Abstract
BACKGROUND
Cardiac fibrosis (CF) is associated with increased ventricular stiffness and diastolic dysfunction and is an independent predictor of long-term clinical outcomes of patients with heart failure (HF). We previously showed that the matricellular CCN5 protein is cardioprotective via its ability to inhibit CF and preserve cardiac contractility.
OBJECTIVES
This study examined the role of CCN5 in human heart failure and tested whether CCN5 can reverse established CF in an experimental model of HF induced by pressure overload.
METHODS
Human hearts were obtained from patients with end-stage heart failure. Extensive CF was induced by applying transverse aortic constriction for 8 weeks, which was followed by adeno-associated virus-mediated transfer of CCN5 to the heart. Eight weeks following gene transfer, cellular and molecular effects were examined.
RESULTS
Expression of CCN5 was significantly decreased in failing hearts from patients with end-stage heart failure compared to nonfailing hearts. Trichrome staining and myofibroblast content measurements revealed that the established CF had been reversed by CCN5 gene transfer. Anti-CF effects of CCN5 were associated with inhibition of the transforming growth factor beta signaling pathway. CCN5 significantly inhibited endothelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation, which are 2 critical processes for CF progression, both in vivo and in vitro. In addition, CCN5 induced apoptosis in myofibroblasts, but not in cardiomyocytes or fibroblasts, both in vivo and in vitro. CCN5 provoked the intrinsic apoptotic pathway specifically in myofibroblasts, which may have been due the ability of CCN5 to inhibit the activity of NFκB, an antiapoptotic molecule.
CONCLUSIONS
CCN5 can reverse established CF by inhibiting the generation of and enhancing apoptosis of myofibroblasts in the myocardium. CCN5 may provide a novel platform for the development of targeted anti-CF therapies.
Keywords: apoptosis, gene therapy, heart failure, NFκB
Heart failure (HF) accounts for approximately 450,000 deaths per year in the United States and is the end result of most pathological cardiac insults. HF is characterized by reduced cardiac contractility and global ventricular remodeling (1,2). Cardiac fibrosis (CF) is associated with increased ventricular stiffness, diastolic dysfunction, combined systolic and diastolic dysfunction (3,4), arrhythmia (5), diabetic cardiomyopathy (6), and impaired coronary blood flow in patients with HF (7). Detection of extensive fibrosis is a predictor of mortality in patients with HF and for the effectiveness of long-term HF therapy (7). Although CF has conventionally been regarded as a secondary phenomenon, it has also been suggested to play a primary role in the progression of HF (8). The clinical outcomes of patients with symptomatic, severe aortic stenosis undergoing aortic valve replacements correlate with CF severity (9). In line with this finding, CF, but not left ventricular (LV), ejection fraction, is associated with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy (10). Thus, CF is a valid target for the treatment of HF; however, effective anti-CF therapies are currently unavailable (11).
CF is mediated primarily by transdifferentiation of fibroblasts to myofibroblasts. Unlike quiescent fibroblasts, myofibroblasts secrete substantial amounts of extracellular matrix proteins and possess contractile properties due to the incorporation of α-smooth muscle actin (α-SMA) into stress fibers (12). The origins of fibroblasts that expand under pathological conditions are diverse (13). Resident fibroblast proliferation may represent the most important source of myofibroblasts. In addition, a lineage tracing study in mouse models of pressure overload and chronic allograft rejection revealed that cardiac endothelial cells contribute to CF through a process called endothelial-mesenchymal transition (EndMT) (14). In a mouse model of dilated cardiomyopathy, a significant fraction of myofibroblasts was shown to originate from hematopoietic cells (15). An in-depth understanding at the molecular level of the diverse paths that lead to the expansion of cardiac myofibroblasts would facilitate the development of anti-CF modalities.
A group of matricellular proteins known as the CCN family (CCN1 to CCN6) are associated with diverse cellular functions (16). We previously showed differential regulation of CCN2 and CCN5 expression during the regression of cardiac hypertrophy. CCN2, also known as connective tissue growth factor, is a well-characterized marker and mediator of fibrosis (17). As expected, hypertrophic responses and CF upon pressure overload are significantly exacerbated in the heart of CCN2 transgenic (Tg) mice. By contrast, hypertrophic responses and CF upon pressure overload are significantly inhibited in the hearts of CCN5 Tg mice. CCN5 also blocks CCN2-induced cardiomyocyte hypertrophy in vitro. These observations suggest that CCN2 and CCN5 play opposing roles during adverse cardiac remodeling: CCN5 is anti-hypertrophic and anti-fibrotic, whereas CCN2 is prohypertrophic and profibrotic (18).
In this study, we found that CCN5 was significantly decreased in the myocardium of patients with severe HF. We evaluated the effects of adeno-associated virus (AAV)-mediated overexpression of CCN5 on established CF with concomitant cardiac dysfunction. CCN5 reversed pre-established CF, as shown by its negative effects on collagen contents and the fraction of cardiac myofibroblasts returning to nearly normal levels. CCN5 inhibited EndMT and transdifferentiation of fibroblasts into myofibroblasts in vivo and in vitro. In addition, CCN5 induced apoptosis in myofibroblasts, but not in cardiomyocytes or fibroblasts in vivo and in vitro. Collectively, these data show that CCN5 could be used for the development of new anti-CF therapies.
