Abstract
This FlyBook chapter summarizes the history and the current state of our understanding of the Wingless signaling pathway. Wingless, the fly homolog of the mammalian Wnt oncoproteins, plays a central role in pattern generation during development. Much of what we know about the pathway was learned from genetic and molecular experiments in Drosophila melanogaster, and the core pathway works the same way in vertebrates. Like most growth factor pathways, extracellular Wingless/Wnt binds to a cell surface complex to transduce signal across the plasma membrane, triggering a series of intracellular events that lead to transcriptional changes in the nucleus. Unlike most growth factor pathways, the intracellular events regulate the protein stability of a key effector molecule, in this case Armadillo/β-catenin. A number of mysteries remain about how the “destruction complex” destabilizes β-catenin and how this process is inactivated by the ligand-bound receptor complex, so this review of the field can only serve as a snapshot of the work in progress.
Keywords: beta-catenin, FlyBook, signal transduction, Wingless, Wnt
Introduction: Origin of the Wnt Name
THE story of the Wingless (Wg)/Wnt signal transduction pathway is a beautiful illustration of both the power of forward genetics and the utility of Drosophila as a genetic model system. The Wnt family of secreted growth factors plays a pivotal role in the embryonic development of all animal species. Wnts direct cell fate specification and morphogenesis in every tissue layer, patterning the central nervous system, the gut, the respiratory and circulatory systems, and various epidermal structures [reviewed in Nusse (2005)]. They also play a role in tumor formation; aberrant Wnt signaling is particularly associated with colorectal cancer in humans (Polakis 2007). Colorectal cancer is a leading cause of cancer deaths and second only to lung cancer, which is mostly attributable to tobacco use (Siegel et al. 2017). Thus, the ability to dissect the Wnt signaling pathway in Drosophila has broad relevance for understanding developmental processes and oncogenesis. Much of what was learned with Drosophila genetics inspired, and was informed by, parallel experiments on the vertebrate Wnt pathway, using mouse and Xenopus as model systems [reviewed in Nusse and Varmus (2012)].
Discovery of the fly gene: the wingless mutant phenotype
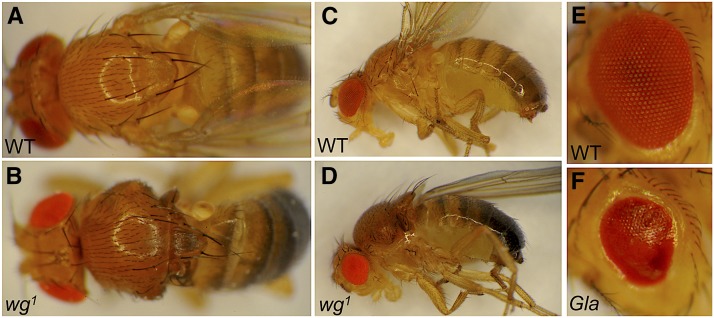
As the name suggests, wingless (wg) gene activity is required for generating the pattern of the adult fly wing, among its many functions during Drosophila development. The wingless mutant phenotype (Figure 1, A–D) was first characterized by R. P. Sharma, working at the Indian Agricultural Research Institute in New Delhi, India, who discovered this mutant in an ethyl methanesulfonate (EMS) mutagenesis (Sharma 1973). The wg1 mutation was recessive and homozygous viable, but there was variable penetrance of winglessness: the homozygous wg1 stock produced flies with no wings, one wing, or two normal wings, in roughly a 2:2:1 ratio. These flies also showed a variable loss of halteres, the pair of small appendages produced by the third thoracic segment, which function to counterbalance the wingbeats during flight. Mutant flies could have no halteres, one haltere, or two normal halteres, in a manner completely independent of the wing status in the second thoracic segment. The wg1 mutation was subsequently shown to result from a small deletion 3′ to the coding region (Baker 1987), identifying an enhancer element that drives expression specifically in the wing and haltere imaginal discs, the developmental precursors to the adult structures (Schubiger et al. 2010). Presumably, this enhancer mutation reduces the level of wg expression to some critical threshold, where sometimes there is enough to pattern the appendage properly and sometimes there is not.
Figure 1.
Viable wg mutant phenotypes. (A) Normal bristle pattern on the notum, the back of a fly’s thorax, with both halteres visible out of focus at the posterior edge (in this and all images, posterior is to the right). (B) Notum of a wg1 homozygous mutant showing disrupted pattern and absence of both wings and one haltere. (C) Side view of wild-type (WT) fly. (D) Side view of wg1 homozygous mutant showing duplicated notum in place of one missing wing, and misshapen eye (cinnabar eye color is not part of the wg phenotype). (E) WT eye shows a regular pattern of ommatidia, the units of the compound eye. (F) The eye of a fly heterozygous for the Gla mutation shows a smooth “glazed” surface.
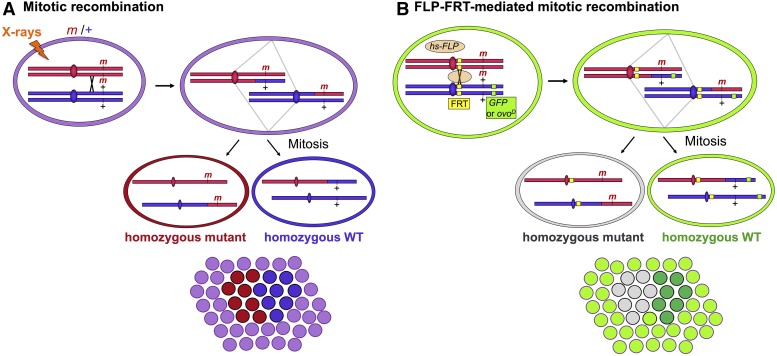
When the wing or haltere is absent in a wg1 fly, the tissue is replaced by a mirror-image duplication of the dorsal thorax, a region called the notum (Figure 1, A and B). This phenotype was interpreted as a homeotic transformation of wing to notum, except that unlike other homeotic mutations, the wg1 mutation behaves in a noncell-autonomous manner in genetic mosaics (Morata and Lawrence 1977). Mosaic flies contain patches of tissue bearing a genotype different from the rest of the fly (Figure 2A). When clones of homozygous mutant tissue were induced by mitotic recombination in a heterozygous wg1/+ animal, small mutant clones were consistently found in completely normal wings. This effect showed that the normal gene product, produced in wild-type tissue, was able to rescue neighboring mutant cells, and thus showed that wg acts nonautonomously.
Figure 2.
Generation of genetic mosaics. (A) Clones of homozygous mutant cells are generated in heterozygous flies when mitotic recombination between the homologs occurs. This rare event can be induced by exposing flies to X-rays, which cause double-stranded DNA breaks that often lead to crossing-over in the process of being repaired. If both mutant chromatids are pulled to the same mitotic spindle pole, the resulting two daughter cells will have different genotypes. Subsequent cell divisions generate mitotic clones from each daughter, producing a “twin spot” of homozygous mutant (red) and homozygous wild-type (WT) (blue) cells within a field of heterozygous cells. (B) The yeast flippase (FLP) and its target sequence (FRT) can be used instead of X-rays to induce mitotic recombination. Heat shock-induced flippase catalyzes site-specific recombination at the FRT target. The presence of a dominant marker, such as green fluorescent protein (GFP), on the WT homolog allows easy detection of mutant clones in somatic tissue. Inclusion of the ovoD dominant female-sterile mutation blocks egg formation in heterozygous ovarian tissue. During the production of germ line clones, the only eggs recovered are derived from tissue homozygous for the non-ovoD-bearing chromosome.
