Abstract
Background
Older adults receiving cancer therapy have heightened risk for treatment-related toxicity. Geriatric Assessment (GA) can identify impairments, which may contribute to vulnerability and adverse outcomes. GA management interventions can address these impairments and have the potential to improve outcomes when implemented.
Methods
We conducted a randomized pilot study comparing GA with management interventions versus usual care in patients with stage III/IV solid tumor malignancies (N=71). In all patients, a trained coordinator conducted and scored a baseline GA with pre-determined cutoffs for impairment. For patients randomized to the intervention arm, an algorithm was used to identify GA management recommendations based upon identified impairments. Recommendations were relayed to the primary oncologist for implementation. GA was repeated at 3 months. The primary outcome was grade 3-5 chemotherapy toxicity. Secondary outcomes included feasibility, hospitalizations, dose reductions, dose delays and early treatment discontinuation.
Results
Mean participant age was 76 (70-89). The total number of GA management recommendations relayed was 409, of which 35.4% were implemented by the primary oncologist. Incidence of grade 3-5 chemotherapy toxicity did not differ between the two groups. Prevalence of hospitalization, dose reductions, dose delays, and early treatment discontinuation also did not differ between the two groups.
Conclusions
An algorithm can be used to guide GA management recommendations in older adults with cancer. However, reliance upon the primary oncologist for execution resulted in a low prevalence of implementation. Future work should aim to understand barriers to implementation and explore alternate models of implementing geriatric-focused care for older adults with cancer.
Keywords: Geriatric Assessment with Management, Cancer, Geriatric Oncology, Geriatric Assessment with Management
Background
Cancer is primarily a disease of older adults, with the majority of cancer diagnoses and cancer-related deaths occurring in patients over the age of 65.1,2 Older adults with cancer commonly have additional age-associated conditions, such as geriatric syndromes or comorbidities,3–5 which may complicate cancer therapy. A GA is recommended to help objectively evaluate the overall health status of an older adult considering cancer therapy.6 GA is a validated assessment tool that evaluates a variety of domains that commonly affect older adults, including evaluation of physical function, comorbidity, polypharmacy, cognition, psychological status, social support and nutritional status. GA has been shown to detect impairments not captured by oncology performance status assessment7 and has demonstrated feasibility for incorporation it is in the oncology setting.8 Domains in GA are predictive of chemotherapy toxicity.9–11 The Cancer and Aging Research Group (CARG) chemotherapy toxicity score is a validated tool that predicts the likelihood of chemotherapy toxicity and incorporates not only cancer- and treatment-specific variables, but also domains from the GA, such as a history of falls and difficulties with medication management.9,11
Rates of treatment-related toxicity for older adults receiving chemotherapy are higher than the younger population, and geriatric factors have been shown to influence the likelihood of toxicity in this population.9–11 Implementing geriatric assessment (GA) with targeted management interventions to address specific impairments may help to improve older adults’ tolerance of therapy and cancer-related outcomes.12 A few select studies in geriatric oncology have evaluated the impact of GA on change in treatment plan and GA management interventions in a non-randomized fashion.13–19 Although GA with management interventions has not yet been formally evaluated in the older cancer population in randomized studies, multiple studies in the non-cancer setting demonstrate benefit from GA management, suggesting potential benefit in the oncology setting as well.20–34 GA can identify specific impairments, such as cognitive impairment or limited social support, and targeted management interventions can be used to support these vulnerabilities in patients receiving cancer therapy.12 GA management interventions are utilized frequently by geriatricians in the non-cancer population with the goal to reverse or provide support to areas of impairment identified on GA. An example of a targeted management intervention based upon GA impairment is implementing physical therapy and home safety evaluations for individuals with impaired physical function or falls. GA with targeted management interventions has been studied extensively in the general geriatrics population and have been shown to reduce mortality,28,31 hospitalizations32 and functional decline.31 A Delphi study was conducted of geriatric oncology experts to develop consensus on high-priority GA-based targeted management interventions for older adults with cancer;35,36 however, to date, no randomized, prospective studies have reported on the feasibility and utility of GA with management interventions in older adults receiving cancer treatment.
With the aging of the population and the limited number of geriatrics-trained health care providers, it is not realistic for all older adults with cancer to be cared for by oncology professionals with have additional geriatric expertise. Therefore, models of care need to be developed to support oncologists in incorporating geriatrics into routine oncology care. An algorithm could be used to guide oncology practitioners in the use of GA management interventions. We conducted a randomized pilot study to assess the feasibility and preliminary efficacy of GA with an algorithm to guide GA management interventions on chemotherapy toxicity and other outcomes (hospitalizations, dose reduction, dose delays, and early termination of treatment) of older adults with advanced cancer starting a new cancer treatment regimen.