METHODS
A detailed description of all experimental procedures is provided in the Online Appendix.
RESULTS
CCN5 IS DOWN-REGULATED IN FAILING HEARTS
To evaluate the clinical relevance of CCN5 in the context of HF, expression of the protein was measured in hearts obtained from patients with HF at the time of cardiac transplantation and compared with that in normal controls (Online Table 1). Western blotting revealed that CCN5 expression was reduced in failing hearts to ~24% of the level detected in control hearts (Figure 1A). By contrast, CCN2 expression was nearly 4-fold higher in the same failing hearts than in control hearts (Online Figure 1A). CCN5 expression was also measured in hearts obtained from mice with HF induced by transverse aortic constriction (TAC) for 8 weeks. In these failing hearts, CCN5 expression was reduced to ~10% of the normal level (Figure 1B), whereas CCN2 expression was 11-fold higher than in the controls (Online Figure 1B). These results indicate that low CCN5 levels are associated with HF in both humans and mice. These data also confirm that CCN2 expression increases during HF (17).
FIGURE 1. CCN5 is Down-Regulated in Failing Hearts.
CCN5 levels were measured by Western blotting in heart tissue samples obtained from patients with HF and from healthy controls (Normal) (A), and mice with TAC-induced HF and sham-operated mice (Sham) (B). Protein extracts (15 µg) were loaded and probed with anti-CCN5 and anti-GAPDH antibodies. **p < 0.01. GAPDH = glyceraldehyde-3-phosphate dehydrogenase; HF = heart failure; TAC = transverse aortic constriction.
CCN5 REVERSES ESTABLISHED CF
To examine the effects of CCN5 in failing hearts, recombinant AAV serotype 9 was generated to drive CCN5 expression (named AAV-CCN5) in the heart, together with a control virus (named AAV-VLP). When injected via tail vein, AAV-CCN5 increased CCN5 expression in a dose-dependent manner and specifically in the heart. AAV-CCN5 prevented TAC-induced HF, which is consistent with the data obtained with CCN5 Tg mice (Online Figure 2). Mice were subjected to TAC for 8 weeks to induce HF. Echocardiography demonstrated significant LV dilation and reduced fractional shortening in these mice, indicating HF. Mice with failing hearts received AAVs (5 × 1010 viral genomes per mouse) through the tail vein and were analyzed 8 weeks later. Although the echocardiographic parameters of mice that received AAV-VLP further deteriorated, those of mice that received AAV-CCN5 were well preserved (Online Figure 3). These results indicate that CCN5 is cardioprotective.
Hearts obtained from the experiments described in the preceding text were subjected to histological analysis. To analyze the components of CF, heart sections were stained with trichrome. Fibrosis in both interstitial and perivascular areas increased after 8 weeks of TAC, and increased further after another 8 weeks of TAC in mice that received AAV-VLP. However, fibrosis in the same areas was markedly reduced in mice that received AAV-CCN5 under the same conditions (Figure 2A). Heart sections were further stained with antibody raised against α-SMA, a marker of myofibroblasts. The fraction of α-SMA-positive myofibroblasts increased after 8 weeks of TAC, and increased further after another 8 weeks of TAC in mice that received AAV-VLP. Once again, the fraction of α-SMA-positive cells was markedly lower in mice that received AAV-CCN5 under the same conditions (Figure 2B). The reduction in the number of α-SMA-positive cells after the administration of AAV-CCN5 was further confirmed by fluorescence-activated cell sorting (FACS) analysis (Figure 2C). These data suggest that CCN5 induces degradation of preformed fibrogenic materials in the heart. Additionally, CCN5 reduced the fraction of α-SMA-positive myofibroblasts that had already expanded in response to TAC.
FIGURE 2. CCN5 Reduces Established CF.
Mice were sham-operated or underwent TAC for 8 weeks. AAV-VLP or AAV-CCN5 (5 × 1010 viral genomes per mouse) was injected via tail vein. Eight weeks later, hearts were analyzed for the extent of CF. (A) Hearts were sectioned and stained with trichrome. Representative images of the interstitial (upper row) and perivascular (lower row) areas are shown. The percentage of fibrotic areas is plotted. (B) Heart sections were immunostained with antibodies against α-SMA. Representative images of interstitial (left panel) and perivascular (right panel) areas are shown. The fraction of α-SMA-positive cells is shown. (C) Fibroblasts were isolated from hearts using the Langendorff perfusion system, and cell fractions that expressed α-SMA were determined using FACS (n = 3). *p < 0.05; **p < 0.01. AAV = adeno-associated virus; CF = cardiac fibrosis; FACS = fluorescence-activated cell sorting; SMA = smooth muscle actin; other abbreviations as in Figure 1.