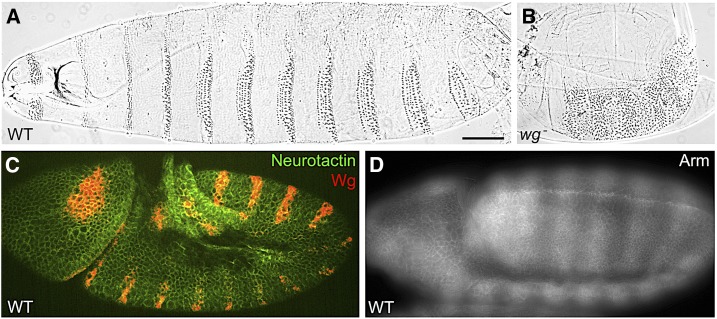
True loss-of-function alleles for wingless were recovered in the large-scale genetic screens for epidermal patterning defects, conducted at the European Molecular Biology Laboratory in Heidelberg (Nüsslein-Volhard and Wieschaus 1980). These screens used the cuticle pattern secreted by the embryonic epidermis as an assay to identify EMS-induced mutations that disrupt embryonic development. Among the many important mutations isolated in this effort were null mutations at the wg locus (Nüsslein-Volhard et al. 1984). The complete absence of Wg activity results in death of the embryo, with severe defects in the anterior–posterior pattern within each segment of the larval cuticle. Thus, wg was classified as a “segment polarity” mutant. The pattern disruption, like the wg1 notum, involves mirror-image duplications. Late in embryogenesis, the ventral epidermal cells produce arrays of hook-like projections, called denticle belts, which are separated by expanses of bare, or naked, cuticle in each segment (Figure 3A). In wg null mutant embryos, these expanses of naked cuticle are replaced by denticles with a reversed polarity (Figure 3B). The behavior of the wg null and wg1 mutant alleles indicated that normal activity of the wg gene promotes the segmental pattern of naked cuticle in the embryo, and plays a noncell-autonomous role in the development of the adult wing.
Figure 3.
Embryonic wg phenotypes. (A) Wild-type (WT) embryos secrete a segmental pattern of denticle belts separated by naked cuticle on their ventral surface. Bar, 50 µm. (B) wg null mutant embryos produce a cuticle pattern with no naked cuticle, only denticles, on the ventral surface. (C) Wg antibody staining (red) shows that the protein is expressed in stripes in WT embryos, and the protein is detected over several cell diameters on either side of the stripe (cell outlines visualized with Neurotactin antibody staining, green). The stripes of wg expression are located within a subset of the epidermal cells that will secrete naked cuticle. (D) Arm antibody staining (white) in WT embryos shows higher levels of Arm in broad stripes that are roughly centered over the Wg-producing cells.
These findings inspired N. Baker, a graduate student in the Lawrence laboratory, to pursue a molecular analysis of the wingless locus. In these early days of cloning, the best way to find the gene sequence was to generate a transposable element (P element) insertion allele, and then use the P element sequence as a hybridization probe to recover recombinant clones that carry both the insertion and chromosomal DNA flanking the element (Rubin et al. 1982; Spradling and Rubin 1982). The non-P element sequence from these clones represents wild-type genomic DNA from the region adjacent to the insertion. This strategy is still relevant today, but has been greatly accelerated by community resource projects with the goal of isolating insertional alleles for every gene in the genome (Spradling et al. 1999; Bellen et al. 2011). Rather than mobilizing P elements and screening for new insertions that fail to complement a mutation of interest, we can now search for an existing insertional allele among the collection generated by the Gene Disruption Project.
The cloned wg gene sequence was used to make RNA in situ hybridization probes, which revealed that wg is expressed in segmental stripes (Figure 3C) in the zone of epidermal cells predicted to produce naked cuticle (Baker 1987). The stripes of wg expression were immediately anterior to the expression stripe of another recently cloned segment polarity gene, engrailed (DiNardo et al. 1985). Expression of engrailed requires wg gene activity (DiNardo et al. 1988; Martinez Arias et al. 1988); wg expression in a nonoverlapping set of cells adjacent to the en stripe was consistent with previous observations that the wg gene acts nonautonomously.
Discovery of wg homology to the mammalian oncogene int-1
The connection between Wingless signaling and cancer was discovered early on, through efforts in the Varmus laboratory to identify cellular oncogenes by insertional mutagenesis (Varmus 1984). Retroviruses, such as Mouse Mammary Tumor Virus (MMTV), carry strong promoters in their long terminal repeats. When the viral cDNA integrates into chromosomal DNA, the promoter in the downstream repeat is positioned next to host genes. If a retrovirus integrates next to a proto-oncogene, the gene is turned on at high levels and drives tumor formation. Proto-oncogenes identified as MMTV integration sites, where the proviral insertion caused breast tumors in mice, were initially named int genes. Characterization of the first of these, int-1, revealed a sequence predicted to encode a secreted, cysteine-rich molecule that was otherwise novel (Nusse and Varmus 1982; Nusse et al. 1984). To understand how the molecule might function to promote tumor formation, the Nusse laboratory searched for sequences homologous to int-1 in Drosophila. They discovered a gene that had 54% amino acid identity with the mouse int-1, and found that it matched the sequence of Baker’s wg clone (Rijsewijk et al. 1987). The Nusse laboratory went on to construct a wild-type wg transgene and introduce it into flies under the control of the heat shock promoter, using the P element-mediated germ line transformation technique (Rubin and Spradling 1982; Spradling and Rubin 1982). Ectopic expression of wg, induced uniformly by heat shock on top of the endogenous stripes of expression, produced embryos with ventral surfaces composed entirely of naked cuticle (Noordermeer et al. 1992). Thus, all of the denticle-secreting cells in every segment are converted to the naked cuticle cell fate when they express high levels of wg, confirming that wg activity is both necessary and sufficient for naked cuticle specification in the embryonic epidermis.
Normal expression of the mouse int-1 was found to be mostly restricted to the developing nervous system in embryos (Shackleford and Varmus 1987; Wilkinson et al. 1987). Knock-out mutations engineered into mice produced severe defects in patterning of the brain, virtually eliminating the cerebellum (Thomas and Capecchi 1990). Indeed, the Capecchi laboratory discovered that an old neurological mutant in the mouse, called swaying, was caused by a lesion at the int-1 locus, with similar effects on cerebellar development (Thomas et al. 1991). Overexpressing the mouse int-1 in Xenopus, by injecting the mRNA into eggs, produced dramatic duplication of the frog embryo’s body axis (McMahon and Moon 1989). Thus loss-of-function mutations for both wg and int-1 severely disrupt the embryonic development of ectodermally derived tissues, and gain-of-function for both molecules transforms cell fates.
Wnts are a conserved gene family found throughout the animal kingdom
Homologs of wg and int-1 were subsequently identified both within the mammalian and Drosophila genomes [reviewed in Nusse and Varmus (1992)], and more broadly in a wide variety of animal species, from leeches and starfish to humans (van ’t Veer et al. 1984; Kostriken and Weisblat 1992; Sidow 1992). There are even some homologs in the sponge genome, but none have yet been found in single-celled organisms, suggesting that this gene family may be as old as multicellularity (Nichols et al. 2006; King et al. 2008; Loh et al. 2016).
The name Wnt, a combination of wg and int-1, was chosen to describe this family of growth factors (Nusse et al. 1991). There are now known to be 19 Wnt genes in most mammalian genomes. A comprehensive list of known Wnt family members, as well as an overview of the pathway and a database of resources for Wnt researchers, are curated by the Nusse laboratory on “The Wnt Homepage” at http://wnt.stanford.edu. Wnt proteins undergo a post-translational lipid modification that is essential for their function (Willert et al. 2003; Takada et al. 2006), as well as N-linked glycosylation as they transit through the secretory pathway (Brown et al. 1987; Papkoff et al. 1987). The covalent attachment of a lipid group, palmitoleic acid, has complicated the study of Wnts because (unlike many other growth factors) they are not freely soluble. Other secreted signals could be purified and crystallized to solve their structures, or used in assays on cultured cells to identify their cell surface receptors and intracellular components. For many years, Wnts were a signal without a receptor. The Drosophila model system proved to be critical in finding the core components of the Wnt pathway (Table 1) and in understanding how the pathway works.