Methods
Study Participants
Eligible subjects were age 70 and over with a diagnosis of an advanced (stage III or IV) solid tumor malignancy. Patients were seen and evaluated by their primary oncologist and received a plan for initiation of first- or second-line treatment. Eligible treatment regimens included chemotherapy, chemoradiotherapy, or targeted therapy with or without chemotherapy. Eligible patients were able to read and understand English, able to provide informed consent (as assessed by their primary oncologist), had a life expectancy of 6 months or greater, and had a planned treatment regimen lasting at least 3 months. Patients were excluded if they had a planned surgery within three months of consent or if they had a referral to a geriatric oncologist.
Study Design (Figure 1)
Figure 1.
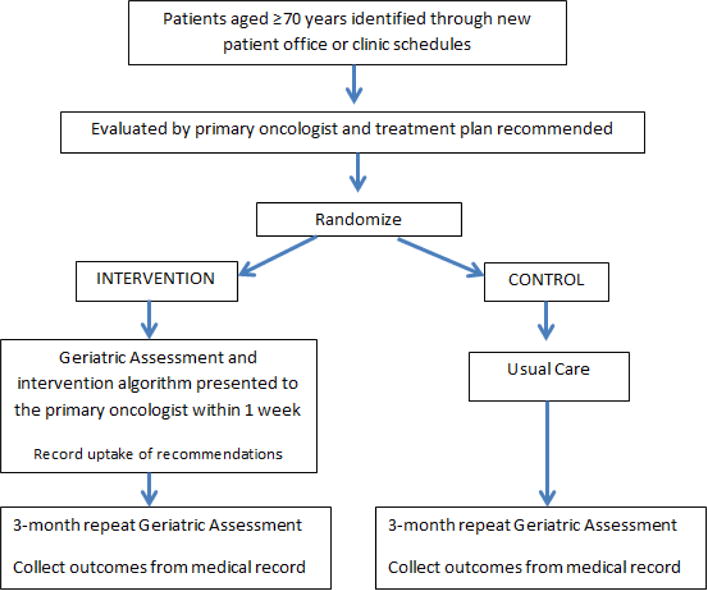
Study Schema
Eligible patients were approached by the study coordinator with permission from the primary oncologist. Following consent, baseline clinical, demographic and GA data was collected. Eighty-three patients consented to participate in the study, however 12 patients did not complete the baseline assessment and thus were not randomized. Following collection of baseline information, patients were randomized using block randomization. Patients were followed for three months after treatment initiation and GA was repeated 3 months after treatment initiation. Following the patients’ completion of all study procedures, their medical records were reviewed by a blinded study investigator in order to capture the administered treatment regimen for each cycle including any supportive care medications, and outcomes including grade 3-5 chemotherapy toxicity, hospitalizations, dose reductions, dose delays, and treatment discontinuation. Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE version 4.0).
Measures
Clinical and demographic information was collected by study coordinators. At baseline, GA was performed, including measures in the following domains: physical function, cognition, nutrition, social support, psychological status, comorbidity, and polypharmacy (Table 1).8 The majority of GA measures were collected using patient self-report questionnaires. A study coordinator conducted objective cognitive and objective physical performance assessment measures. Tumor and treatment characteristics were collected from the medical record. GA measures were evaluated again at 3 months after treatment initiation. A CARG chemotherapy toxicity score was calculated at baseline using clinical and GA measures.9
Table 1.
Geriatric Assessment domains, scoring tools and management interventions
| Assessment Tool | Scoring Cutoff | Targeted GA Management Recommendations | |
|---|---|---|---|
| Physical Function | |||
|
|
|
|
| Nutrition | |||
|
|
|
|
| Social Support | |||
|
|
|
|
| Cognition | |||
|
|
|
|
| Polypharmacy | |||
|
|
|
|
| Comorbidity | |||
|
|
|
|
| Psychological | |||
|
|
|
|
Intervention: GA with targeted management recommendations
A previously developed algorithm, based upon expert consensus,35 was used to formulate management recommendations for specific GA impairments with minor adaptations based upon availability of local resources (Table 1). For patients randomized to the intervention arm, the coordinator scored the GA and identified impairments (defined by pre-determined scoring cutoffs for each GA domain). The coordinator then summarized GA impairments with management recommendations and delivered recommendations to the patient’s primary oncologist within one week of assessment. At the 3-month follow-up time point, the primary oncologist reported whether these recommendations had been implemented.