CCN5 INHIBITS TGF-β SIGNALING
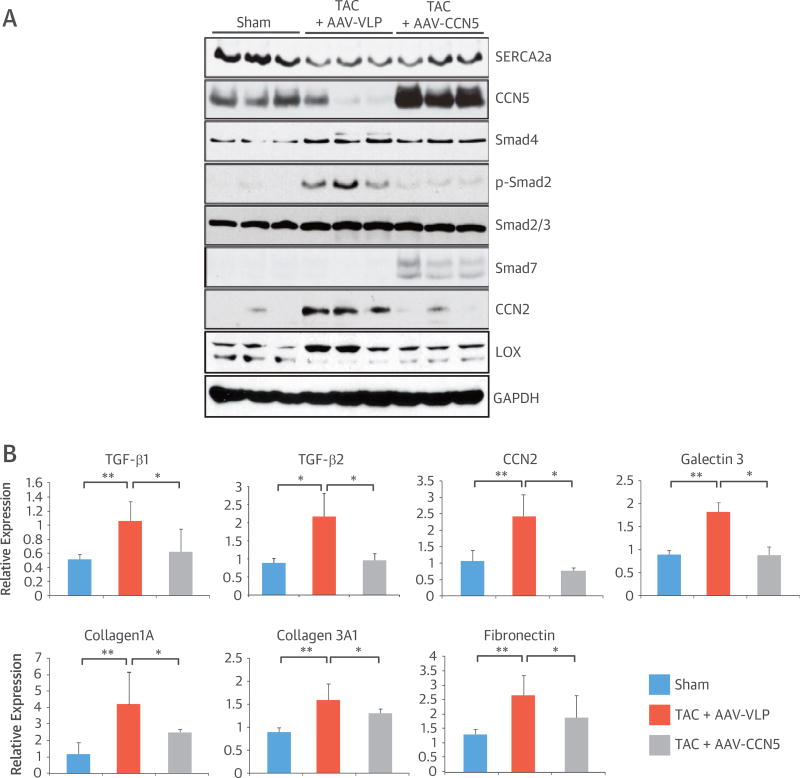
Transforming growth factor (TGF)-β is a critical mediator of CF. The effect of CCN5 on TGF-β signaling was determined by Western blotting (Figure 3A). Consistent with previous reports, TAC increased TGF-β signaling, as shown by the increased phosphorylation of SMAD2, an activating SMAD. In mice that received AAV-CCN5, TAC-induced SMAD2 phosphorylation was markedly reduced, and moreover, the level of SMAD7, an inhibitory SMAD, was increased. CCN2 is a downstream target of TGF-β signaling (19). The higher level of CCN2 in failing hearts was not observed in mice that received AAV-CCN5. Lysyl oxidase mediates crosslinking of collagen fibers during fibrosis (20). TAC increased the expression of lysyl oxidase in mice, but its expression returned to normal following AAV-CCN5 treatment. Quantitative real-time polymerase chain reaction (qRT-PCR) also revealed that TAC induced higher expression of TGF-β1 and -β2 and that AAV-CCN5 significantly inhibited the expression of CF-associated genes, including CCN2, galectin 3, collagen1A, collagen3A1, and fibronectin (Figure 3B and Online Table 2). These data suggest that CCN5 inhibits TGF-β signaling.
FIGURE 3. CCN5 Inhibits TGF-β Signaling.
Mice were sham-operated or underwent TAC for 8 weeks. AAV-VLP or AAV-CCN5 was injected. (A) Eight weeks later, heart extracts (50 µg) were subjected to Western blotting. (B) Heart extracts were subjected to qRT-PCR. *p < 0.05; **p < 0.01. qRT-PCR = quantitative real-time polymerase chain reaction; TGF = transforming growth factor; other abbreviations as in Figures 1 and 2.
CCN5 INHIBITS EndMT
Cardiac fibroblasts are derived from many different cellular origins. Endothelial cells significantly contribute to CF through EndMT (14). Thus, we tested whether CCN5 inhibits EndMT using the Scl-Cre-ERT; R26RstopYFP double-Tg mice. In these double-Tg mice, Cre-ERT is expressed specifically in endothelial cells. Therefore, tamoxifen-induced yellow fluorescent protein (YFP) expression persists in cells of endothelial origin, regardless of subsequent phenotypic alterations. Tamoxifen was administered for 5 consecutive days to these double-Tg mice. At 4 weeks after the first tamoxifen administration, mice were subjected to TAC and injected with AAV-CCN5 or AAV-VLP simultaneously. After an additional 8 weeks, cardiac function and anatomy were examined (Figure 4A). Severe cardiac dysfunction and fibrosis were evident in mice that received AAV-VLP, whereas they were less evident in mice that received AAV-CCN5 (data not shown). Cardiac tissue sections were then prepared and immunostained with anti-YFP and anti-vimentin antibodies (Figure 4B). Cells positive for both YFP and vimentin, a fibrotic marker, represent cells of endothelial origin that had acquired fibrotic phenotypes through EndMT. Approximately 8.5% of the vimentin-positive cells were also YFP-positive in mice that received AAV-VLP, whereas in mice that received AAV-CCN5, it was <1% (Figure 4C), indicating that CCN5 significantly inhibited EndMT in vivo.