Table 1. Known components of the Wingless pathway and their human counterparts.
| Drosophila gene | Human homolog | Activity | |
|---|---|---|---|
| Production | wingless | WNT1, 2, 2B(13), 3, 3A, 4, 5A, 5B, 6, 7A, 7B, 8A, 8B, 9A, 9B, 10A, 10B, 11, 16 | Secreted signal (ligand) |
| porcupine | PORCN | O-acyltransferase | |
| Wntless/evenness interrupted/sprinter | WLS | Multipass transmembrane protein, chaperone? | |
| Swim | TINAG, TINAGL1 | Polysaccharide binding | |
| Activation | arrow | LRP5,6 | Receptor |
| frizzled, frizzled2 | FZD1, 2, 3, 4, 5, 6, 7, 8, 9, 10 | Receptor | |
| dishevelled | DVL1, 2, 3, L1 | ? | |
| armadillo | CTNNB1 | β-catenin (effector) | |
| Tcf/pangolin | TCF1, 3, 4, LEF-1 | Transcription factor | |
| legless | BCL9 | Transcriptional cofactor | |
| pygopus | PYGO1, 2 | Transcriptional cofactor | |
| Inhibition | naked cuticle | NKD1, 2 | ? |
| discs overgrown/CK1e | CK1e | Casein kinase | |
| zeste-white3/shaggy | GSK3A, B | Glycogen synthase kinase | |
| Apc, Apc2 | APC1, 2 | Destruction complex | |
| Axin | AXIN1, 2 | Destruction complex | |
| groucho | TLE1, 2, 3, 4, 6 | Transcriptional corepressor | |
| slimb | BTRC, FBXW7, 11 | F-box of ubiquitin ligase | |
| Modulation | sugarless | UGDH | UDP-glucose 6-dehydrogenase |
| sulfateless | NDST1, 2, 3, 4 | N-deacetylase and N-sulfotransferase | |
| Dally, dally-like | GPC1, 2, 3, 4, 5, 6 | Glypican | |
| Notum | NOTUM | Palmitoleoyl-protein carboxylesterase | |
| CtBP | CTBP1, 2 | Transcriptional cofactor |
Based on information from FlyBase (http://flybase.org/) and the Human Genome Organisation Gene Nomenclature Committee (https://www.genenames.org/).
Identifying the Components of the Wingless/Wnt Pathway
Forward genetic screens for embryonic pattern disruption
“Heidelberg” screens for zygotic patterning phenotypes:
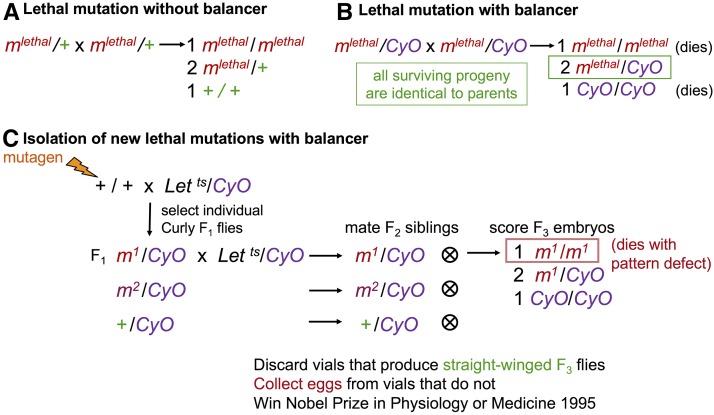
The long history of Drosophila genetics was critical to gene discovery in the Wnt pathway. Starting at the turn of the last century, T. H. Morgan’s laboratory, first at Columbia University and then at Caltech, generated and mapped hundreds of fly mutations to produce rough maps of the four fly chromosomes. The fly community was blessed with many generous individuals who created and shared fly stocks and information, and laid the groundwork for the large-scale screens that were to follow. Two features were particularly important for these screens: a comprehensive catalog of existing mutations and the availability of balancer chromosomes (Lindsley and Grell 1968). Balancer chromosomes are versions of the three largest fly chromosomes—the X, second, and third chromosomes—which contain multiple inversions that suppress recombination. In addition, they carry at least one dominant marker mutation, and at least one homozygous lethal mutation (or in the case of the X chromosome, a female-sterile mutation). This prevents the chromosome from surviving (or contributing to the next generation) in the homozygous state (Figure 4, A and B). For lethal mutations on the fourth chromosome, only a dominant marker mutation is needed because the fourth chromosome is mostly heterochromatic and does not undergo recombination (Ashburner 1989).
Figure 4.
The magic of balancer chromosomes. (A) A lethal mutation must be maintained in the heterozygous state, with a wild-type allele of the gene on the other homolog. If the other homolog is entirely wild-type, this wild-type chromosome will predominate in future generations, and the lethal mutation may be lost. (B) Balancers, such as Curly-O (CyO), were designed so that homozygous lethal mutations could be maintained in heterozygous, balanced, fly stocks that are stable over many generations. Flies carrying a lethal mutation on one homolog and a balancer chromosome as the other homolog would produce progeny where one-quarter are homozygous lethal due to the first mutation, one-quarter are homozygous lethal (or are sterile, in the case of X chromosome balancers) due to homozygosity for the balancer, and the remaining half survive to adulthood as heterozygotes with a genotype identical to the parents. Multiple inversions on the balancer disrupt pairing between the homologs, preventing a recombination event between the desired lethal mutation and the recessive lethal mutation carried by the balancer, which could otherwise generate an entirely wild-type chromosome. (C) Balancer chromosomes can be used in genetic screens to isolate new lethal mutations. Flies are mutagenized and crossed to a stock carrying a balancer chromosome. The Heidelberg screens made use of a balancer stock carrying a dominant temperature-sensitive lethal mutation (here designated Let ts) to eliminate the nonbalancer chromosome in the next generation. However, any dominant mutation different from the marker on the balancer could be used and selected against in the next generation. Each F1 individual is then crossed back to a fly of the opposite sex from the balancer stock. F2 progeny from these individual crosses are then selected for the presence of the balancer dominant marker and the absence of the nonbalancer dominant marker, or incubated at high temperature to eliminate the nonbalancer progeny if using a dominant temperature-sensitive lethal stock. In this way, F2 individuals of the opposite sex, each heterozygous for the same mutagenized chromosome, are generated and can be crossed together to assess the homozygous phenotype of the mutagenized chromosome.
The presence of a dominant visible marker mutation also makes balancer chromosomes useful for recovering newly induced mutations that might be homozygous lethal (Figure 4C). In a mutagenesis, each chromosome exposed to the mutagen would have a unique set of mutations. To find mutations that produce autosomal recessive phenotypes requires a breeding program that produces two flies of the opposite sex that carry exactly the same mutagenized chromosome. Balancer chromosomes facilitate such breeding programs. The basic strategy used by the Heidelberg group was to cross mutagenized flies to flies carrying a balancer for a particular chromosome, so that each individual fly in the next generation carried a uniquely mutagenized chromosome balanced with an unmutagenized balancer chromosome. These flies were then bred through two generations (see Figure 4C) to produce embryos homozygous for each new mutation, which could then be examined for patterning defects.
This strategy, however tedious, was enormously successful in generating a collection of EMS-induced embryonic-lethal mutations for each of the four chromosomes (Jürgens et al. 1984; Nüsslein-Volhard et al. 1984; Wieschaus et al. 1984). Some embryonic-lethal mutations showed cuticle pattern disruptions that fell into one of three classes: gap, pair-rule, and segment polarity; these mutations revealed the basic mechanics of early embryonic development in the fly. The segment polarity class was particularly important to the Wnt story, because not only were wg loss-of-function mutations recovered in this screen, but so too were two other segment polarity mutations that produced all-denticle phenotypes similar to wg mutants. The two genes disrupted by these mutations, armadillo (arm) and arrow (arr), encode core components required to activate the Wnt pathway; thus, their loss-of-function produced embryonic pattern disruptions similar to loss of the signal itself. Conversely, other mutations isolated in the Heidelberg screens, such as naked cuticle (nkd) and shaven-baby (svb), produced the opposite effect on patterning: the secretion of all-naked cuticle. This mimics the effects of overexpressing wg, and suggested that the wild-type gene products play a role in opposing Wg pathway activity.