Statistical Analysis
Descriptive analyses were performed to summarize patient characteristics, GA results and feasibility measures. T-test (continuous data) and Chi square tests (categorical data) were used to compare baseline characteristics and GA results between usual care and intervention groups including age (continuous), gender (male vs female), race (white versus other), marital status (single vs married vs divorced vs separated), income status (<20K vs 20-50K vs 50-100K vs >100K), education (<high school vs high school vs >high school), impaired activities of daily living (none vs ≥1), impaired instrumental activities of daily living (none vs ≥1), history of falls (none vs ≥1), self-reported limitations in physical activity (yes vs no), comorbidities (≤3 vs >3), impaired vision (yes vs no), impaired hearing (yes vs no), Geriatric Depression Screen (≤5 versus >5), impaired social activities (a little/none vs all/most/some of the time), distress score (<5 vs ≥5), self-reported Karnofsky performance status (>80 vs ≤70), Blessed-Orientation-Memory-Concentration test (≤10 vs >10), Short Physical Performance Battery Score (≤9 vs >9), and Mini-cog score (normal vs abnormal). Outcome variables, including incidence of NCI CTCAE grades ≥3 chemotherapy toxicity (yes vs no), hospitalizations (yes vs no), dose reductions (yes vs no), dose delays (yes vs no), early treatment terminations (yes vs no), and hospice enrollments (yes vs no), were calculated for both arms. Logistic regression was used to examine each variable in a univariate analysis and stepwise selection using entry and retention of P values of >0.1 and clinical significant variables. Separate regression models were used for each outcome variable. Investigators assessing outcomes and statisticians were blinded to allocation. All analysis were performed using SAS statistical software (SAS 9.3, NC). All statistical tests were 2-sided and p values <0.05 were considered statistically significant. The Institutional Review Board at the University of Rochester approved the study.
Results
Patient Characteristics
Seventy-one patients were randomized between November 2013 and January 2015. The mean age of study participants was 76 (range 70-89, SD 4.98). At baseline, patient demographics and GA variables were not significantly different between the two arms, except for a greater percentage of patients in the intervention group with Instrumental Activities of Daily Living (IADL) impairment (Table 2). Additionally, there was a significant difference in the CARG toxicity score, with the intervention arm having more patients in the high-risk CARG group (p=0.04).
Table 2.
Patient demographics and baseline geriatric assessment results
| Patient Demographics | |||
|---|---|---|---|
| Control | Intervention | P Value | |
| Gender | Male – 54% Female – 46% |
Male – 59% Female – 41% |
0.66 |
| Income Level | ≤ 50K – 74% > 50K – 26% |
≤ 50K – 83% > 50K – 17% |
0.37 |
| Insurance Coverage | Medicare – 37% Medicare + Medicaid – 3% Medicare + Private – 43% Medicare + Private – 17% |
Medicare – 38% Medicare + Medicaid – 5% Medicare + Private – 41% Medicare + Private – 16% |
0.96 |
| Race | Caucasian – 97% Other – 3% |
Caucasian – 91% Other – 9% |
0.36 |
| Education Level | ≤ High School – 60% > High School – 40% |
≤ High School – 57% > High School – 43% |
0.96 |
| Marital Status | Married – 65% Other – 35% |
Married – 57% Other – 43% |
0.63 |
| Tumor type | GI – 38% Lung – 47% GU – 15% Other – 0% |
GI – 48% Lung – 37% GU – 8% Other – 5% |
0.16 |
| Tumor Stage | III – 32% IV – 68% |
III – 27% IV – 72% |
0.80 |
| Line of therapy | First – 94% Second – 6% |
First – 95% Second -5% |
1.00 |
| Baseline Geriatric Assessment Results | |||
| Control | Intervention | P value | |
| Activities of Daily Living (ADL) impairment | 20% | 27% | 0.48 |
| Instrumental Activities of Daily Living (IADL) impairment | 31% | 59% | 0.02 |
| ≥3 Comorbidities | 29% | 41% | 0.29 |
| Physical Activity Impairment | 60% | 65% | 0.67 |
| Geriatric Depression Screen >5 | 12% | 19% | 0.52 |
| Vision Impairment (self-report fair or worse) | 14% | 8% | 0.47 |
| Hearing Impairment (self-report fair or worse) | 29% | 19% | 0.34 |
| History of Falls | 23% | 27% | 0.77 |
| Self-rated Karnofsky Performance Status <70 | 14% | 22% | 0.32 |
| Short Physical Performance Battery <9 | 69% | 73% | 0.68 |
| Abnormal Mini-Cog score | 19% | 33% | 0.12 |
| Blessed Orientation-Memory-Concentration Test score >10 | 6% | 3% | 0.61 |
| Baseline Cancer and Aging Research Group (CARG) Toxicity Score | Low-9% Medium-76% High-15% |
Low-19% Medium-46% High-35% |
0.04 |
Study Feasibility and Intervention Uptake (Table 3)
Table 3.
