p=0·004). There was no evidence of interaction between the indoor residual spraying time period and house type.
Table 3.
Household risk factors for parasitaemia in children aged 6 months to 10 years in Nagongera, Uganda, before and after the introduction of indoor residual spraying
|
Before indoor residual spraying (Aug 19, 2011, to Jan 31, 2015) |
After indoor residual spraying (Feb 1, 2015, to June 30, 2017) |
||||||
|---|---|---|---|---|---|---|---|
| Parasite rate* (total blood smears) | OR†(95% CI) | p value | Parasite rate* (total blood smears) | OR†(95% CI) | p value | ||
| Age | |||||||
| 6 months to <2 years | 17·6 (375) | 1 (ref) | ·· | 2·8 (218) | 1 (ref) | ·· | |
| 2 to <5 years | 26·0 (1152) | 1·64 (1·15–2·33) | 0·006 | 6·7 (1635) | 2·52 (0·95–6·69) | 0·06 | |
| 5 to 10 years | 37·2 (2358) | 2·76 (1·93–3·94) | <0·0001 | 19·0 (2903) | 8·16 (2·92–22·81) | <0·0001 | |
| Sex | |||||||
| Female | 30·8 (1764) | 1 (ref) | ·· | 10·5 (2285) | 1 (ref) | ·· | |
| Male | 33·0 (2121) | 1·10 (0·88–1·37) | 0·40 | 17·2 (2471) | 1·80 (1·27–2·56) | 0·001 | |
| 2013 household data | |||||||
| Wealth index tertile | |||||||
| Poorest | 40·0 (1274) | 1 (ref) | ·· | 20·6 (1707) | 1 (ref) | ·· | |
| Middle | 30·5 (1353) | 0·63 (0·47–0·85) | 0·003 | 9·4 (1617) | 0·40 (0·25–0·63) | <0·0001 | |
| Least poor | 26·2 (1158) | 0·51 (0·35–0·75) | 0·001 | 11·2 (1432) | 0·52 (0·30–0·92) | 0·03 | |
| Main roof material | |||||||
| Thatched | 29·5 (1537) | 1 (ref) | ·· | 12·5 (2052) | 1 (ref) | ·· | |
| Tiles or metal | 33·6 (2348) | 1·33 (0·99–1·78) | 0·06 | 15·1 (2704) | 1·34 (0·75–2·37) | 0·32 | |
| Main wall material | |||||||
| Mud | 34·2 (3236) | 1 (ref) | ·· | 15·3 (4062) | 1 (ref) | ·· | |
| Cement, wood, or metal | 21·0 (649) | 0·59 (0·42–0·83) | 0·003 | 6·1 (694) | 0·45 (0·24–0·83) | 0·01 | |
| Eaves | |||||||
| Open | 32·5 (1910) | 1 (ref) | ·· | 14·1 (2457) | 1 (ref) | ·· | |
| Closed | 31·5 (1975) | 1·04 (0·78–1·39) | 0·77 | 13·8 (2299) | 1·13 (0·69–1·86) | 0·63 | |
| 2016 household data | |||||||
| Wealth index tertile | |||||||
| Poorest | 36·3 (1165) | 1 (ref) | ·· | 16·1 (1659) | 1 (ref) | ·· | |
| Middle | 35·5 (1292) | 0·91 (0·64–1·29) | 0·58 | 15·9 (1618) | 0·86 (0·51–1·46) | 0·58 | |
| Least poor | 24·6 (1031) | 0·57 (0·40–0·81) | 0·002 | 8·7 (1432) | 0·50 (0·25–0·99) | 0·05 | |
| Main roof material | |||||||
| Thatched | 30·9 (955) | 1 (ref) | ·· | 12·6 (1345) | 1 (ref) | ·· | |
| Tiles or metal | 33·2 (2533) | 1·18 (0·83–1·68) | 0·35 | 14·2 (3364) | 1·13 (0·57–2·22) | 0·73 | |
| Main wall material | |||||||
| Mud | 32·6 (1862) | 1 (ref) | ·· | 15·0 (2430) | 1 (ref) | ·· | |
| Cement, wood, or metal | 32·6 (1626) | 1·09 (0·81–1·46) | 0·58 | 12·5 (2279) | 0·87 (0·54–1·39) | 0·55 | |
| Eaves | |||||||
| Open | 31·4 (1199) | 1 (ref) | ·· | 13·3 (1704) | 1 (ref) | ·· | |
| Closed | 33·2 (2289) | 1·08 (0·78–1·50) | 0·63 | 14·0 (3005) | 1·00 (0·54–1·86) | 1·00 | |
| All household data | |||||||
| House typeठ| |||||||
| Traditional (2013 and 2016) | 33·2 (1912) | 1 (ref) | ·· | 15·1 (2523) | 1 (ref) | ·· | |
| Traditional (2013) and modern (2016) | 36·6 (1062) | 1·33 (0·94–1·87) | 0·11 | 15·2 (1497) | 1·06 (0·64–1·75) | 0·82 | |
| Modern (2013 and 2016) | 23·3 (450) | 0·79 (0·57–1·11) | 0·17 | 6·4 (624) | 0·43 (0·24–0·77) | 0·004 | |
Parasite rate=total positive blood smears/total blood smears.
