Abstract
Subcutaneous emphysema may aggravate traumatic pneumothorax treatment, especially when mechanical ventilation is required. Expectative management usually suffices, but when respiratory function is impaired surgical treatment might be indicated. Historically relevant methods are blowhole incisions and placement of various drains, often with related wound complications. Since the first report of negative pressure wound therapy for the treatment of severe subcutaneous emphysema in 2009, only few publications on use of commercially available sets were published. We report on patient injured in a motor vehicle accident who had serial rib fractures and bilateral pneumothorax managed initially in another hospital. Due to respiratory deterioration, haemodynamic instability and renal failure patient was transferred to our Intensive Care Unit. Massive and persistent subcutaneous emphysema despite adequate thoracic drainage with respiratory deterioration and potentially injurious mechanical ventilation with high airway pressures was the indication for active surgical treatment. Negative-pressure wound therapy dressing was applied on typical blowhole incisions which resulted in swift emphysema regression and respiratory improvement. Negative pressure wound therapy for decompression of severe subcutaneous emphysema represents simple, effective and relatively unknown technique that deserves wider attention.
Keywords: Subcutaneous emphysema, Negative-pressure wound therapy, Vacuum-assisted closure, Pneumothorax, Respiratory insufficiency
Background
Subcutaneous emphysema (SE) is usually benign air leak through parietal pleura after thoracic surgery or trauma involving rib fracture and pneumothorax. Most cases of SE are self-limiting and resolve spontaneously after adequate thoracic drainage. When massive SE develops, it disfigures the patient due to chest, neck and face swelling and can cause respiratory insufficiency. Massive SE, especially in mechanically ventilated patient, can endanger tracheal patency, aggravate ventilation and obstruct venous return from head and neck. Open or video-assisted thoracoscopic surgery (VATS) in critically ill patients might be detrimental. Less invasive treatment options include blowhole incisions and placement of various drains, often with related wound complications. There are only few reports in literature on use of vacuum-assisted closure device for treatment severe SE in patients with secondary pneumothorax or after pulmonary resections. We report on a critically ill mechanically ventilated patient with persistent severe SE after thoracic trauma treated successfully with negative-pressure wound therapy (NPWT) dressing.
Case report
Sixty-year-old male was injured in a motor-vehicle accident while driving a van unrestrained. He sustained 4th to 9th right side rib fractures with bilateral pneumothorax, left hip dislocation and fractures of spinous processes of 5th to 8th thoracic vertebrae. He was obese with BMI = 39 kg/m2 and has quit smoking after left lower lobectomy for carcinoid 12 years before the accident. The patient was initially attended in a regional hospital where both sides of thoracic cavity were drained. Hip dislocation was reduced under the general anaesthesia. This turned to be significant due to subsequent mechanical ventilation dependence. Four days later the patient was transferred to our Intensive Care Unit (ICU) due to respiratory insufficiency, haemodynamic instability and acute renal failure necessitating haemodialysis. On admission massive SE of face, neck, thoracic and abdominal wall was the hallmark (Fig. 1). CT scans upon arrival revealed persistent bilateral pneumothorax, pneumomediastinum, pneumoperitoneum (without signs of viscus perforation) and subcutaneous emphysema extending to the pelvis. Both thoracic drains were replaced for unsatisfactory position but it did not yield improvement. Continuous venovenous haemodiafiltration was initiated. The patient was septic and multi-resistant Acinetobacter baumanii was isolated from tracheal aspirate. Bilevel positive airway pressure (BPAP) mode of mechanical ventilation was used with high inspiratory airway pressure (Pinsp) 30 mbar), high positive end-expiratory pressure (PEEP 10 mbar) and low dynamic compliance (Cdyn 33–40 mL/mbar). Five days after admission to our ICU patient's respiratory status further deteriorated and high inspired oxygen fraction (FiO2 100%) was required for sufficient oxygenation. Control CT confirmed correct position of thoracic drains but also persistent massive SE which was the indication for surgical decompression (Fig. 2). At the bedside in ICU after sterile prepping and draping, two subclavicular blowhole incisions up to 3 cm in length were created through skin, subcutaneous tissue and pectoral fascia. After meticulous haemostasis, routinely available NPWT dressing set was applied (V.A.C.®GranuFoam Medium Dressing Kit, KCI Acelity, San Antonio TX USA). After insertion of trimmed polyurethane foam in the wounds, first layer of adhesive drape was placed. Two separate fenestrations were bridged with V shape foam stripe covered with second drape layer allowing suction through solitary V.A.C. pad. The described technique allowed distancing from the patient's swollen neck and economic use of only one pad (Fig. 3). Continuous negative pressure of 100 mm Hg was used. Swift regression of SE was noticed over 12 h followed by improvement in ventilatory parameters (Pinsp 24 mbar, Cdyn 40–47 mL/mbar, FiO2 50–60%) which allowed transition to pressure support mode (PSV) of mechanical ventilation. NPWT dressing was removed after 72 h and the wounds were sutured which allowed healing with primary intention. Surgical tracheostomy was performed and the patient was weaned off ventilator after prolonged struggle with sepsis. Renal function gradually improved. Further recovery was uneventful.