FIGURE 4. CCN5 Inhibits EndMT.
(A) Experimental schema are shown for B and C. (B) Heart sections were co-immunostained with antibodies against YFP (green) and vimentin (red). Cells positive for both YFP and vimentin (arrows) underwent EndMT. (C) Fractions of vimentin-positive cells that were YFP-positive are plotted (n = 3). (D) HCAECs were cultured in CM-Con or CM-CCN5 in the presence or absence of TGF-β. Cells were then co-stained with VE-cadherin or vimentin. (E) HCAECs were scratched and then cultured under the same conditions as shown in E. After 48 h, cells were fixed and stained with DAPI. Representative images are shown (left), and migration distances are plotted (right) (n = 4). (F) qRT-PCR analyses of α-SMA, collagen I, collagen III, Tie2, and CD31 were performed using cells cultured under the same conditions as shown in D (n = 6). *p < 0.05; **p < 0.01. CM-CCN5 = CCN5-containing conditioned medium; CM-Con = control-conditioned medium; DAPI = 4’,6-diamidino-2-phenylindole; EndMT = endothelial-mesenchymal transition; YFP = yellow fluorescent protein; HCAEC = human coronary artery endothelial cell; VE = vascular-endothelial; other abbreviations as in Figures 2 and 3.
CCN5 function was analyzed in vitro by using CCN5-containing conditioned medium prepared from cultures of HEK293 cells transiently transfected with CCN5-expressing plasmids. The conditioned medium contained ~200 ng/ml of CCN5, as determined by enzyme-linked immunoabsorbent assay (ELISA) (data not shown). When added to the culture of neonatal cardiomyocytes, CCN5-containing conditioned medium, but not the control conditioned medium, inhibited phenylephrine-induced hypertrophy (Online Figure 4). This indicates that conditioned medium containing CCN5, but not control conditioned medium, contained functionally active CCN5. Treatment of human coronary artery endothelial cells (HCAECs) with TGF-β resulted in acquisition of vimentin and loss of vascular-endothelial (VE)-cadherin, an endothelial cell marker. This TGF-β-induced EndMT was completely prevented by co-treatment with CCN5-containing conditioned medium (Figure 4D). Endothelial cells that have undergone transition are characterized by increased migratory capability (21). Treatment of HCAECs with TGF-β increased cellular migratory activity, as assessed by an in vitro scratch assay, and this was prevented by incubation with CCN5-containing conditioned medium (Figure 4E). qRT-PCR revealed that fibroblast-associated genes including α-SMA, collagen I, and collagen III were up-regulated and endothelial-associated genes, including Tie2 and CD31, were down-regulated in HCAECs after treatment with TGF-β, and that these changes were completely prevented by CCN5 (Figure 4F). These results indicate that CCN5 inhibits EndMT in vitro.
CCN5 INHIBITS FIBROBLAST-TO-MYOFIBROBLAST TRANSDIFFERENTIATION
Cardiac fibroblasts transdifferentiate into myofibroblasts during CF. Myofibroblasts are characterized by the expression of α-SMA. Mice were subjected to TAC and injected with AAV-CCN5 or AAV-VLP simultaneously (Figure 5A). Eight weeks after this procedure, cardiac dysfunction and fibrosis were evident in mice that received AAV-VLP, whereas they were considerably less evident in mice that received AAV-CCN5 (data not shown). Cardiac tissue sections were then prepared and immunostained with anti-vimentin and anti-α-SMA antibodies (Figure 5B). The cells positive for both vimentin and α-SMA represent cells of fibroblast origin that had transdifferentiated into myofibroblasts. Approximately 17% of the vimentin-positive cells were also α-SMA-positive in mice that received AAV-VLP. This was reduced to ~4% in mice that received AAV-CCN5 (Figure 5C). These data indicate that CCN5 inhibited fibroblast-to-myofibroblast transdifferentiation in vivo.
FIGURE 5. CCN5 Inhibits Transdifferentiation of Fibroblasts Into Myofibroblasts.
(A) Experimental schema for B and C. (B) Heart sections were co-immunostained with anti-vimentin (red) and anti-α-SMA (green). Cells positive for both vimentin and α-SMA (arrows) underwent fibroblast-to-myofibroblast transdifferentiation. (C) The fraction of α-SMA-positive cells that were vimentin-positive is plotted (n = 3). (D) Fibroblasts were cultured in CM-Con or CM-CCN5 in the presence or absence of TGF-β. Cells were co-stained with anti-α-SMA or DAPI. (E) Collagen gel lattices were made and cultured under the same conditions as shown in D. After 48 h, areas of the collagen lattice were measured. Representative images of collagen gel lattices are shown (left), and areas of the collagen gel relative to those of the control gel are plotted (right) (n = 3). (F) RNA was obtained from the same sets of cells as in D. qRT-PCR analyses of α-SMA and collagen I were performed (n = 6). *p < 0.05; **p < 0.01. Abbreviations as in Figures 2 to 4.