Odds ratio (OR) adjusted for age at the time of the blood smear (continuous variable), sex, and household socioeconomic position.
Modern houses were defined as those with a cement, wood, or metal wall, tiled or metal roof, and closed eaves; all other houses were defined as traditional.
Before indoor residual spraying, OR adjusted for 2013 household socioeconomic position and after indoor residual spraying, OR adjusted for 2016 household socioeconomic position.
A total of 2907 episodes of uncomplicated malaria were diagnosed after 1412·5 person-years of follow-up, yielding an overall incidence of 2·1 episodes per person-year at risk. Two participants were withdrawn immediately after enrolment without contributing time at risk. Incidence declined from 3·0 episodes per person-year (884·7 person-years) before indoor residual spraying to 0·5 episodes per person-year (527·8 person years) after indoor residual spraying (p<0·0001). Socioeconomic position was not associated with malaria incidence before indoor residual spraying, but after indoor residual spraying there was a 45% reduction in malaria incidence associated with 2013 socioeconomic position (highest vs lowest wealth tertile: IRR 0·55, 95% CI 0·39–0·78, p=0·001) and a 42% reduction associated with 2016 socioeconomic position (highest vs lowest wealth tertile: IRR 0·58, 0·42–0·82, p=0·002; table 4). House type and malaria incidence were not associated either before or after indoor residual spraying.
Table 4.
Household risk factors for clinical malaria in children aged 6 months to 10 years in Nagongera, Uganda, before and after the introduction of indoor residual spraying
|
Before indoor residual spraying (Aug 19, 2011, to Jan 31, 2015) |
After indoor residual spraying (Feb 1, 2015, to June 30, 2017) |
||||||
|---|---|---|---|---|---|---|---|
| Malaria incidence* (total person years) | IRR (95% CI)† | p value | Malaria incidence* (total person years) | IRR (95% CI)† | p value | ||
| Age | |||||||
| 6 months to <2 years | 4·71 (7·2) | 1 (ref) | ·· | 0·43 (14·0) | 1 (ref) | ·· | |
| 2 to <5 years | 4·18 (257·2) | 0·87 (0·74–1·03) | 0·10 | 0·56 (246·7) | 1·37 (0·60–3·10) | 0·46 | |
| 5 to 10 years | 2·43 (620·3) | 0·48 (0·40–0·58) | <0·0001 | 0·54 (267·1) | 1·62 (0·72–3·68) | 0·25 | |
| Sex | |||||||
| Female | 2·73 (398·8) | 1 (ref) | ·· | 0·52 (250·4) | 1 (ref) | ·· | |
| Male | 3·14 (486·0) | 1·14 (0·99–1·31) | 0·07 | 0·57 (277·4) | 1·10 (0·81–1·49) | 0·54 | |
| 2013 household data | |||||||
| Wealth index tertile | |||||||
| Poorest | 2·94 (286·9) | 1 (ref) | ·· | 0·72 (185·2) | 1 (ref) | ·· | |