GA management recommendations and implementation rates
| GA management recommendations | |
|---|---|
| Intervention | % Uptake |
| PT/OT Referral | 24% (6/25) |
| Fall Precautions Counseling Handout | 44% (11/25) |
| Home Safety Evaluation | 28% (7/25) |
| Vitamin D Screen With Repletion | 12% (3/25) |
| Consider Initial Dose Reduction | 55% (17/31) |
| Minimize Psychoactive Medications in cognitive impairment | 40% (4/10) |
| Personal Emergency Response Device | 0% (0/10) |
| Energy Conservation Handout | 44% (8/18) |
| Exercise Regimen Handout | 44% (8/18) |
| Home Nursing/Home Health Aid Referral | 41% (7/17) |
| Nutrition Counseling Handout | 63% (5/8) |
| Nutrition Referral | 50% (4/8) |
| Meals-On-Wheels Referral | 21% (3/11) |
| Ride Assistance Programs | 33% (3/9) |
| Social Work Referral | 71% (15/21) |
| Identification of Health Care Proxy | 31% (4/13) |
| Co-signature For Treatment Consents in cognitive impairment | 8% (1/13) |
| Delirium Education Handout | 31% (4/13) |
| Pillbox Recommendation | 18% (5/28) |
| Minimization of High Risk Medications | 36% (5/14) |
| Polypharmacy Medication Reduction | 50% (1/2) |
| Avoidance of Neurotoxic Agents (Diabetes Comorbidity) | 73% (8/11) |
| Avoidance of Nephrotoxic Agents | 40% (2/5) |
| Consider Pharmacologic Therapy for depression | 14% (1/7) |
| Referral for Psychotherapy | 14% (1/7) |
| Support Group Information | 58% (7/12) |
| Chaplaincy/Spiritual Counseling | 33% (4/12) |
Among all eligible patients who were approached and asked to participate in this study, 75% consented. Twelve patients withdrew following consent but prior to completion of baseline assessment and were not randomized; three ultimately declined chemotherapy prior to starting treatment, one transitioned to hospice prior to initiating treatment and eight felt overwhelmed with the study procedures and declined to participate further.
GA at 3-month follow-up was completed on 89% of patients who had completed baseline assessment (N=63/71). Reasons for lack of 3-month follow-up data include withdrawal from study (3 patients), death due to disease (2 patients), or loss to follow-up (2 patients). Of the 37 patients randomized to the intervention group, recommendations for GA management interventions were relayed to the primary oncologist within the target time-frame in 34 patients (92%). Overall uptake of recommended interventions by the primary oncologist was 35.4% (145/409). The most frequently implemented interventions were avoidance of neurotoxic agents in patients with a history of neuropathy risk (73%), social work referral (71%) and nutrition counseling information (63%). The lest frequently implemented interventions were personal emergency response device implementation (0%), co-signature for chemotherapy consents in patients with cognitive impairment (8%) and vitamin D screen and repletion in patients with a fall history (12%). (Table 3)
Follow-Up Assessment, Chemotherapy Toxicities, and Treatment Outcomes (Figure 2)
Figure 2.
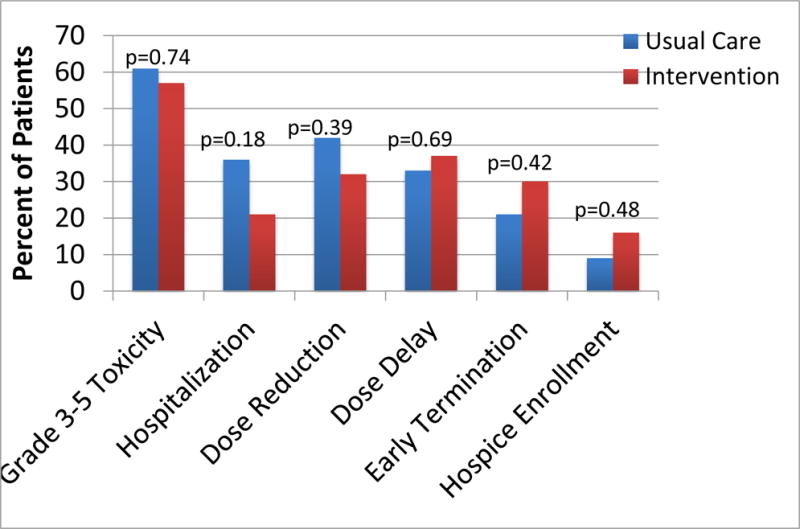
Summary of primary and secondary outcomes
Repeat GA at 3-month follow-up showed no significant differences between patients in the two arms in any of the self-reported measures. The difference in IADL status between the two arms was no longer present at 3 months (usual care = 38% impaired, intervention = 49% impaired; p=0.38). The occurrence of grade 3-5 toxicities did not differ significantly between the two arms (61% in usual care versus 57% in the intervention arm, p=0.74). No differences were observed between the two arms in the number of hospitalizations, dose reductions, dose delays, early treatment terminations, or enrollments in hospice.