An in vitro experiment recapitulating fibroblast-to-myofibroblast transdifferentiation was performed using rat adult cardiac fibroblasts. Treatment of these fibroblasts with TGF-β markedly increased α-SMA expression, as shown by immunostaining, whereas co-treatment with CCN5-containing conditioned medium completely blocked α-SMA expression (Figure 5D). Increased α-SMA expression significantly increases myofibroblast contractile activity, which can be observed in a collagen gel lattice assay (22). Treatment of cardiac fibroblasts with TGF-β significantly induced collagen gel contraction, which was attenuated by co-treatment with CCN5-containing conditioned medium (Figure 5E). qRT-PCR revealed that TGF-β significantly increased α-SMA and collagen I mRNA expression in cardiac fibroblasts, and that CCN5-containing conditioned medium completely reversed this effect of TGF-β (Figure 5F). These results indicate that CCN5 completely inhibits TGF-β-mediated transdifferentiation of fibroblasts in vitro.
CCN5 INDUCES APOPTOSIS IN MYOFIBROBLASTS
The preceding data suggest that CCN5 prevents expansion and transdifferentiation of fibroblasts under fibrotic conditions. We tested whether enhanced clearance of myofibroblasts also contributes to CCN5-mediated reversal of CF. Mice were subjected to TAC and injected with AAV-CCN5 or AAV-VLP simultaneously (Figure 6A). Two weeks after this procedure, cardiac tissue sections were examined for apoptosis with terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) staining. Immunostaining with anti-α-SMA antibody was also performed simultaneously (Figure 6B). Either TUNEL-positive or α-SMA-positive cells were rare in sham-operated mice but were significantly prominent in TAC-operated mice. Approximately 9% of the α-SMA-positive cells were also TUNEL-positive in mice that received AAV-VLP. This level increased to ~72% in mice that received AAV-CCN5 (Figure 6C). Taken together, these data indicate that CCN5 induces apoptosis in myofibroblasts in vivo. CCN5-mediated apoptosis was not observed in either cardiomyocytes or fibroblasts (data not shown).
FIGURE 6. CCN5 Induces Myofibroblast Apoptosis.
(A) Experimental schema for B and C. (B) Heart sections were co-stained with TUNEL (red) and α-SMA (green). Cells positive for both TUNEL and α-SMA (arrows) underwent myofibroblast apoptosis. (C) The fraction of TUNEL-positive cells that were α-SMA-positive is plotted (n = 3). (D) Cardiomyocytes (Myo), fibroblasts (FB), and myofibroblasts (MyoFB) were cultured in CM-Con or CM-CCN5 for 48 h and then stained with DAPI. Arrows indicate cells with pyknotic nuclei. Pyknotic nuclei were counted and plotted (n = 3). (E) Cells were cultured under the same conditions as in (D) and subjected to TUNEL staining. Arrows indicate TUNEL-positive nuclei. TUNEL-positive nuclei were counted and plotted (n = 3). (F) Fibroblasts and myofibroblasts were cultured in CM-con or CM-CCN5, and harvested after 48 h. Cells were analyzed by FACS; n = 3. **p < 0.01. TUNEL = terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling; other abbreviations as in Figures 2 to 4.
Cardiomyocytes and fibroblast (nonmyocyte) fractions were separately obtained from rat hearts. Part of the fibroblast fraction was further differentiated into myofibroblasts in vitro by treatment with TGF-β (23). Fractions were subjected to Western blotting, using antibodies against α-actinin, vimentin, and α-SMA to confirm cell type and differentiation status (Online Figure 5) before treatment with control or CCN5-containing conditioned medium. Cells undergoing necrosis or apoptosis are characterized by shrinking nuclei, a phenomenon called pyknosis. Fluorescence microscopy revealed that CCN5 increased pyknosis in myofibroblasts, but not in cardiomyocytes or fibroblasts (Figure 6D). CCN5 also increased TUNEL-positive nuclei in myofibroblasts but not in cardiomyocytes or fibroblasts (Figure 6E). Fibroblasts and myofibroblasts were subjected to FACS analysis to quantify cells with surface expression of annexin V, a marker of apoptosis. CCN5 significantly increased the number of annexin V-positive myofibroblasts, but had no effect on fibroblasts (Figure 6F). We could not obtain consistent FACS data with cardiomyocytes, probably because of their rod-like shapes (data not shown). Taken together, these results indicate that CCN5 induces apoptosis specifically in myofibroblasts.