The arm gene encodes the fly β-catenin protein (Peifer and Wieschaus 1990), an intracellular effector that drives target gene expression in response to the Wnt signal. Activity of the Wg pathway hinges on the stabilization of Arm protein: cytosolic Arm is continually turned over by a set of proteins dedicated to its destruction, which is inhibited when Wg binds and activates its receptor complex (Peifer et al. 1994b), described in Function of the Wingless/Wnt Pathway. The arrow gene encodes part of the receptor complex, but this was not recognized until much later, mostly because the mutant phenotype was not as severe as the wg mutant phenotype. This brings us to one of the limitations of forward genetic screens. The Heidelberg screens were designed to identify zygotic phenotypes, that is, phenotypes that result exclusively from the embryo’s own genotype. However, many gene activities important for fly development are prepackaged in the egg by the mother. The maternal contribution of a gene product may allow homozygous mutant embryos to develop normally, and thus a role for that gene product in patterning would not be detected.
In some cases, the maternal product is used up during embryogenesis and the homozygous mutant may survive to later stages, where defects in imaginal disc patterning can be detected. This was the case for mutations in casein kinase 1 (CK1), which were first identified in flies as alleles of lethal(3)discs overgrown (dco) (Jursnich et al. 1990). Homozygous dco mutant larvae survive but remain in the larval stage for extended periods, with dramatic overgrowth of the imaginal discs and eventual death. The dco gene was shown to encode the Drosophila CK1ε, a Ser/Thr kinase (Zilian et al. 1999), which had also been identified as the gene disrupted by doubletime mutations that affect circadian rhythm (Kloss et al. 1998). Overexpression of dco in Drosophila S2 cells, a cultured cell line, showed that CK1 phosphorylates Arm and that this correlates with increased Arm degradation (Yanagawa et al. 2002). However, subsequent experiments in vivo indicated that CK1ε can play a positive role in promoting Wg signaling (Klein et al. 2006). This discrepancy was resolved when other casein kinases in the fly genome were tested for roles in Wg signaling. Reducing the function of the closely related CK1α produced strong hyperactivity of the Wg pathway in vivo, indicating that CK1α has a profound negative regulatory role (Zhang et al. 2006). These observations suggested complex relationships between CK1 family members and the Wg pathway, but hinted at involvement in the destruction complex, which adds phosphate tags to Arm/β-catenin, making it a target for degradation by the proteasome (Figure 5).
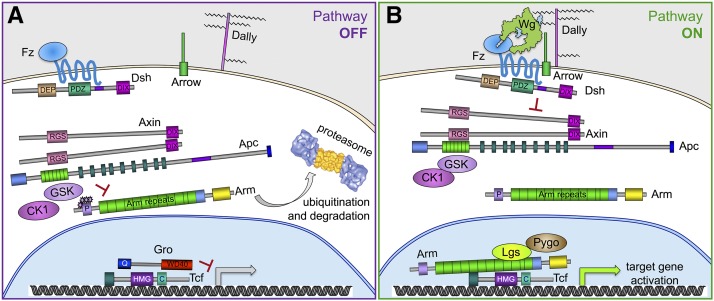
Figure 5.
Diagram of core components in the Wg pathway. (A) In the absence of Wg signaling, Arm is presented by Apc and Axin to CK1 and Zw3/GSK for phosphorylation, targeting it for ubiquitination and degradation by the proteasome. Tcf binds target genes and, with the Gro transcriptional corepressor, keeps expression repressed. (B) Wg, concentrated at the cell surface by glycosaminoglycans on the glypican Dally, binds the Fz and Arrow receptors and causes them to cluster. This allows polymerization of Dsh and Axin at the plasma membrane, inactivating the kinase complex so that it cannot target Arm for destruction. Stabilized Arm translocates into the nucleus, binds to Tcf, and recruits the transcriptional activation complex, which includes Lgs and Pygo. Structural features of proteins depicted here are based on data from Wodarz and Nusse (1998), Janda et al. (2012).
Maternal-effect screens for embryonic patterning mutants:
Specific techniques can be employed to hunt for gene products that are maternally contributed but are also required zygotically. These techniques were employed very effectively to identify other critical components of the Wg pathway. Maternal-effect mutations, where homozygous mutant female flies produce embryos with disrupted pattern, identified genes that are required during oogenesis (Perrimon et al. 1986; Schupbach and Wieschaus 1986). However, mutations in genes that are required both maternally and zygotically were not recovered, because the zygotic requirement prevents survival of homozygous mutant females to egg-laying adulthood. Finding mutations in this class of gene required the generation of a clone of homozygous mutant tissue in the gonad of a heterozygous female fly. Eggs produced from the mutant cells would then lack the maternally loaded gene product. N. Perrimon pioneered the use of an X-linked dominant female-sterile mutation, ovoD, to produce germ line clones of homozygous mutant tissue (Perrimon 1984). The ovoD mutation blocks oogenesis in heterozygous ovarian tissue, so the only eggs produced are derived from mitotic clones homozygous for the non-ovoD-bearing X chromosome.
The ability to remove the maternal contribution from developing embryos revealed several critical components of the Wg signaling pathway that are maternally provided and zygotically required. Several lethal alleles had been found for dishevelled (dsh), an X-linked gene originally identified by a weak mutation that is adult viable and causes tissue polarity disruption. Lethal dsh homozygous mutants have normal cuticle pattern and die during larval stages, but when germ line clones of these dsh mutations were generated, they resulted in embryonic lethality, with homozygous embryos showing an all-denticle phenotype identical to zygotic loss of wg function (Perrimon and Mahowald 1987). This discovery led to a large-scale screen for X-linked maternal-effect mutations that cause pattern disruption. This screen identified two important components of the Wg pathway in addition to new alleles of dsh (Perrimon et al. 1989). Germ line clones of porcupine (porc) mutations, like dsh, produced embryos with wg-like pattern defects. The porc gene product was later shown to control proper secretion of the Wg protein (van den Heuvel et al. 1993). Germ line clones of zeste-white3 mutations (zw3, also known as shaggy) produced the opposite effect—all-naked cuticle—suggesting that its wild-type function was to inhibit Wg signaling activity. Indeed, zw3/shaggy was found to encode the fly homolog of glycogen synthase kinase 3 β (GSK), which phosphorylates Arm protein and targets it for degradation (Siegfried et al. 1990, 1992). The action of GSK requires prior Arm phosphorylation by CK1. Experiments in human cells (Amit et al. 2002) and in the Xenopus model system (Liu et al. 2002) showed that CK1 phosphorylates Ser45 in β-catenin, and that this is required to “prime” Arm/β-catenin for the GSK phosphorylations that target it for degradation.
The germ line clone technique was later improved (Chou and Perrimon 1992) by combining it with the FLP-FRT system (Figure 2B). The yeast site-specific recombinase FLP and its target, FRT, were transferred into flies (Golic and Lindquist 1989) to produce a more reliable and precise means of generating mitotic crossovers than X-irradiation. The Perrimon laboratory engineered fly strains with an FRT recombination target site inserted at the base of each chromosome arm and an inducible source of FLP recombinase, allowing easy generation of mitotic clones. They also cloned the ovoD mutant gene, and used P element-mediated transformation to create fly lines with the ovoD transgene inserted on each of the autosomal chromosome arms (Chou et al. 1993). Autosomal ovoD transgenes extended the efficient production of germ line clones beyond X-linked mutations, allowing germ line clone production for mutations on the second and third chromosomes of Drosophila. These experiments yielded more mutations that produced wg-like all-denticle patterns when removed both maternally and zygotically (Perrimon et al. 1996). Among the autosomal genes identified were sugarless and sulfateless, which encode enzymes that function in the biosynthesis of heparan sulfate glycosaminoglycans. This implicated cell surface/extracellular matrix proteoglycans in modulating reception of the Wg signal (Häcker et al. 1997; Lin and Perrimon 1999). Another gene identified using this strategy was sprinter, since renamed Wntless, which encodes a multiple transmembrane domain protein that acts to chaperone Wg during its journey through the secretory pathway (Goodman et al. 2006).