Toxicity based upon CARG chemotherapy score
The baseline CARG chemotherapy toxicity score was used to evaluate the likelihood of chemotherapy toxicity for each patient and averaged for each arm.9,11 The average CARG chemotherapy toxicity score for the usual care arm was 8.06, with a mean likelihood of toxicity of 58%. Compared to the anticipated toxicity of 58%, observed toxicity in the usual care arm was 61% (p = 0.56). The average CARG chemotherapy toxicity score for the intervention group was 8.78, with a mean likelihood of toxicity of 60%. Compared to the anticipated toxicity of 60%, observed toxicity in the intervention group was 57% (p=0.55).
Discussion
In this prospective, randomized pilot study of a GA management intervention, it was feasible to use a pre-specified algorithm to develop GA management recommendations independent of geriatrician input, with recommendations relayed to the primary oncologist in >90% of intervention subjects. However, only a third of these recommendations were ultimately implemented by the primary oncologist into care plans for the patients. Recommendations that were more oncology-based, such as “consider initial dose reduction” in patients with multiple impairments and “consider avoiding neurotoxic agents” in patients with diabetes mellitus or neuropathy were more frequently implemented by the oncologists. Recommendations for referrals that were logistically more feasible (i.e., cancer center-based, such as social work referral or nutrition consult), were also implemented at higher frequency. Recommendations for interventions that were more “geriatric-specific” and may have been less familiar to oncologists were less likely to be implemented, such as “recommend a personal emergency response system (PERS)” for patients with impaired physical function.
The patients in this study represent a vulnerable population, with 74% scoring impaired on the objective physical performance measures, nearly 30% screening positive for cognitive impairment, and 36% having >3 comorbidities. Despite the vulnerability of this population, we were able to accrue patients; 75% of approached patients consented to participation. Considering the vulnerability of this population, retention of patients over the 3-month course of the study was also reasonably high with only 11% drop out mainly due to progressive illness. There were a significant number of patients who consented to participate in the study and then withdrew prior to completing the baseline assessment due to feeling overwhelmed with study procedures (8 patients). Given the pilot nature of the study, a large battery of assessment tools was used and this could potentially be limited for future studies to minimize this dropout. Older adults are frequently underrepresented in clinical trials, and enrolling vulnerable older adults in oncology clinical trials is an important step toward increasing the knowledge base for this population.37
The intervention did not demonstrate a significant impact on cancer-related outcomes, including chemotherapy toxicity, hospitalizations, dose reductions, dose delays, early treatment terminations, or hospice enrollments. Older adults with cancer are at increased risk for treatment-related toxicity. Prior studies evaluating chemotherapy toxicity in older adults demonstrated rates of grade 3-5 toxicity between 53% and 64%.9–11 Our study is consistent with these results; 59% of patients experienced toxicity within the first three months of treatment. The current study was designed as a pilot study with a modest sample size, and did not have adequate power to detect significant difference in outcomes between the two arms. This limits the conclusions to be drawn from a negative result. A larger sample size with greater power would have the ability to detect a smaller difference between the control and intervention groups, and a larger, multi-site study evaluating the impact of GA with management recommendations in this population is underway (NCT02054741; Geriatric Assessment Intervention for Reducing Toxicity in Older Patients With Advanced Cancer). Kalsi and colleagues observed benefit from comprehensive geriatric assessment interventions on tolerance to chemotherapy in a non-randomized fashion.14 In this study, they compared subjects who received a GA with recommended interventions as compared to a historical cohort. Investigators determined that the intervention group was more likely to complete cancer treatment as planned, had fewer required treatment modifications, and had a trend towards lower toxicity rates.
This study design explored a model of care for implementing geriatric interventions in the management of older adults with cancer, independent of a geriatrics provider. To our knowledge, this is the first study in geriatric oncology to evaluate the feasibility of implementing GA management recommendations independent of a geriatrics provider using an algorithm. We determined that it is feasible to utilize an algorithm to guide GA management recommendations, although ultimate implementation of the more geriatrics-based recommendations by oncologists was low and low uptake of the recommendations may have diminished the strength of the intervention for improving outcomes. Limited uptake of GA management recommendations by non-geriatrics providers has been demonstrated in other studies as well.38,39 Wildiers, et al evaluated the implementation of GA management recommendations in older adults with cancer in Belgium. In this study, patients aged 70 and over were enrolled at diagnosis or disease progression and underwent GA. Concrete geriatric recommendations were developed and relayed to the treating physicians. In this study, 35.3% of all the geriatric recommendations were performed, which is similar to our study. In a meta-analysis of controlled trials of comprehensive GA in the non-cancer population, studies in which geriatricians retained control over implementation of medical recommendations were more likely to be effective than those that relied upon the primary care provider for implementation.33 Therefore, limited implementation of GA management recommendations is the most likely reason for the lack of improvement of the intervention on outcomes in this pilot study.