| Middle | 3·05 (309·4) | 1·10 (0·89–1·36) | 0·38 | 0·50 (190·7) | 0·70 (0·51–0·95) | 0·02 | |
| Least poor | 2·88 (265·3) | 1·05 (0·83–1·32) | 0·70 | 0·40 (151·8) | 0·55 (0·39–0·78) | 0·001 | |
| Main roof material | |||||||
| Thatched | 3·24 (347·4) | 1 (ref) | ·· | 0·58 (231·3) | 1 (ref) | ·· | |
| Tiles or metal | 2·77 (537·3) | 0·94 (0·76–1·16) | 0·56 | 0·53 (296·5) | 1·10 (0·85–1·42) | 0·47 | |
| Main wall material | |||||||
| Mud | 3·02 (736·2) | 1 (ref) | ·· | 0·56 (452·1) | 1 (ref) | ·· | |
| Cement, wood, or metal | 2·67 (148·5) | 0·93 (0·66–1·30) | 0·66 | 0·49 (75·7) | 1·20 (0·84–1·71) | 0·33 | |
| Eaves | |||||||
| Open | 3·11 (433·9) | 1 (ref) | ·· | 0·58 (277·1) | 1 (ref) | ·· | |
| Closed | 2·81 (450·8) | 0·95 (0·78–1·15) | 0·58 | 0·51 (250·7) | 1·01 (0·78–1·31) | 0·95 | |
| 2016 household data | |||||||
| Wealth index tertile | |||||||
| Poorest | 3·19 (264·4) | 1 (ref) | ·· | 0·65 (191·3) | 1 (ref) | ·· | |
| Middle | 2·98 (294·3) | 0·97 (0·76–1·23) | 0·79 | 0·59 (176·7) | 0·92 (0·66–1·27) | 0·61 | |
| Least poor | 3·07 (234·1) | 0·99 (0·77–1·27) | 0·93 | 0·38 (154·5) | 0·58 (0·42–0·82) | 0·002 | |
| Main roof material | |||||||
| Thatched | 2·97 (212·9) | 1 (ref) | ·· | 0·55 (149·9) | 1 (ref) | ·· | |
| Tiles or metal | 3·11 (579·8) | 1·16 (0·92–1·46) | 0·22 | 0·55 (372·7) | 1·09 (0·80–1·49) | 0·59 | |
| Main wall material | |||||||
| Mud | 3·16 (422·5) | 1 (ref) | − | 0·60 (274·5) | 1 (ref) | ·· | |
| Cement, wood, or metal | 2·98 (370·2) | 0·97 (0·79–1·19) | 0·74 | 0·49 (248·1) | 0·89 (0·67–1·17) | 0·40 | |
| Eaves | |||||||
| Open | 3·15 (269·6) | 1 (ref) | ·· | 0·59 (187·2) | 1 (ref) | ·· | |
| Closed | 3·04 (523·1) | 1·01 (0·80–1·28) | 0·91 | 0·52 (335·4) | 0·90 (0·68–1·20) | 0·48 | |
| All household data | |||||||
| House typeठ| |||||||
| Traditional (2013 and 2016) | 3·15 (434·3) | 1 (ref) | ·· | 0·61 (282·8) | 1 (ref) | ·· | |
| Traditional (2013) and modern (2016) | 2·93 (242·3) | 0·90 (0·73–1·11) | 0·34 | 0·47 (164·5) | 0·81 (0·57–1·15) | 0·24 | |
| Modern (2013 and 2016) | 2·80 (101·7) | 0·84 (0·58–1·23) | 0·37 | 0·50 (68·6) | 0·93 (0·65–1·35) | 0·72 | |
Malaria incidence per person-years=new malaria episodes/person years of observation.
Incidence rate ratio (IRR) adjusted for mean age during follow-up (continuous variable), sex, and household socioeconomic position.
Modern houses were defined as those with a cement, wood, or metal wall, tiled or metal roof, and closed eaves; all other houses were defined as traditional.
Before indoor residual spraying, IRR adjusted for 2013 household socioeconomic position and after indoor residual spraying, IRR adjusted for 2016 household socioeconomic position.