CCN5 TRIGGERS THE INTRINSIC PATHWAY OF APOPTOSIS IN MYOFIBROBLASTS
To further characterize CCN5-induced apoptosis of myofibroblasts, cells were prepared as described previously and incubated with CCN5-containing conditioned medium for 1 or 2 days. Western blotting revealed lower expression of Bcl-2, an antiapoptotic molecule, and higher expression of BAX, a proapoptotic molecule, after treatment with CCN5. The executioner caspases, caspase 3 and 7, were activated by CCN5, as shown by their cleavage. These data further support our claim that CCN5 induces apoptosis in myofibroblasts. Whereas caspase 8 is involved in the extrinsic pathway, caspase 9 is involved in the intrinsic apoptotic pathway. CCN5 induced activation of caspase 9 but not caspase 8, indicating that CCN5 induced the intrinsic pathway of apoptosis (Figure 7A). Release of cytochrome c from mitochondria is another hallmark of the intrinsic pathway of apoptotic cell death (24). Immunostaining of the myofibroblasts revealed that cytochrome c release was increased after CCN5 treatment (Figure 7B).
FIGURE 7. CCN5 Triggers the Intrinsic Apoptotic Pathway.
(A) Total cell lysates from myofibroblasts cultured in CM-Con or CM-CCN5 were immunoblotted with antibodies against apoptotic marker proteins. (B) Myofibroblasts were cultured under the same conditions as in A and stained with anti-cytochrome c and anti-VDAC. Arrows indicate cytochrome c release. (C) Fibroblasts and myofibroblasts were subjected to Western blotting using antibodies against NFκB, vimentin, and α-SMA. (D) Fibroblasts and myofibroblasts were cotransfected with the NFκB reporter plasmids pBIIx and pRenilla concomitantly with pcDNA (empty plasmid) or pcDNA-hCCN5 (CCN5 plasmid). Promoter activities were determined by measuring dual-luciferase activity (n = 4). (E) Myofibroblasts were cultured under the same conditions as in B and stained with antibody against NFκB. **p < 0.01. VDAC = voltage-dependent anion channel; other abbreviations as in Figures 1, 2, and 4.
Myofibroblasts exhibited higher expression levels of the antiapoptotic molecule NFκB than fibroblasts did (Figure 7C). We reasoned that higher levels of NFκB are needed to maintain the viability of apoptosis-prone myofibroblasts. In line with this hypothesis, the higher transcriptional activity of NFκB observed in myofibroblasts was markedly reduced by CCN5 (Figure 7D). NFκB exerts its antiapoptotic functions in the nucleus. The nuclear localization of NFκB in myofibroblasts was completely reversed after treatment with CCN5 (Figure 7E). These data suggest that CCN5 may induce apoptosis in myofibroblasts by inhibiting the nuclear localization of NFκB.
DISCUSSION
The CCN family consists of 6 secreted matricellular proteins, of which CCN2, the best characterized, has prominent profibrotic activity in a variety of tissues, including liver and kidney (25). We previously reported that CCN2 is prohypertrophic and profibrotic, whereas CCN5 is antihypertrophic and antifibrotic in the heart. CCN2 and CCN5 are regulated in opposite ways during the progression and regression of cardiac hypertrophy, suggesting that CCN2 and CCN5 play a “tug-of-war” during cardiac remodeling under pathological conditions.
In this study, we focused on specific antifibrotic mechanisms and showed that AAV-mediated delivery of CCN5 regressed established CF in a mouse model of pressure overload-induced HF. This observation is consistent with previous reports showing that CF is reversible in certain cases (26,27). However, in these previous studies, it was unclear whether CF was merely prevented from further deterioration or was truly reversed. In the current study, we clearly show that CCN5 reversed established CF using trichrome staining and analysis of myofibroblast contents before and after CCN5 gene transfer (Figure 2).
Endothelial cells provide an important source of cells for the expanded fibroblast population in the failing heart and under other adult fibrotic conditions through EndMT (14,28). In addition, fibroblast transdifferentiation into myofibroblasts plays a major role in the progression of CF. Our data show that CCN5 inhibits EndMT and the transdifferentiation of fibroblasts to myofibroblasts both in vivo and in vitro (Figures 4 and 5). It was previously shown that the TGF-β signaling pathway is involved in both EndMT and fibroblast transdifferentiation. We showed that CCN5 inhibits this pathway (Figure 3), indicating that CCN5 blocks EndMT and fibroblast transdifferentiation via inhibition of the TGF-β signaling pathway. In addition, CCN5 induced apoptosis in myofibroblasts (Figures 6 and 7). To the best of our knowledge, this study is the first report to show the loss of myofibroblasts during CF regression. A role for TGF-β in protecting rat lung fibroblasts from interleukin (IL)-1β-induced apoptosis was previously reported (29). Therefore, it is possible that CCN5 induces apoptosis of myofibroblasts indirectly, by inhibiting TGF-β signaling (Central Illustration). Besides its function in inhibiting the TGF-β signaling pathway, CCN5 is also thought to exert its functions via other mechanisms.
CENTRAL ILLUSTRATION. CCN5 Reverses Established Cardiac Fibrosis: Effect in the Myocardium.