Alternate models for implementing these recommendations need to be explored. The ELCAPA study evaluated the influence of a geriatrician-led GA on cancer treatment decision-making by oncologists and found that GA results prompted modification of the cancer treatment plan in 20% of patients enrolled onto the study.13 Clearly, a useful model would be direct involvement of a geriatrics provider; however, given the limited number of geriatric practitioners and the increasing numbers of older adults with cancer, this model of care would not be feasible for the majority of patients. An alternative model would be to engage other care team members, such as nursing and social work, into the implementation of GA management recommendations. A trial is in progress evaluating the feasibility of a nurse practitioner in developing and implementing GA management interventions (NCT02517034). Studies in non-cancer settings have evaluated the feasibility and impact of non-physician driven interventions and have demonstrated benefit. For example, the DEED II study evaluated the effects of a GA with multidisciplinary management intervention on elderly patients discharged from the emergency department (ED).32 In this study, a research team member, typically a nurse, evaluated subjects randomized to the intervention and initiated immediate interventions or referrals based upon the GA. Investigators determined that patients randomized to the intervention had lower rates of admissions to the hospital in the 30 days after the initial ED visit and lower rates of repeat ED visits in the 18-month follow-up period. Additionally, enhanced geriatrics education for oncologists may be another mechanism to increase the implementation of geriatric-specific interventions into oncology practice. Prior studies of educational programs for non-geriatrics providers have demonstrated improvements in clinician knowledge about geriatric issues and may alter treatment practices.40,41
There are limitations to this study. The study was performed at a single institution, and was not performed as a cluster randomization; oncologists who enrolled multiple patients onto the study may have observed patterns of GA management recommendations from patients enrolled on the intervention arm and may have later applied similar recommendations to subsequent patients enrolled on the usual care arm, thus minimizing the observed effect of the intervention. However, given the limited uptake of recommendations in this study, this explanation is less likely. Nonetheless, future studies could be designed as cluster randomized studies to minimize this potential bias. The two arms in our study were not perfectly balanced, with patients in the intervention arm having higher rates of IADL impairment and greater frequency of high-risk CARG chemotherapy toxicity scores, potentially minimizing the benefits of the intervention. Future studies should enroll a larger sample size to achieve more balanced arms. Additionally, as described, the low implementation of GA management recommendations limits interpretation of the ability of the intervention to improve cancer-related outcomes; future studies should explore alternate methods for implementation.
In conclusion, this pilot study demonstrated that utilizing an algorithm to guide GA management recommendations to oncologists in older adults with cancer is feasible. In this study, we were able to recruit older, vulnerable adults with cancer readily to a clinical trial and observed a high prevalence of geriatric issues and chemotherapy toxicity in this population. This demonstrates a need for further supportive care interventions for this population. Rates of implementation of GA-based recommendations by the primary oncologists were low and this study adds to the literature that reliance on oncologists to implement GA-based interventions limits feasibility. Further research is needed to understand barriers to implementation of GA-based interventions in oncology and to develop alternative models of care to implement GA management recommendations in older adults with cancer.
Acknowledgments
Funding/Support: The work was funded by Wilmot Research Fellowship Award and R03 AG042342 and with support from the National Cancer Institute R25 CA102618.
Footnotes
Previous Presentation of Results: Some results of this study were presented at the 2016 American Society of Clinical Oncology (ASCO) Annual Meeting.