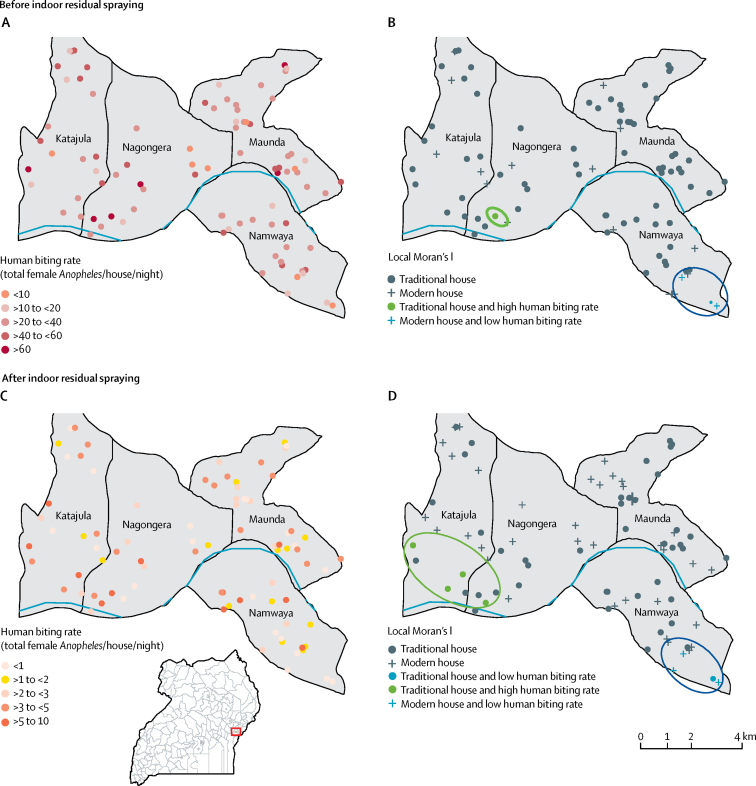
There was evidence of local clustering of human biting rate and house type, with a cluster of modern housing and low human biting rate in the southeast of the study area both before and after indoor residual spraying and a cluster of traditional housing and high human biting rate in the southwest of the study area after indoor residual spraying (figure 4).
Figure 4.
Local cluster maps of human biting rate and house type in 86 households in Nagongera, Uganda, before and after the introduction of indoor residual spraying
Houses were classified as modern (cement, wood, or metal walls, a tiled or metal roof, and closed eaves) or traditional (all other houses). Positive spatial autocorrelation (spatial clustering) occurs when a household with a specific outcome value is surrounded by neighbouring households with similar outcome value (low–low, high–high). (A) Human biting rate before indoor residual spraying (Oct 1, 2011, to Jan 31, 2015). (B) Univariate local indicator of spatial association (LISA) analysis of house type and human biting rate before indoor residual spraying. (C) Human biting rate after indoor residual spraying (Feb 1, 2015, to June 30, 2017). (D) Univariate LISA analysis of house type and human biting rate after indoor residual spraying. The implementation of indoor residual spraying was associated with study-wide declines in human biting rate. Before and after indoor residual spraying, a cluster of modern housing and low human biting rate is observed in the southeast of the study area. After indoor residual spraying, a cluster of traditional housing and high human biting rate is observed in the southwest of the study area.
Discussion
We have provided the first quantification of temporal changes in house design in the context of malaria control in Uganda and evaluated their association with malaria in relation to a mass scale-up of control efforts. Between 2013 and 2016, there was a rapid increase in the prevalence of modern houses with a cement, wood, or metal wall, tiled or metal roof, and closed eaves. Modern houses were associated with a 48% reduction in human biting rate before indoor residual spraying, rising to a 73% reduction after indoor residual spraying, and we found evidence of local spatial clustering of modern housing and reduced human biting rate. There was no association between house type and parasitaemia before indoor residual spraying, but after indoor residual spraying there was a 57% reduction in the odds of parasitaemia in modern compared with traditional houses. Our study provides evidence of rapid changes to house design in rural Uganda and confirms house design as a risk factor for malaria from intense to reduced transmission.
Well designed housing can help to reduce malaria transmission by lowering house entry by mosquitoes and therefore reducing human exposure to infectious bites.8 Protective features might include the presence of a ceiling, closed eaves,18 and screening on doors and windows.4, 8 Our findings concur with previous studies of the same population in Nagongera, which found a strong association between house type and human biting rate and parasite prevalence, but not consistently with malaria incidence.9, 11 A similar association between house type and parasitaemia was observed in a systematic review and meta-analysis, which showed that improved housing was associated with 47% lower odds of parasitaemia in a range of African settings,7 as well as an analysis of survey data from 21 countries, which found modern housing across Africa to be associated with a 9–14% reduction in the odds of parasitaemia after controlling for socioeconomic position, similar to the reduction associated with the use of insecticide-treated nets.6 Since the house improvements assessed in this study did not approach universal coverage targets nor were they implemented as part of a malaria control programme, it is possible that a greater effect might be achieved with deliberate and planned intervention.