Other genetic tricks to identify pathway components
Suppressor screens for mutations that modify loss-of-function wg phenotypes:
One strategy for finding mutations that compromise a particular genetic pathway is to mutagenize a fly line that bears a weak mutation in one pathway component and screen for mutants that show an enhanced or suppressed phenotype. This “modifier” screen strategy was used to great effect in dissecting the Ras signaling pathway, through suppressor/enhancer screens with a weak mutation in the Sevenless receptor tyrosine kinase, which controls cell fate specification during Drosophila eye development (Rogge et al. 1991; Simon et al. 1991). My laboratory used partially functional wg mutant alleles (Bejsovec and Wieschaus 1995; Dierick and Bejsovec 1998) to provide a “sensitized” background for identifying modifier mutations that affect Wg signaling. We isolated a number of mutations that partly suppressed the pattern defects of the wg mutants, including mutations in the genes encoding the transcription factor Tcf, also known as Pangolin, and the negative Wg/Wnt regulator, Apc2 (van de Wetering et al. 1997; McCartney et al. 1999). Tcf, and its close relative LEF-1, had been characterized as HMG-box-containing transcription factors important for vertebrate T cell development. Tcf/LEF had been found to bind β-catenin in yeast two-hybrid protein–protein interaction screens, and to form a transcriptional activation complex with β-catenin (Behrens et al. 1996; Molenaar et al. 1996). Our recovery of Tcf/pan mutations as suppressors of wg loss-of-function phenotypes revealed that Tcf is not simply required for transcriptional activation of Wnt target genes, but also acts to repress those genes in the absence of Wg signaling (Cavallo et al. 1998). A connection between the fly Wg pathway and Tcf/LEF-1 was also found in the Bienz laboratory, who worked backward from a Wg response element they had defined upstream of Ultrabithorax, a target gene regulated by embryonic Wg signaling in the Drosophila intestine (Riese et al. 1997). They had noted that the DNA sequence of this element was similar to the consensus binding site for LEF-1, tested for binding of the mouse LEF-1 to this Drosophila sequence, and concluded that flies must have a LEF-1 homolog.
Our isolation of an Apc2 mutation in the suppressor screen was particularly serendipitous because loss-of-function Apc2 mutations are homozygous viable in the first generation. Maternally and zygotically mutant embryos derived from these Apc2 homozygous mothers produced the all-naked cuticle phenotype that indicates deregulation of the Wg pathway (McCartney et al. 1999). Our mutation, Apc2∆S, was temperature sensitive, allowing us to maintain a homozygous mutant fly stock at low temperature, then shift these flies to the higher temperature to characterize the effects of the mutation. Thus, we could demonstrate, without having to make germ line clones, that Apc2 was a maternal-effect gene, and that the mutant phenotype implicates Apc2 gene activity in the Arm destruction process. Other groups had identified the first Apc homolog in flies, based on similarity to the human tumor suppressor gene responsible for Adenomatous polyposis coli, a familial form of colon cancer (Polakis 1997). This first gene, when mutated, showed defects in the nervous system, such as degeneration of photoreceptor cells in the retina, but had no effect on embryonic Wg signaling (Hayashi et al. 1997; Ahmed et al. 1998). Thus flies, like humans, have two Apc genes that differ in their levels of expression in different tissues, which accounts for their distinct phenotypes when mutated (Hamada et al. 1999a; McCartney et al. 1999). However, both Apc molecules contribute to Wg regulation because doubly mutant Apc Apc2 embryos or mitotic clones in various tissues show more profound hyperactivation of Wg signaling (Ahmed et al. 2002; Akong et al. 2002). This appears to be the case also with the two mammalian Apc molecules; disruption of both Apc and Apc2 drives tumorigenesis in mouse mammary tissue and leads to higher Wnt target gene expression in human breast tumor samples, compared with singly mutant tissue (Daly et al. 2017).
Suppressor screens for mutations that modify gain-of-function wg phenotypes:
An alternative strategy to identify pathway components was to screen for mutations that suppress an artificial phenotype produced by overexpressing wg. The Basler laboratory took advantage of a construct that fused the sevenless promoter to a wild-type wg transgene, driving wg overexpression exclusively in the developing fly eye. This construct disrupted the very precise organization of the adult compound eye, producing a rough-eye phenotype that is easily observed but does not otherwise affect the health of adult flies (Cadigan and Nusse 1996). Dominant suppression of this rough-eye phenotype could then be assayed by crossing EMS-mutagenized flies to the sev-wg fly line. This screen identified mutations in Tcf/pan (Brunner et al. 1997), as well as mutations in a new gene, named legless (lgs), which encodes a protein essential for transcriptional activation of the Tcf-Arm complex (Kramps et al. 2002). The Cadigan laboratory used a variation on this theme to find gene products that, when overexpressed themselves, could suppress the rough-eye phenotype of eye-specific wg overexpression (Parker et al. 2002). This gain-of-function screen used the EP lines developed by P. Rorth (Rorth 1996). These randomly inserted upstream activating sequence (UAS) target sequences, recognized by the yeast Gal4 transcription factor, can be activated with the Gal4-UAS binary expression system, which uses a variety of fly promoters to drive Gal4 in defined domains (Brand and Perrimon 1993). Using eye-specific Gal4 drivers, the Cadigan laboratory identified zw3/sgg, and a second gene called pygopus (pygo). Pygo had also been identified in the Basler laboratory as a yeast two-hybrid interactor with Lgs (Kramps et al. 2002), and thus it forms part of the transcriptional complex that controls Wg target gene activation. The Cadigan laboratory also identified C‐terminal‐binding protein (CtBP) and CREB-binding protein (CBP) in their gain-of-function screens, and showed that these transcriptional regulators have dual roles, both positive and negative, in controlling Wg target gene expression (Fang et al. 2006; Li et al. 2007). CtBP had also been identified by the Bienz laboratory as a binding partner of Apc2, and shown to act as a transcriptional corepressor of Wg target genes (Hamada and Bienz 2004).
Broader gain-of-function screens for molecules that disrupt wing pattern also yielded Wg pathway components. A screen of the Rorth EP collection using a wing-specific Gal4 driver identified Notum, which, when overexpressed, causes a wg1-like duplication of the notum at the expense of the wing, suggesting that it antagonizes Wg signaling (Giraldez et al. 2002). Notum was also identified in the Basler laboratory using a different approach: constructing a collection of randomly inserted Gal4 “enhancer traps,” which will express Gal4 under the control of genomic regulatory elements close to the site of insertion, and screening for insertions that mimic the wg pattern of expression in the wing imaginal disc (Gerlitz and Basler 2002; Gerlitz et al. 2002). The similarity of expression patterns between wg and Notum suggested that Notum is part of a negative feedback loop in the wing disc. Indeed, Notum was found to be a direct target of Wg signaling (Hoffmans et al. 2005; Parker et al. 2008), and encodes an extracellular enzyme that can inactivate Wg by cleaving off its lipid group (Kakugawa et al. 2015).
Ironically, a gain-of-function wg phenotype in the eye defined the very first published report of a wg mutation, although it took 63 years to understand this. The dominant Glazed (Gla) mutation, which narrows the eye and smoothens its surface (Figure 1, E and F), was isolated in the Morgan laboratory (Morgan et al. 1936). The Basler laboratory was able to revert this phenotype by X-ray mutagenesis and by P element insertion, indicating that the phenotype resulted from a gain-of-function (Brunner et al. 1999). Their realization that the reverting P element was inserted very close to the wg gene led them to discover that the Gla phenotype was produced by ectopic wg expression during pupal development of the eye. This abnormal wg expression was caused by insertion of a roo retrotransposon, with a strong promoter in its long terminal repeat, making it analogous to the MMTV insertion that defined the first mouse Wnt, int-1.
Mosaic screens:
Mitotic recombination, induced by X-irradiation, can be used to assess adult phenotypes of genes that are essential for embryonic development. For example, when lethal wg loss-of-function alleles were tested by clonal analysis, the mutant clones were mostly rescued by surrounding wild-type tissue (Baker 1988a), as was found for the wg1 allele (Morata and Lawrence 1977). However, large clones of wg mutant tissue resulted in notching of the adult wing margin (Baker 1988a), correlating with a stripe of wg mRNA expression in this region of the wing imaginal disc (Baker 1988b) and revealing a role for Wg signaling in specifying this part of the wing structure.