Fig. 1.
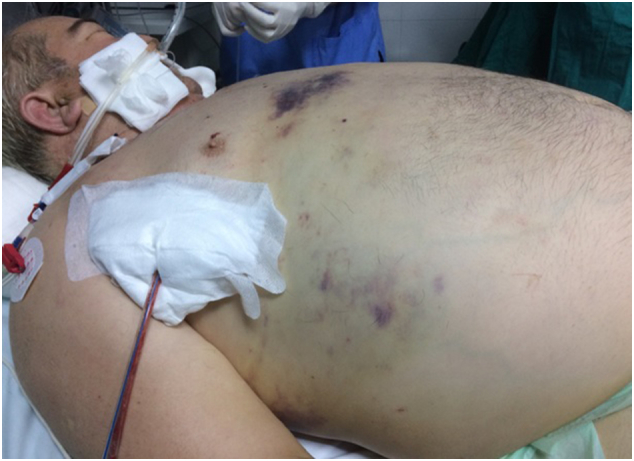
Patient on admission to our ICU with marked thoraco-abdominal wall distension and facial disfigurement due to massive subcutaneous emphysema.
Fig. 2.
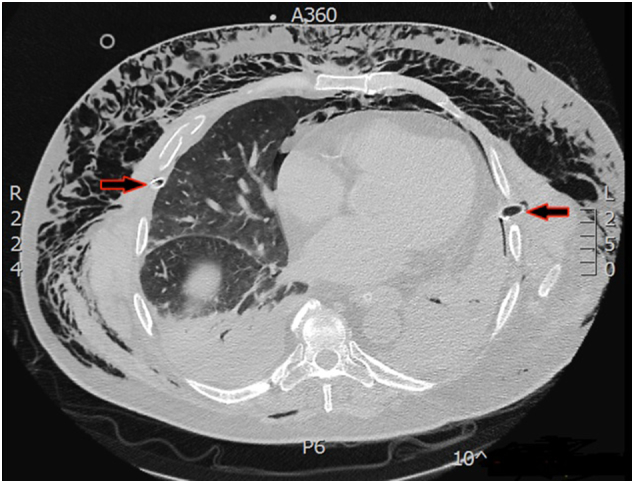
CT showing massive and persistent subcutaneous emphysema despite appropriate thoracic drain placement (red arrows). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
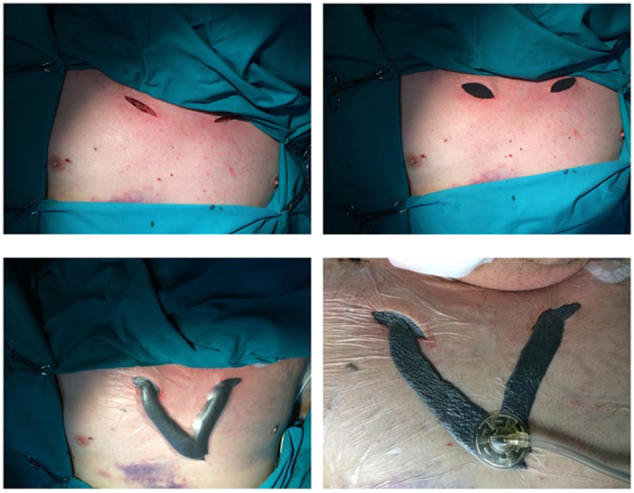
Technique of NPWT dressing for bilateral subclavicular blowhole incisions.