Few studies have evaluated the effect of house design on malaria in the context of other malaria interventions.19 Our study confirms previous observations that the introduction of indoor residual spraying was associated with significant reductions in malaria burden in Nagongera.12, 13 Here we observed an increase in the strength of association between house design and mosquito density and parasite prevalence following the introduction of indoor residual spraying, possibly because any effect of house design on mosquito house entry and parasitaemia is density dependent (ie, exacerbated when overall transmission levels are reduced). There might also be synergy between the effect of house design and indoor residual spraying, due to a lower residual effect of bendiocarb20, 21 and Actellic22 on mud than brick and cement walls, or a combined effect of increased mosquito mortality, excito-repellency, and reduced house entry in sprayed, modern homes. This observation is discrepant from a study in The Gambia, which found a greater reduction in mosquito entry following indoor residual spraying in thatched than metal-roofed houses; however, that finding might have resulted from the exclusion of metal roofs from the surfaces sprayed.23 Further studies are needed to clarify the potential interaction between house design and indoor residual spraying.
There is growing evidence that house design is rapidly changing in many parts of endemic Africa alongside wider economic and demographic change.5, 7 To our knowledge, our study is the first to quantify changes in house design in the context of malaria control in Uganda. We observed a striking increase in the prevalence of modern housing within less than 3 years. It is possible that participation in an intensive prospective study led to health and therefore economic benefits that increased the ability of study households to invest in their homes. However, the observed changes are consistent with broader shifts in consumption patterns across Africa, where per capita spending on housing is projected to increase by an average of 4·4% annually between 2017 and 2025.24
Homeowner investments, together with increased housing demand stemming from population growth and urbanisation,25 the general suppression of malaria transmission in urban environments,26 and impetus from the New Urban Agenda for sustainable global urban development,2 provide a prime opportunity to build healthier housing to integrate malaria control more closely with other aspects of socioeconomic development. Indeed, the geographical cluster of modern housing and reduced human biting rate found in the small town in the southeast of the study area highlights the potential overlap between urbanisation, housing improvements, and malaria control. In particular, in the context of possible rebound in transmission if interventions such as indoor residual spraying are discontinued, housing improvements might offer more long-term protection. Our study concurs with recent findings from urban Dar es Salaam that rapid improvements to housing linked to economic change, alongside primary malaria interventions, were associated with reductions in community-level vector densities.27 Of course, improving housing is not a panacea and it is important to establish whether malaria risk increases in unscreened houses through the deflection of bites. Metal-roofed housing, although proliferating, might not be a universally sympathetic design and there remains a need to identify comfortable and culturally acceptable house designs that reduce exposure to vectors.28, 29
This study had several limitations. First, the sample size was calculated to compare temporal changes in malaria incidence in the cohort with temporal changes in malaria test positivity rate from health facility based surveillance and the study was therefore not powered specifically for the analyses undertaken. Second, since socioeconomic position and house design were strongly associated, residual confounding by socioeconomic position is likely. The wealth index is also highly influenced by the variables included and is an imperfect representation of socioeconomic position.16 Third, our hypothesis is rooted in the assumption of causality from house design to malaria, but a reverse effect is plausible if households with a higher burden of malaria are less able to invest in housing. Despite that, the reduced human biting rate in modern houses is consistent with a direct effect via suppressed vector house entry. Fourth, the measurements of house type at two timepoints might not be representative of changes to house design that occurred outside the surveyed period. Fifth, our findings relate to a small sample of households in rural eastern Uganda, and might have limited generalisability to urban areas or to other rural settings with different eco-epidemiological characteristics. Sixth, a large number of statistical tests were done, increasing the risk of false positive results. Last, the lack of consideration of window screening or glazing is a substantial limitation because mosquitoes are unlikely to be deterred by closed eaves alone if the windows are completely open. For all these reasons, our findings require future validation in this and other settings. Nonetheless, our observations are consistent over two household survey years (2013 and 2016), and with an increasing body of research that identifies house design as an important risk factor for malaria.6, 8, 9, 30
In conclusion, we provide the first evidence of rapid improvements to house design in rural Uganda and show that house design remains a key risk factor for malaria as transmission declines. Large-scale changes to housing in endemic Africa present an opportunity to further the gains achieved with more widely accepted malaria control interventions and to keep countries malaria free after elimination.