References
- 1.Balducci L. Epidemiology of cancer and aging. The Journal of oncology management : the official journal of the American College of Oncology Administrators. 2005 Spring;14(2):47–50. [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015 Jan-Feb;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Koroukian SM, Murray P, Madigan E. Comorbidity, disability, and geriatric syndromes in elderly cancer patients receiving home health care. J Clin Oncol. 2006 May 20;24(15):2304–2310. doi: 10.1200/JCO.2005.03.1567. [DOI] [PubMed] [Google Scholar]
- 4.Koroukian SM, Xu F, Bakaki PM, Diaz-Insua M, Towe TP, Owusu C. Comorbidities, functional limitations, and geriatric syndromes in relation to treatment and survival patterns among elders with colorectal cancer. The journals of gerontology. Series A, Biological sciences and medical sciences. 2010 Mar;65(3):322–329. doi: 10.1093/gerona/glp180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohile SG, Fan L, Reeve E, et al. Association of cancer with geriatric syndromes in older Medicare beneficiaries. J Clin Oncol. 2011 Apr 10;29(11):1458–1464. doi: 10.1200/JCO.2010.31.6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurria A, Browner IS, Cohen HJ, et al. Senior adult oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2012 Feb;10(2):162–209. doi: 10.6004/jnccn.2012.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002 Jan 15;20(2):494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 8.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005 Nov 1;104(9):1998–2005. doi: 10.1002/cncr.21422. [DOI] [PubMed] [Google Scholar]
- 9.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011 Sep 1;29(25):3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2011 Nov 9; doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- 11.Hurria A, Mohile S, Gajra A, et al. Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults With Cancer. J Clin Oncol. 2016 Jul 10;34(20):2366–2371. doi: 10.1200/JCO.2015.65.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magnuson AAH, Cohen HJ, Mohile SG, Williams GR, Chapman A, Extermann M, Olin RL, Targia V, Mackenzie A, Holmes HM, Hurria A. Geriatric Assessment with Management in Cancer Care: Current Evidence and Potential Mechanisms for Future Research. Journal of Geriatric Oncology. 2016 doi: 10.1016/j.jgo.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caillet P, Canoui-Poitrine F, Vouriot J, et al. Comprehensive geriatric assessment in the decision-making process in elderly patients with cancer: ELCAPA study. J Clin Oncol. 2011 Sep 20;29(27):3636–3642. doi: 10.1200/JCO.2010.31.0664. [DOI] [PubMed] [Google Scholar]
- 14.Kalsi T, Babic-Illman G, Ross PJ, et al. The impact of comprehensive geriatric assessment interventions on tolerance to chemotherapy in older people. British journal of cancer. 2015 Apr 28;112(9):1435–1444. doi: 10.1038/bjc.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourdel-Marchasson I, Blanc-Bisson C, Doussau A, et al. Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: a two-year randomized controlled trial. PloS one. 2014;9(9):e108687. doi: 10.1371/journal.pone.0108687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodwin JS, Satish S, Anderson ET, Nattinger AB, Freeman JL. Effect of nurse case management on the treatment of older women with breast cancer. J Am Geriatr Soc. 2003 Sep;51(9):1252–1259. doi: 10.1046/j.1532-5415.2003.51409.x. [DOI] [PubMed] [Google Scholar]
- 17.McCorkle R, Strumpf NE, Nuamah IF, et al. A specialized home care intervention improves survival among older post-surgical cancer patients. J Am Geriatr Soc. 2000 Dec;48(12):1707–1713. doi: 10.1111/j.1532-5415.2000.tb03886.x. [DOI] [PubMed] [Google Scholar]
- 18.Rao AV, Hsieh F, Feussner JR, Cohen HJ. Geriatric evaluation and management units in the care of the frail elderly cancer patient. The journals of gerontology. Series A, Biological sciences and medical sciences. 2005 Jun;60(6):798–803. doi: 10.1093/gerona/60.6.798. [DOI] [PubMed] [Google Scholar]
- 19.Nipp R, Sloane R, Rao AV, Schmader KE, Cohen HJ. Role of pain medications, consultants, and other services in improved pain control of elderly adults with cancer in geriatric evaluation and management units. J Am Geriatr Soc. 2012 Oct;60(10):1912–1917. doi: 10.1111/j.1532-5415.2012.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillespie LD, Gillespie WJ, Robertson MC, Lamb SE, Cumming RG, Rowe BH. Interventions for preventing falls in elderly people. Cochrane Database Syst Rev. 2003;(4):CD000340. doi: 10.1002/14651858.CD000340. [DOI] [PubMed] [Google Scholar]
- 21.Beswick AD, Rees K, Dieppe P, et al. Complex interventions to improve physical function and maintain independent living in elderly people: a systematic review and meta-analysis. Lancet. 2008 Mar 1;371(9614):725–735. doi: 10.1016/S0140-6736(08)60342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patterson SM, Cadogan CA, Kerse N, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2014 Oct 07;(10):CD008165. doi: 10.1002/14651858.CD008165.pub3. [DOI] [PubMed] [Google Scholar]