Discussion
SE results from ongoing air leak from simultaneously severed visceral and parietal pleura into subfascial and subcutaneous tissue planes. In mechanically ventilated patients leak is boosted by positive pressures required. Treatment sine qua non is proper thoracic drainage which removes inter-pleural air allowing apposition of two pleurae thus sealing the leaking spot. In patients with thoracic trauma demonstration of high-flow lung leak is indication for surgery, favourably by VATS in experienced hands [1]. Open surgery and even VATS might be intolerable by critically ill patients, especially regarding necessary lung isolation. Less invasive treatment may be suitable for this group of patients. SE that affects respiratory and/or circulatory dynamics, especially with fulminant onset mandates drainage of trapped air collection. Available interventions range from multiple needle venting, placement of fenestrated drain or angiocatheter, to the most effective blowhole incisions in peracute cases [2]. Created blowhole incisions can be supplied with a simple rubber/silicone drains or gauze packing. Drawbacks are the same: it is a two-way street that enables air traffic but also leaves the door open for bacteria access, especially in ICU environment. The second disadvantage is the need for regular, at least daily, wound dressing that consumes time and resources, underlining previously mentioned infection risks. NPWT represents revolution in treatment of variety of complex wounds and the spectrum of application widens constantly [3]. Commercially available sets offer simple yet effective solutions. However, although first described eight years ago, NPWT system model for treatment of SE influencing respiration has not been widely used. PubMed search for “subcutaneous emphysema” and “negative pressure wound therapy” or “vacuum assisted closure” yielded only four papers. Total of 16 patients were treated with NPWT system but technique and indications vary. Original technique by Sciortino et al. published in 2009 was observed in our case because we consider it the most advantageous [4]. Main points are subclavicular incision through skin, fat and pectoral fascia and negative pressure of 100 mm Hg. Indication for the intervention was persistent severe postoperative SE with high positive end-expiratory ventilatory pressure required to maintain adequate oxygenation [4]. Two South Korean multicenter case series studies from 2013 and 2014 followed. Report by Byun et al. on four patients with pneumothorax secondary to chronic obstructive pulmonary disease (COPD) and intractable SE despite chest tube drainage does not mention patients' respiratory status [5]. Negative pressure of 150 mm Hg might have generated wound site pain declared as a common complication. Son et al. retrospectively analyzed 10 patients who developed severe SE during ventilator care [6]. The suggestion for NPWT use was pneumomediastinum-induced cardiac tamponade and airway obstruction. Again, negative pressure of 150 mm Hg was applied. Well-illustrated feature is creation of subcutaneous space to facilitate air removal. We find this wound pocketing disadvantageous with increased risk of complications such as bleeding, infection and soreness especially during sponge removal. This technique failed to hasten the process of air extraction with reported mean duration of NPWT of 7.3 ± 4.8 (range 3–14) days which is explained with pathological lung status and the presence of positive-pressure mechanical ventilation [6]. The most recent report on NPWT use for the treatment of recalcitrant SE after thoracoscopic right middle lobe resection causing vision loss and emotional distress, without dyspnoea or respiratory insufficiency was published in October 2014 by Towe and colleagues [7]. Treatment consisted of 6 cm subclavicular incision extended to the pectoral fascia with 125 mm Hg negative pressure leading to SE regression and vision improvement within 4 h, but reportedly NPWT dressing frequently obstructed, losing suction. Two days later the dressing was extended to a contralateral side with complete response within the next 24 h [7]. All of the above-mentioned authors agree on safety and efficacy of NPWT use as an alternative and advantageous management of wounds created to relieve poorly controlled SE especially when thoracic surgical intervention is considered contraindicated or too risky. Technical aspects of the NPWT model are rather obvious: broad contact of polyurethane foam surface with the wound in conjunction with continuous high negative pressure efficiently and swiftly removes the trapped air, not allowing clotting or blocking as described with simple catheters or drains. Superiority in sterility and speed of SE regression strongly supports negative pressure use. NPWT provides a model by which this might be achieved. In the light of our experience with presented case we encourage NPWT use in conjunction with appropriately indicated decompression incisions for selected patients. Limitation for the stronger recommendation is paucity of published supporting evidence. Further clinical studies might yield enough data to modify current clinical practice for respiratory compromised thoracic trauma patients and/or patients after thoracic surgery with severe SE.