CCN5 exerts its anti-CF effects by inhibiting both EndMT and the transdifferentiation of fibroblasts into myofibroblasts. In addition, CCN5 induces apoptosis specifically in myofibroblasts. CCN5 mediates these functions at least partly by inhibiting the TGF-β signaling pathway. CF = cardiac fibrosis; EndMT = endothelial-mesenchymal transition; TGF = transforming growth factor.
Myofibroblasts are innately unstable cells that are transiently generated under pathological conditions and they must be rapidly destroyed once the pathological stimuli are removed. We suggest that both survival and death signaling pathways are delicately balanced in activated states in myofibroblasts and that a disturbance in this balance can potentially lead to apoptosis of myofibroblasts. We found that the expression of the proapoptotic molecule p53 was elevated in myofibroblasts (data not shown). The apoptosis associated with this elevated level of p53 could be counteracted by the elevated level of the antiapoptotic molecule NFκB. CCN5 did not affect the expression or activity of p53 (data not shown), but inhibited NFκB signaling (Figure 7). It is possible that more proapoptotic and antiapoptotic molecules are involved in CCN5-mediated apoptosis of myofibroblasts. Further in-depth studies will be required to address this issue in more detail.
In addition to reducing the number of myofibroblasts, CCN5 also reduced the levels of collagens and related extracellular matrix proteins. This may be explained by the increased activities of matrix metalloproteases (MMPs) and/or reduced activities of tissue inhibitors of metalloproteases (TIMPs) (30). We found that MMP-2, MMP-3, and MMP-9 were up-regulated by CCN5, whereas TIMP2, TIMP3, and TIMP4 were down-regulated by CCN5 (Online Figure 6). CCN5 also normalized the hyperphosphorylation of Akt and mammalian target of rapamycin (mTOR) induced by TGF-β in cardiac fibroblasts, which is consistent with the findings observed in lung fibroblasts (Online Figure 7).
STUDY LIMITATIONS
The mouse model used in this study involved a surgical procedure that is widely used in preclinical studies of HF. Although this model exhibits an array of typical HF phenotypes, it may represent only the acute model of HF. In clinical scenarios, CF (in particular, nonreparative CF) is more often associated with HF that has been developing over a long period of time. Therefore, it may be necessary to test the efficacy of CCN5 in more chronic models of HF. For example, a protocol with less intense TAC for a period of 4 to 6 months might provide such a chronic model. It is notable that CCN5 improved the survival of mice that were subjected to TAC for up to 4.5 months (Online Figure 8).
CONCLUSIONS
Our data demonstrate that CCN5 can reverse established CF in a mouse model of HF, probably via its ability to inhibit both EndMT and the transdifferentiation of fibroblasts into myofibroblasts, and its capacity to increase apoptosis specifically in myofibroblasts. Collectively, these actions of CCN5 result in lower numbers of myofibroblasts in the myocardium, resulting in reversal of CF. Although further studies are required to fully understand the molecular mechanisms, we propose that CCN5 exerts these effects, at least in part, by inhibiting the TGF-β signaling pathway (Central Illustration). CCN5 could represent a new target for the development of novel therapies for CF.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
CF develops in several types of heart disease including HF, ventricular arrhythmias, and atrial fibrillation. The matricellular protein CCN5 can reverse fibrosis in preclinical models.
TRANSLATIONAL OUTLOOK
clinical studies are needed to evaluate the efficacy and safety of CCN5 in patients with HF and other cardiac diseases associated with progressive fibrosis.
Acknowledgments
Dr. Kho was supported by National Institutes of Health grant R00116645. Dr. D’Escamard was supported by NIH grant T32HL007824. Dr. Kovacic was supported by NIH grant K08HL111330, and a grant from AstraZeneca. Dr. Hajjar was funded by NIH grants R01 HL117505, HL093183, and P50 HL112324 and a National Heart, Lung, and Blood Institute Program of Excellence in Nano-Technology award, HHSN268201000045C. Dr. Park was supported by Bio & Medical Technology Development Program grant NRF-2015 M3A9E6028951, and Global Research Laboratory Program grant M6-0605-00-0001 of National Research Foundation; and funded by the Korean government and a grant from the Systems Biology Infrastructure Establishment provided by Gwangju Institute of Science and Technology (GIST).
ABBREVIATIONS AND ACRONYMS
- AAV
adeno-associated virus
- CF
cardiac fibrosis
- EndMT
endothelial-mesenchymal transition
- FACS
fluorescence-activated cell sorting
- HCAEC
human coronary artery endothelial cell
- HF
heart failure
- LV
left ventricle/ventricular
- TAC
transverse aortic constriction
- TUNEL
terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling
APPENDIX
For an expanded Materials section and supplemental tables and figures, please see the online version of this article.