- 23.Cuijpers P, Karyotaki E, Pot AM, Park M, Reynolds CF., 3rd Managing depression in older age: psychological interventions. Maturitas. 2014 Oct;79(2):160–169. doi: 10.1016/j.maturitas.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klainin-Yobas P, Oo WN, Suzanne Yew PY, Lau Y. Effects of relaxation interventions on depression and anxiety among older adults: a systematic review. Aging & mental health. 2015;19(12):1043–1055. doi: 10.1080/13607863.2014.997191. [DOI] [PubMed] [Google Scholar]
- 25.Reijnders J, van Heugten C, van Boxtel M. Cognitive interventions in healthy older adults and people with mild cognitive impairment: a systematic review. Ageing research reviews. 2013 Jan;12(1):263–275. doi: 10.1016/j.arr.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Bibas L, Levi M, Bendayan M, Mullie L, Forman DE, Afilalo J. Therapeutic interventions for frail elderly patients: part I. Published randomized trials. Progress in cardiovascular diseases. 2014 Sep-Oct;57(2):134–143. doi: 10.1016/j.pcad.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Millen BE, Ohls JC, Ponza M, McCool AC. The elderly nutrition program: an effective national framework for preventive nutrition interventions. Journal of the American Dietetic Association. 2002 Feb;102(2):234–240. doi: 10.1016/s0002-8223(02)90055-6. [DOI] [PubMed] [Google Scholar]
- 28.Frese T, Deutsch T, Keyser M, Sandholzer H. In-home preventive comprehensive geriatric assessment (CGA) reduces mortality–a randomized controlled trial. Arch Gerontol Geriatr. 2012 Nov-Dec;55(3):639–644. doi: 10.1016/j.archger.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Fabacher D, Josephson K, Pietruszka F, Linderborn K, Morley JE, Rubenstein LZ. An in-home preventive assessment program for independent older adults: a randomized controlled trial. J Am Geriatr Soc. 1994 Jun;42(6):630–638. doi: 10.1111/j.1532-5415.1994.tb06862.x. [DOI] [PubMed] [Google Scholar]
- 30.Bula CJ, Berod AC, Stuck AE, et al. Effectiveness of preventive in-home geriatric assessment in well functioning, community-dwelling older people: secondary analysis of a randomized trial. J Am Geriatr Soc. 1999 Apr;47(4):389–395. doi: 10.1111/j.1532-5415.1999.tb07228.x. [DOI] [PubMed] [Google Scholar]
- 31.Ellis G, Whitehead MA, O’Neill D, Langhorne P, Robinson D. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2011;(7):CD006211. doi: 10.1002/14651858.CD006211.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caplan GA, Williams AJ, Daly B, Abraham K. A randomized, controlled trial of comprehensive geriatric assessment and multidisciplinary intervention after discharge of elderly from the emergency department–the DEED II study. J Am Geriatr Soc. 2004 Sep;52(9):1417–1423. doi: 10.1111/j.1532-5415.2004.52401.x. [DOI] [PubMed] [Google Scholar]
- 33.Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet. 1993 Oct 23;342(8878):1032–1036. doi: 10.1016/0140-6736(93)92884-v. [DOI] [PubMed] [Google Scholar]
- 34.Kuo HK, Scandrett KG, Dave J, Mitchell SL. The influence of outpatient comprehensive geriatric assessment on survival: a meta-analysis. Arch Gerontol Geriatr. 2004 Nov-Dec;39(3):245–254. doi: 10.1016/j.archger.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Mohile SG, Velarde C, Hurria A, et al. Geriatric Assessment-Guided Care Processes for Older Adults: A Delphi Consensus of Geriatric Oncology Experts. Journal of the National Comprehensive Cancer Network : JNCCN. 2015 Sep;13(9):1120–1130. doi: 10.6004/jnccn.2015.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Donovan A, Mohile SG, Leech M. Expert consensus panel guidelines on geriatric assessment in oncology. European journal of cancer care. 2015 Jul;24(4):574–589. doi: 10.1111/ecc.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurria A, Levit LA, Dale W, et al. Improving the Evidence Base for Treating Older Adults With Cancer: American Society of Clinical Oncology Statement. J Clin Oncol. 2015 Jul 20; doi: 10.1200/JCO.2015.63.0319. [DOI] [PubMed] [Google Scholar]
- 38.Epstein AM, Hall JA, Fretwell M, et al. Consultative geriatric assessment for ambulatory patients. A randomized trial in a health maintenance organization. Jama. 1990 Jan 26;263(4):538–544. [PubMed] [Google Scholar]
- 39.Baitar A, Kenis C, Moor R, et al. Implementation of geriatric assessment-based recommendations in older patients with cancer: A multicentre prospective study. J Geriatr Oncol. 2015 Sep;6(5):401–410. doi: 10.1016/j.jgo.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Fisher E, Hasselberg M, Conwell Y, et al. Telementoring Primary Care Clinicians to Improve Geriatric Mental Health Care. Population health management. 2017 Jan 20; doi: 10.1089/pop.2016.0087. [DOI] [PubMed] [Google Scholar]
- 41.Christmas C, Park E, Schmaltz H, Gozu A, Durso SC. A model intensive course in geriatric teaching for non-geriatrician educators. Journal of general internal medicine. 2008 Jul;23(7):1048–1052. doi: 10.1007/s11606-008-0585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