The FLP-FRT system provided a much more convenient and reliable means of producing mitotic clones than the X-irradiation technique (Figure 2, A and B), and enabled large-scale genetic screens to be conducted. The Xu laboratory, using this approach to identify genes involved in growth and patterning of imaginal discs, recovered mutations in supernumerary limbs (slimb), an F-box protein that forms part of the E3 ubiquitin ligase complex that targets Arm for degradation (Xu et al. 1995; Theodosiou et al. 1998). The Struhl laboratory also found alleles of slimb using a similar approach, but screening specifically for mutant clones that alter the pattern of adult structures, as slimb mutant clones produce dramatic duplications of wing and leg tissue (Jiang and Struhl 1998). The first mutant allele of the fly CK1α was identified in a similar mosaic screen for disrupted eye development, supporting a role for CK1α in regulating Wg signaling (Legent et al. 2012).
The FLP-FRT system can also be used in suppression screens: the Basler laboratory adapted this strategy to find recessive mutations that suppress the rough-eye phenotype of the sev-wg fly line. They crossed the sev-wg transgene into flies carrying a FLP transgene driven specifically in the eye, so that only eye tissue would produce homozygous mutant clones of EMS-induced mutations. This strategy yielded lethal mutations in a gene that they named Wntless (Banziger et al. 2006), which was allelic with the sprinter locus identified through germ line clone screens for embryonic wg-like cuticle defects (Goodman et al. 2006).
Reverse genetic approaches:
Wntless was also identified in a cell-based screen. The Perrimon laboratory had generated a library of double-stranded RNA constructs to knock down mRNA levels for > 90% of the predicted genes in the fly genome (Boutros et al. 2004). This RNA interference (RNAi) strategy was used to screen wg-expressing Drosophila cell lines for knockdowns that altered expression of a Wg reporter transgene (Bartscherer et al. 2006). In addition to some known pathway components such as arrow, the Boutros laboratory discovered a gene that they initially named evenness interrupted (evi), and which is now known as Wntless.
Large-scale RNAi screens for whole fly phenotypes are also possible, primarily through the efforts of the Vienna Drosophila Resource Center (Dietzl et al. 2007) and Harvard’s Transgenic RNAi Project (Perkins et al. 2015), which have produced fly lines carrying double-stranded RNA transgenes for most of the protein-coding genes in the genome. Screens using these RNAi transgenes have also detected Wg pathway modulators. For example, the Basler laboratory screened RNAi transgene lines using wing-specific Gal4 drivers, and identified the armless gene by its ability to disrupt wing margin pattern when knocked down (Reim et al. 2014). This gene encodes a ubiquitin regulatory molecule that appears to protect Arm from degradation by the proteasome. The Verheyen laboratory used a similar approach to identify phosphatases and kinases that modulate Wg signaling during wing imaginal disc patterning (Swarup et al. 2015).
Protein–protein interaction with known components:
A key component of the Arm destruction complex, Axin, was identified in a yeast two-hybrid screen for Arm-binding proteins, and was subsequently shown to bind to both Zw3/GSK and Apc (Nakamura et al. 1998; Hamada et al. 1999b). The Akiyama laboratory identified a loss-of-function Axin mutation in a collection of lethal P element insertion alleles: maternal/zygotic mutants produced an all-naked cuticle phenotype, similar to zw3/sgg and Apc2 maternal/zygotic loss-of-function. Likewise, mutant clones in imaginal disc tissues showed ectopic wing margin bristles and leg duplications, which are symptomatic of Wg pathway hyperactivity. The Nusse laboratory had also identified the Drosophila homolog of Axin, and used RNAi knockdown and overexpression of Axin in Drosophila embryos to demonstrate that Axin is required for Arm degradation (Willert et al. 1999).
Yeast two-hybrid screens conducted in the Bienz laboratory had found Apc as an Arm-interacting protein that can also bind to Zw3/GSK (Yu et al. 1999), and they identified CBP as a Tcf-interacting protein (Waltzer and Bienz 1998), thus establishing direct connections between these core pathway components. Yeast two-hybrid screens and GST pull-down assays also demonstrated contact between Arm and Lgs, and between Lgs and Pygo (Kramps et al. 2002), to establish direct connections among components of the transcriptional activation complex. Yeast two-hybrid screens for Tcf-interacting proteins in the Xenopus model system identified the vertebrate homolog of Groucho, a transcriptional corepressor in the fly (Roose et al. 1998). This physical interaction and corepressor function was verified in cultured cells; Drosophila Tcf was able to move Groucho into the nucleus, where it showed dose-dependent inhibition of a Tcf-Arm activated reporter gene (Cavallo et al. 1998). The Weis laboratory was able to recapitulate interactions of Tcf with β-catenin and with Groucho/TLE using purified proteins in vitro (Daniels and Weis 2005; Chodaparambil et al. 2014). How the Tcf transcriptional machinery switches from a repressor complex with Groucho to an activator complex with Arm/β-catenin is still unclear, but may involve post-translational modifications of Tcf and/or Groucho (Hikasa et al. 2010; Hanson et al. 2012), or rearrangement of the transcriptional complex proteins at Wg target gene promoters (van Tienen et al. 2017).
Noncanonical Wnt signaling and its connection to the canonical pathway
Tissue polarization in the epidermis and adult eye:
Some components of the Wnt pathway were hiding in plain sight. A weak mutation in dsh had long been known to disrupt the orientation of bristles on the back of the fly’s thorax and on the legs, as well as the organization of ommatidia in the compound eye (Lindsley and Grell 1968; Held et al. 1986). Thus, dsh gene activity was first associated with polarizing the actin cytoskeleton in epidermal cells for bristle and hair production [reviewed in Adler (1992)], and then subsequently was found to mediate the response to Wg signal in a cell-autonomous fashion (Perrimon et al. 1989; Klingensmith et al. 1994). Dsh is a core component in the establishment of planar cell polarity (PCP), a process that is conserved across the animal kingdom and is important in such diverse morphogenetic events as gastrulation and hair follicle orientation [reviewed in Goodrich and Strutt (2011)].
As with dsh, historic mutations in frizzled (fz) disrupted ommatidial organization, bristle polarity on the back of the fly, and hair polarity on the wing blade (Bridges and Brehme 1944; Lindsley and Grell 1968). However, unlike dsh, fz mutations were found to have noncell-autonomous effects on PCP as well as cell-autonomous effects: clones of mutant cells could influence the polarity of neighboring wild-type cells (Gubb and Garcia-Bellido 1982). The Adler laboratory showed that there is a clear directionality to this nonautonomy, where only the wild-type cells distal to fz mutant clones in the wing showed disruption (Vinson and Adler 1987). This observation suggested the presence of graded information along the proximal–distal axis of the developing wing. The Adler laboratory went on to clone and characterize the gene, finding that fz encodes a predicted cell surface molecule with seven transmembrane-spanning regions (Vinson et al. 1989). This structure suggested similarity to G protein-coupled receptors, but it was not clear what the ligand might be. The connection to the Wg pathway was made serendipitously when the Nathans laboratory found a mammalian Fz homolog among their collection of human retina cDNA clones, and searched for other mammalian Fz homologs using degenerate PCR primers (Wang et al. 1996). This led to their identification and characterization of many members of this large gene family in a variety of species, including a second Drosophila fz gene, which they named fz2 (also known as Dfz2). In collaboration with the Andrew and Nusse laboratories, they showed that this gene is expressed in segmental stripes in the embryo in a pattern similar to the wg expression pattern, and that when transfected into Drosophila S2 cells, it confers on them both the ability to bind to Wg and to respond to it by stabilizing Arm protein (Bhanot et al. 1996). Finally, the Wg signal had an excellent candidate for a receptor.