Footnotes
Dr. Hajjar has ownership interest in Celladon and Nanocor. Drs. Hajjar and Park have co-ownership interest in Bethphagen. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Houser SR, Margulies KB. Is depressed myocyte contractility centrally involved in heart failure? Circ Res. 2003;92:350–8. doi: 10.1161/01.RES.0000060027.40275.A6. [DOI] [PubMed] [Google Scholar]
- 2.Konstam MA, Kramer DG, Patel AR, et al. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. J Am Coll Cardiol Img. 2011;4:98–108. doi: 10.1016/j.jcmg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Wong TC, Piehler K, Meier CG, et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206–16. doi: 10.1161/CIRCULATIONAHA.111.089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schelbert EB, Fonarow GC, Bonow RO, et al. Therapeutic targets in heart failure: refocusing on the myocardial interstitium. J Am Coll Cardiol. 2014;63:2188–98. doi: 10.1016/j.jacc.2014.01.068. [DOI] [PubMed] [Google Scholar]
- 5.Dzeshka MS, Lip GY, Snezhitskiy V, et al. Cardiac fibrosis in patients with atrial fibrillation: mechanisms and clinical implications. J Am Coll Cardiol. 2015;66:943–59. doi: 10.1016/j.jacc.2015.06.1313. [DOI] [PubMed] [Google Scholar]
- 6.Wong TC, Piehler KM, Kang IA, et al. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J. 2014;35:657–64. doi: 10.1093/eurheartj/eht193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.López B, González A, Ravassa S, et al. Circulating biomarkers of myocardial fibrosis: the need for a reappraisal. J Am Coll Cardiol. 2015;65:2449–56. doi: 10.1016/j.jacc.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Thum T, Gross C, Fiedler J, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–4. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 9.Weidemann F, Herrmann S, Störk S, et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577–84. doi: 10.1161/CIRCULATIONAHA.108.847772. [DOI] [PubMed] [Google Scholar]
- 10.Gulati A, Jabbour A, Ismail TF, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896–908. doi: 10.1001/jama.2013.1363. [DOI] [PubMed] [Google Scholar]
- 11.Izawa H, Murohara T, Nagata K, et al. Mineralocorticoid receptor antagonism ameliorates left ventricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy: a pilot study. Circulation. 2005;112:2940–5. doi: 10.1161/CIRCULATIONAHA.105.571653. [DOI] [PubMed] [Google Scholar]
- 12.Tomasek JJ, Gabbiani G, Hinz B, et al. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 13.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105:1164–76. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeisberg EM, Tarnavski O, Zeisberg M, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–61. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 15.Chu PY, Mariani J, Finch S, et al. Bone marrow-derived cells contribute to fibrosis in the chronically failing heart. Am J Pathol. 2010;176:1735–42. doi: 10.2353/ajpath.2010.090574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holbourn KP, Acharya KR, Perbal B. The CCN family of proteins: structure-function relationships. Trends Biochem Sci. 2008;33:461–73. doi: 10.1016/j.tibs.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen MM, Lam A, Abraham JA, et al. CTGF expression is induced by TGF- beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol. 2000;32:1805–19. doi: 10.1006/jmcc.2000.1215. [DOI] [PubMed] [Google Scholar]
- 18.Yoon PO, Lee MA, Cha H, et al. The opposing effects of CCN2 and CCN5 on the development of cardiac hypertrophy and fibrosis. J Mol Cell Cardiol. 2010;49:294–303. doi: 10.1016/j.yjmcc.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Song JJ, Aswad R, Kanaan RA, et al. Connective tissue growth factor (CTGF) acts as a downstream mediator of TGF-beta1 to induce mesenchymal cell condensation. J Cell Physiol. 2007;210:398–410. doi: 10.1002/jcp.20850. [DOI] [PubMed] [Google Scholar]
- 20.Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–72. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- 21.Widyantoro B, Emoto N, Nakayama K, et al. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation. 2010;121:2407–18. doi: 10.1161/CIRCULATIONAHA.110.938217. [DOI] [PubMed] [Google Scholar]
- 22.Dobaczewski M, Bujak M, Li N, et al. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–28. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrov VV, Fagard RH, Lijnen PJ. Stimulation of collagen production by transforming growth factor-beta1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension. 2002;39:258–63. doi: 10.1161/hy0202.103268. [DOI] [PubMed] [Google Scholar]
- 24.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipson KE, Wong C, Teng Y, et al. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5(Suppl 1):S24. doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102:1388–93. doi: 10.1161/01.cir.102.12.1388. [DOI] [PubMed] [Google Scholar]
- 27.Díez J, Querejeta R, López B, et al. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–7. doi: 10.1161/01.cir.0000017264.66561.3d. [DOI] [PubMed] [Google Scholar]
- 28.Kovacic JC, Mercader N, Torres M, et al. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease. Circulation. 2012;125:1795–808. doi: 10.1161/CIRCULATIONAHA.111.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang HY, Phan SH. Inhibition of myofibroblast apoptosis by transforming growth factor β1. Am J Respir Cell Mol Biol. 1999;21:658–65. doi: 10.1165/ajrcmb.21.6.3720. [DOI] [PubMed] [Google Scholar]
- 30.Turner NA, Porter KE. Regulation of myocardial matrix metalloproteinase expression and activity by cardiac fibroblasts. IUBMB Life. 2012;64:143–50. doi: 10.1002/iub.594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.