For more on the MRC/DFID Concordat agreement see http://www.mrc.ac.uk
Acknowledgments
Acknowledgments
We are grateful to the study participants and their families. We thank the Infectious Disease Research Collaboration for administrative and technical support. This work was supported by NIH/NIAID (U19AI089674), Research and Policy for Infectious Disease Dynamics (RAPIDD) programme of the Science and Technology Directorate, US Department of Homeland Security, the Fogarty International Center (US National Institutes of Health), and the Bill & Melinda Gates Foundation (OPP1053338). LST is a Skills Development Fellow (#N011570) jointly funded by the UK Medical Research Council (MRC) and the UK Department for International Development under the MRC/DFID Concordat agreement.
Contributors
MRK, SWL, SGS, and GD conceived and designed the study. JCR, EA, MRK, AK, MK, SGS, GD, and LST collected the data. JCR, VA, GD, and LST analysed the data. JT and EC advised on the analysis. JCR, VA, GD, and LST drafted the manuscript. All authors saw drafts and provided input. All authors approved the final version of the Article.
Declaration of interests
We declare no competing interests.
References
- 1.UN . United Nations Department of Economic and Social Affairs Population Division; New York: 2015. World population prospects: key findings and advance tables 2015 revison. [Google Scholar]
- 2.Habitat III. New urban agenda. 2016. https://habitat3.org/the-new-urban-agenda/ (accessed Sept 21, 2017).
- 3.Tusting LS, Willey B, Lines J. Building malaria out: improving health in the home. Malaria J. 2016;15:1–3. doi: 10.1186/s12936-016-1349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogoma SB, Kannady K, Sikulu M. Window screening, ceilings and closed eaves as sustainable ways to control malaria in Dar es Salaam, Tanzania. Malaria J. 2009;8:1. doi: 10.1186/1475-2875-8-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu JX, Bousema T, Zelman B. Is housing quality associated with malaria incidence among young children and mosquito vector numbers? Evidence from Korogwe, Tanzania. PLoS One. 2014;9:e87358. doi: 10.1371/journal.pone.0087358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tusting LS, Bottomley C, Gibson H. Housing improvements and malaria risk in sub-Saharan Africa: a multi-country analysis of survey data. PLoS Med. 2017;14:e1002234. doi: 10.1371/journal.pmed.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tusting LS, Ippolito M, Kleinschmidt I. The evidence for improving housing to reduce malaria: a systematic review and meta-analysis. Malaria J. 2015;14:209. doi: 10.1186/s12936-015-0724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirby MJ, Ameh D, Bottomley C, Green C, Jawara M, Milligan PJ. Effect of two different house screening interventions on exposure to malaria vectors and on anaemia in children in The Gambia: a randomised controlled trial. Lancet. 2009;374:998–1009. doi: 10.1016/S0140-6736(09)60871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wanzira H, Tusting LS, Arinaitwe E. Mind the gap: house construction and the risk of malaria in Ugandan children. PLoS One. 2015;10:e0117396. doi: 10.1371/journal.pone.0117396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snyman K, Mwangwa F, Bigira V. Poor housing construction associated with increased malaria incidence in a cohort of young Ugandan children. Am J Trop Med Hyg. 2015;92:1207–1213. doi: 10.4269/ajtmh.14-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tusting LS, Rek J, Arinaitwe E. Why is malaria associated with poverty? Findings from a cohort study in rural Uganda. Infect Dis Poverty. 2016;5:78. doi: 10.1186/s40249-016-0164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katureebe A, Zinszer K, Arinaitwe E. Measures of malaria burden after long-lasting insecticidal net distribution and indoor residual spraying at three sites in Uganda: a prospective observational study. PLoS Med. 2016;13:e1002167. doi: 10.1371/journal.pmed.1002167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alegana VA, Kigozi SP, Nankabirwa J. Spatio-temporal analysis of malaria vector density from baseline through intervention in a high transmission setting. Parasit Vectors. 