Genetic redundancy hindered discovery of the Wg receptor Fz2:
Why was the Wg receptor not identified earlier? The dirty little secret of genetics is that mutant phenotypes can be obscured by overlapping activity from a related gene. That is exactly what happens with fz and fz2. When mutations were made in the fz2 gene, by mobilizing a P transposable element inserted in the promoter region and screening for an imprecise excision event that deleted part of the coding sequence, no significant cuticle pattern disruption was observed in the homozygous mutant embryos (Bhanot et al. 1999). However, a perfect wg-like lawn of denticles phenotype could be generated by combining the fz2 mutation with both maternal and zygotic loss of fz function. Other signs of Wg signaling activity, such as stabilization of Arm protein and maintenance of en expression within each segment, were likewise affected in maternal/zygotic fz combined with zygotic fz2 mutation (Bhanot et al. 1999; Chen and Struhl 1999; Muller et al. 1999). Thus, the maternally contributed fz product is able to compensate for zygotic loss of fz2, even though it appears to be fz2 that is best able to bind to the Wg protein (Rulifson et al. 2000). Indeed, this capacity may be part of the reason that fz is able to compensate for loss of fz2: in fz2 single-mutant embryos, Wg protein accumulates to levels much higher than those seen in wild-type embryos, and may therefore drive a suboptimal interaction with Fz receptor to produce normal Wg signaling (Moline et al. 2000).
fz and fz2 transgenic flies clarified distinctions between polarity and Wg signaling:
The ease with which transgenes can be introduced into flies allowed structural comparison of the two Fz proteins (Boutros et al. 2000; Strapps and Tomlinson 2001). Frizzled structure can be subdivided into an extracellular domain, including a cysteine-rich domain known to bind Wg (Rulifson et al. 2000), a seven transmembrane-spanning region with both extracellular and intracellular loops, and an intracellular C-terminal domain that is not strongly conserved between the two Drosophila Frizzleds (Bhanot et al. 1996). These domains were swapped to create chimeric molecules that were then tested for their ability to rescue PCP vs. Wg signaling defects. The C-terminal domain of Fz correlated with polarity phenotypes, whereas the C-terminal domain of Fz2 correlated with Wg signaling activity (Boutros et al. 2000), consistent with earlier work showing that deletion of the Fz2 C-terminus produced a dominant negative effect on Wg signaling in the wing imaginal disc (Cadigan et al. 1998). Chimeras between Fz and Fz2 also demonstrated that C-terminal domains control apical–basal membrane targeting within epithelial cells, with basally localized Fz2 being essential for its Wg signaling function and apical Fz essential for polarization (Wu et al. 2004). Some of the phenotypic effects of Fz vs. Fz2 overexpression can be modulated by changing the dosage of Dsh, suggesting that the two Frizzleds compete for the cellular pool of Dsh molecules (Wu et al. 2004). Although both Fz and Fz2 can bring Dsh to the membrane when overexpressed (Boutros et al. 2000), it is Fz’s asymmetric placement of Dsh on distal cell membranes that correlates with tissue polarization (Axelrod et al. 1998; Axelrod 2001, 2009). Fz and Fz2 share a small conserved domain within their C-terminal regions that directly binds the PDZ domain of Dsh (Wong et al. 2003).
The extracellular cysteine-rich domain (CRD) of Fz correlated with rescue of fz mutant effects on PCP (Strapps and Tomlinson 2001). The CRD, even though it binds Wg, appears to be dispensable for Wg signaling. fz and fz2 transgenes that lack the CRD are still able to rescue Wg signaling (Chen et al. 2004; Povelones and Nusse 2005). Furthermore, molecular characterization of fz mutations found missense mutations in the extra- and intracellular loops and transmembrane domains, but none within the CRD (Povelones et al. 2005). Thus, the Wg-binding domain relevant to signaling may reside elsewhere, in the Fz2 extracellular N-terminus or in the extracellular loops of the transmembrane-spanning regions. However, Wg binding to the CRD of Fz may affect PCP. The Mlodzik laboratory showed that the Fz CRD binds to Van Gogh (Vang), a cell surface protein that localizes to proximal membranes, and that this interaction is essential for PCP (Wu and Mlodzik 2008). They went on to discover that Wg and one of its homologs in Drosophila, Wnt4, have redundant functions in modulating the interaction between Fz and Vang (Wu et al. 2013). Loss-of-function wg mutant clones were created with a temperature-sensitive allele of wg (Baker 1988a; Bejsovec and Martinez Arias 1991), which provides normal Wg activity to pattern the wing disc and can then be removed by shifting to higher temperature after patterning is complete. The wgts mutant clones have normal PCP, but when the wgts allele is combined with a mutation in the Wnt4 gene, the doubly mutant clones show strong PCP defects. Thus, these two secreted Wnt molecules, both expressed along the distal margin of developing wings, could provide a redundant source of graded information that might account for the nonautonomy observed in fz PCP phenotypes.
Other components of the canonical Wg pathway were identified through their PCP phenotypes in the fly eye, where photoreceptor cells are precisely arranged in each ommatidium with mirror symmetry across the dorsal–ventral midline [reviewed in Axelrod (2009)]. For example, nemo, which encodes a Ser/Thr kinase, was identified in the Benzer laboratory through its mutant effects disrupting polarity in the eye (Choi and Benzer 1994). nemo mutations were also found in a genetic screen for modifiers of signaling pathways (Verheyen et al. 1996), and the Verheyen laboratory showed that Nemo kinase acts as a negative regulator of Wg signaling, as well as of other signaling pathways (Verheyen et al. 2001).
Function of the Wingless/Wnt Pathway
Fly genetics showed how the pathway works
Stabilization of Arm protein as the key effector:
Although the arm gene product is contributed maternally (Wieschaus and Noell 1986), there is not enough to rescue embryonic patterning, so zygotic loss of arm function produces strong segment polarity mutant phenotypes. The Wieschaus laboratory set out to clone the arm gene with the same strategy used to isolate the wg gene sequence, by mobilizing P elements to generate new insertional alleles of the gene and then using the P element sequence as a hybridization probe for clone recovery (Riggleman et al. 1989). Cloning the arm gene allowed the Wieschaus laboratory to perform mRNA in situ hybridization in embryos, which revealed that the gene is broadly expressed in a uniform pattern during development (Riggleman et al. 1989). The breakthrough came when antibodies were made against the Arm protein. By contrast to the uniform mRNA distribution in embryos, Arm antibody staining showed a dramatic striped pattern (Figure 3D), which mirrored the striped pattern of wg expression and depended on wild-type Wg activity (Riggleman et al. 1990). Similar responsiveness to Wg was demonstrated in wing imaginal disc tissue (Peifer et al. 1991), confirming that wg gene activity caused a post-transcriptional stabilization of the arm gene product. The status of Arm protein stability correlated with cell fates: wg mutant embryos that produce no naked cuticle show no striping (Riggleman et al. 1990), whereas wg overexpression produces entirely naked cuticle and causes high uniform accumulation of Arm protein (Noordermeer et al. 1992). Other mutants that produce excess naked cuticle, such as naked and zw3/sgg, show corresponding increases in Arm protein accumulation as well (Noordermeer et al. 1992; Peifer et al. 1994b).
The Wieschaus laboratory then discovered that the Arm protein was homologous to human plakoglobin and β-catenin (Peifer and Wieschaus 1990; Peifer et al. 1992). These molecules are critically important for cell–cell adhesion, and indeed, so is Arm when maternal production is disrupted (Peifer et al. 1993). Germ line clones removing arm gene activity in the ovaries produce defective eggs, because of disrupted cell–cell adhesion and defects in the actin cytoskeleton, which is essential for transfer of maternal components into the developing oocyte (Cooley et al. 1992). Characterization of the arm sequence and the position of mutations within that sequence showed that Arm is a modular protein, with conserved N- and C-terminal domains and a more highly conserved repetitive middle region (Figure 5). This middle region contains 12 copies of a 42-amino acid motif, called Arm repeats, which forms a “superhelix” of helices with an extended positively charged groove (Huber et al. 1997). Within the groove are binding sites for the Arm/β-catenin-binding domains of various interacting molecules, such as cadherins, Tcf/LEF, and Apc [reviewed in Valenta et al. (2012)]. The C-terminal Arm domain appears essential for Arm’s role in Wg signaling activity, as many arm mutant alleles encode truncated proteins lacking this domain (Peifer and Wieschaus 1990). The Peifer laboratory corroborated this with structure/function analyses of Arm. Using arm transgenes, they showed that at least some Wg signaling function resides in the C-terminus, and found that this region can interact with the Arm repeat region in a yeast two-hybrid assay (Orsulic and Peifer 1996; Cox et al. 1999). The vertebrate β-catenin shows the same intramolecular interaction, with the C-terminus folding back to contact the repeat region, where it may regulate access of potential binding partners to favor adhesion vs. signaling function (Gottardi and Gumbiner 2004).