Conclusion
NPWT was used to manage the two wounds created to relieve pressure in the treatment of severe persistent SE in critically ill patient. SE regressed swiftly which directly facilitated ventilation. We believe that potential benefit outweighs risks of this generally safe albeit invasive procedure. Technique might be especially beneficial for patients unfit for surgery and therefore represents a strategy that can be more readily resorted to. NPWT dressing for active treatment of severe SE is simple, effective and relatively unknown technique that deserves wider attention.
Disclosure
JM travelled and participated in Acelity Surgical Wound Forum in Frankfurt 15–17 June 2017 at the expense of Acelity™, San Antonio TX USA.
Footnotes
Case was presented as a second award winning abstract at the Acelity Surgical Wound Forum, organised and sponsored by Acelity, San Antonio TX USA, held in Frankfurt am Main, Germany, 15–17 June 2017.
References
- 1.Cerfolio R.J., Bryant A.S., Maniscalo L.M. Management of subcutaneous emphysema after pulmonary resection. Ann. Thorac. Surg. 2008;85:1759–1763. doi: 10.1016/j.athoracsur.2007.12.079. [DOI] [PubMed] [Google Scholar]
- 2.Herlan D.B., Landreneau R.J., Ferson P.F. Massive spontaneous subcutaneous emphysema. Acute management with infraclavicular “blow holes”. Chest. 1992;102:503–505. doi: 10.1378/chest.102.2.503. [DOI] [PubMed] [Google Scholar]
- 3.Huang C., Leavitt T., Bayer L.R., Orgill D.P. Effect of negative pressure wound therapy on wound healing. Curr. Probl. Surg. 2014;51:301–331. doi: 10.1067/j.cpsurg.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Sciortino C.M., Mundinger G.S., Kuwayama D.P. Case report: treatment of severe subcutaneous emphysema with a negative pressure wound therapy dressing. Eplasty. 2009;9 [PMC free article] [PubMed] [Google Scholar]
- 5.Byun C.S., Choi J.H., Hwang J.J. Vacuum-assisted closure therapy as an alternative treatment of subcutaneous emphysema. Korean J. Thorac. Cardiovasc. Surg. 2013;46:383–387. doi: 10.5090/kjtcs.2013.46.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Son Bong Soo, Lee Sungsoo, Cho Woo Hyun, Hwang Jung Joo, Kim Kil Dong, Kim Do Hyung. Modified blowhole skin incision using negative pressure wound therapy in the treatment of ventilator-related severe subcutaneous emphysema. Interact. Cardiovasc. Thorac. Surg. 2014;19(6):904–907. doi: 10.1093/icvts/ivu287. [DOI] [PubMed] [Google Scholar]
- 7.Towe C., Solomon B., Donington J.S., Pass H.I. Treatment of recalcitrant subcutaneous emphysema using negative pressure wound therapy dressings. BMJ Case Rep. 2014 doi: 10.1136/bcr-2014-205577. [DOI] [PMC free article] [PubMed] [Google Scholar]