2016;9:637. doi: 10.1186/s13071-016-1917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maxwell K, Smith DL, Hutchinson R. Estimating the annual entomological inoculation rate for Plasmodium falciparum transmitted by Anopheles gambiae s.l. using three sampling methods in three sites in Uganda. Malaria J. 2014;13:111. doi: 10.1186/1475-2875-13-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamya MR, Arinaitwe E, Wanzira H. Malaria transmission, infection and disease at three sites with varied transmission intensity in Uganda: implications for malaria control. Am J Trop Med Hyg. 2015;92:903–912. doi: 10.4269/ajtmh.14-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tusting LS, Rek JC, Arinaitwe E. Measuring socioeconomic inequalities in relation to malaria risk: a comparison of metrics in rural Uganda. Am J Trop Med Hyg. 2016;94:650–658. doi: 10.4269/ajtmh.15-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anselin L. Local indicators of spatial association—LISA. Geogr Anal. 1995;27:93–115. [Google Scholar]
- 18.Njie M, Dilger E, Lindsay SW, Kirby MJ. Importance of eaves to house entry by Anopheline, but not Culicine, mosquitoes. J Med Entomol. 2009;46:977–984. doi: 10.1603/033.046.0314. [DOI] [PubMed] [Google Scholar]
- 19.Bradley J, Rehman AM, Schwabe C. Reduced prevalence of malaria infection in children living in houses with window screening or closed eaves on Bioko Island, equatorial Guinea. PLoS One. 2013;8:e80626. doi: 10.1371/journal.pone.0080626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Etang J, Nwane P, Mbida JA. Variations of insecticide residual bio-efficacy on different types of walls: results from a community-based trial in south Cameroon. Malaria J. 2011;10:333. doi: 10.1186/1475-2875-10-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeebiyo Y, Dengela D, Tesfaye AG. Short persistence of bendiocarb sprayed on pervious walls and its implication for the indoor residual spray program in Ethiopia. Parasit Vector. 2016;9:266. doi: 10.1186/s13071-016-1549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chanda E, Chanda J, Kandyata A. Efficacy of ACTELLIC 300 CS, pirimiphos methyl, for indoor residual spraying in areas of high vector resistance to pyrethroids and carbamates in Zambia. J Med Entomol. 2013;50:1275–1281. doi: 10.1603/me13041. [DOI] [PubMed] [Google Scholar]
- 23.Tangena J-AA, Adiamoh M, D'Alessandro U. Alternative treatments for indoor residual spraying for malaria control in a village with pyrethroid- and DDT-resistant vectors in The Gambia. PLoS One. 2013;8:e74351. doi: 10.1371/journal.pone.0074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKinsey Global Institute . McKinsey Global Institute; 2016. Lions on the move II: realizing the potential of Africa's economies. [Google Scholar]
- 25.UN Habitat . UN Habitat; Nairobi: 2014. State of African cities 2014: re-imagining sustainable urban transitions. [Google Scholar]
- 26.Tatem A, Gething P, Smith D, Hay S. Urbanization and the global malaria recession. Malaria J. 2013;12:133. doi: 10.1186/1475-2875-12-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Msellemu D, Namango HI, Mwakalinga VM. The epidemiology of residual Plasmodium falciparum malaria transmission and infection burden in an African city with high coverage of multiple vector control measures. Malaria J. 2016;15:288. doi: 10.1186/s12936-016-1340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knudsen J, von Seidlein L. Axel Menges; Stuttgart/London: 2014. Healthy homes in tropical zones: improving rural housing in Asia and Africa. [Google Scholar]
- 29.von Seidlein L, Ikonomedis K, Mshamu S. Affordable house designs to improve health in rural Africa: a field study from northeastern Tanzania. Lancet Planet Health. 2017;1:e188–e199. doi: 10.1016/S2542-5196(17)30078-5. [DOI] [PubMed] [Google Scholar]
- 30.Ogoma SB, Lweitoijera DW, Ngonyani H. Screening mosquito house entry points as a potential method for integrated control of endophagic filariasis, arbovirus and malaria vectors. PLoS Negl Trop Dis. 2010;4:e773. doi: 10.1371/journal.pntd.0000773. [DOI] [PMC free article] [PubMed] [Google Scholar]