The N-terminal domain of Arm has a particularly important role in Wg signaling, as this is the portion of the protein that regulates its stability. The discovery that zw3/sgg encodes a Ser/Thr kinase strongly suggested that phosphorylation plays a role in activating the Wg pathway (Siegfried et al. 1990, 1992). Indeed, Arm protein was shown to be phosphorylated by GSK, and consensus sites for GSK phosphorylation were found in the N-terminal domain (Peifer et al. 1994a). The Peifer laboratory deleted the putative phosphorylation domain and found that ectopic expression of this armS10 transgene showed higher-than-wild-type accumulation of the transgenic Arm protein, along with uniform naked cuticle production in the embryo (Pai et al. 1997). This phenotype mimicked the effect of knocking down GSK or CK1 function (Peifer et al. 1994b; Yanagawa et al. 2002). Thus, Arm is stabilized artificially when it cannot be phosphorylated by GSK, the precursor step to Slimb-mediated ubiquitination and destruction by the proteasome. Under normal conditions, Apc and Axin, which each have multiple protein–protein interaction domains, bring the kinases into close proximity with Arm (Figure 5A). The group of interacting scaffold and kinase proteins is known collectively as the destruction complex, since it is dedicated to the phosphorylation and degradation of constitutively synthesized Arm [reviewed in Stamos and Weis (2013)]. This central feature of Wnt pathway control is conserved in humans; mutations in the corresponding GSK and CK1 phosphorylation sites of β-catenin, as well as loss-of-function mutations disrupting Apc, have been found in a variety of cancers [reviewed in Oving and Clevers (2002) and Polakis (2007)].
Genetic epistasis experiments determined the order of steps in the pathway:
The opposite cuticle patterns produced by loss and gain of Wg activity, all-denticle vs. all-naked cuticle, respectively, allowed researchers to deduce the molecular steps of pathway activation. The heat shock-driven wg+ transgene was used to determine that porc acts upstream of Wg, as the porc mutant phenotype was partially rescued by ectopic wg expression, whereas dsh and arm mutant phenotypes were not rescued (Noordermeer et al. 1994). This order matched expectations, as porc mutations disrupt Wg secretion but had no effect in Wg-responding cells (van den Heuvel et al. 1993; Kadowaki et al. 1996), whereas dsh and arm both showed cell-autonomous loss of Wg signaling in responding cells (Wieschaus and Riggleman 1987; Klingensmith et al. 1994). The excess naked cuticle phenotype of zw3 mutants (Perrimon and Smouse 1989) was used to test the order of intracellular gene activities. Removing arm function, in arm zw3 double-mutant embryos, resulted in the all-denticle arm mutant phenotype, indicating that Arm is required for the all-naked zw3 phenotype. By contrast, removing dsh function, in the zw3 dsh double-mutant embryos, did not eliminate the all-naked zw3 phenotype (Siegfried et al. 1994). This positioned Dsh as the most upstream cytosolic component, acting between receptor activation and destruction complex inhibition.
The nkd gene product may also act at this upstream position. Double-mutant experiments indicated that both dsh;nkd and arm;nkd showed the all-denticle phenotype (Rousset et al. 2001). However, the all-denticle phenotype was also observed in wg;nkd double mutants, with only a slight rescue of segmentation in the denticle pattern (Bejsovec and Wieschaus 1993), suggesting that nkd gene activity is most important when the Wg pathway is active. Indeed, Wg signaling induces nkd gene expression, suggesting that Nkd is an inducible negative feedback inhibitor of the pathway (Zeng et al. 2000). Overexpression of nkd suppressed dsh overexpression phenotypes in the eye, and Nkd bound to Dsh in yeast two-hybrid assays, indicating a direct interaction between them (Rousset et al. 2001). Experiments with the vertebrate homologs of Nkd and Dsh corroborated this, showing that Nkd and Dsh colocalize at the plasma membrane, where Nkd may influence the stability of Dsh (Hu et al. 2010; Schneider et al. 2010). In addition, Nkd has two nuclear localization sequences, and its localization to the nucleus correlated with partial rescue of the nkd mutant phenotype by a mechanism that is still unclear (Waldrop et al. 2006; Chan et al. 2008).
The arrow mutation, although it was identified in the Heidelberg screens, was not connected to the Wg pathway until the DiNardo laboratory tested it for maternal contribution. Homozygous mutant embryos from germ line clones produced the all-denticle cuticle pattern typical of lost Wg signaling (Wehrli et al. 2000). This phenotype was not altered by overexpression of wg, but was substantially rescued by overexpressing dsh, indicating that this predicted transmembrane protein acts between the Wg signal and its intracellular effectors. The vertebrate homologs of Arrow, LDL receptor-related proteins LRP5 and 6, were found to mediate Wnt signaling in Xenopus (Tamai et al. 2000). The intracellular domain of LRP5 was also shown to bind to Axin in mouse cells (Mao et al. 2001), providing another link between the cell surface and cytosolic components of the Wg/Wnt pathway. These results suggested that Arrow/LRP acts as a coreceptor with Fz to transduce the Wnt signal (Figure 5B). Perhaps the most convincing evidence of this was the construction of transgenic Drosophila lines carrying a chimeric molecule, with the intracellular domain of Arrow fused to the C-terminus of Fz2 (Tolwinski et al. 2003). The chimeric transgene was able to rescue naked cuticle formation in wg null mutant and in fz fz2 maternal/zygotic mutant embryos, but not in dsh or arm maternal/zygotic mutants. The behavior of the chimera suggested that Wg binding to the cell surface receptors may bring Fz2 and Arrow together, and that close proximity of the Arrow cytoplasmic domain with some or all of Fz’s three intracellular loops, and/or with Fz’s C-terminus, may trigger the intracellular response. This idea was corroborated in cultured Drosophila cells and in human cells by the Varmus laboratory, who engineered mutations into the rat Fz1 and human Fz5 genes, and demonstrated that residues in all three Fz intracellular loops and in the C-terminal tail were important for Wg-induced reporter gene expression (Cong et al. 2004). These Fz mutations also reduced binding to Dsh and reduced the activity of a Fz-LRP chimeric molecule, confirming that Wg/Wnt binding induced the clustering of Fz with LRP for intracellular transduction of the signal. Subsequent experiments with an artificial ligand designed to cross-link the extracellular portions of Fz and LRP also produced Wg pathway activation (Janda et al. 2017).
Downstream steps in the transcriptional activation of wg target genes were also deduced in genetic epistasis experiments. The all-naked cuticle phenotype produced by the armS10 transgene, which lacks the phosphorylation sites targeting Arm for degradation, was suppressed by Tcf/pan loss-of-function (van de Wetering et al. 1997). Thus, Tcf gene activity is essential for the phenotypic effects of Arm stabilization, indicating that the physical interaction detected in yeast two-hybrid assays is relevant to in vivo target gene activation. Conversely, deleting the predicted Arm-binding domain of Tcf yields a dominant negative molecule that strongly represses target gene expression, replacing naked cuticle with denticles when expressed in otherwise wild-type embryos. Even a wild-type Tcf transgene can increase denticle specification when overexpressed in sensitized embryos with low levels of wg expression (Cavallo et al. 1998). In both cases, the effect was weakened when the transgene was expressed in embryos from mothers (but not fathers) that were heterozygous for groucho mutations. This confirmed the identity of Groucho as the transcriptional corepressor that mediates Tcf’s repressive mode in vivo (Figure 5A), and demonstrated that it is maternally provided.
Properties of the Wg signal
Lipid modification and glycosylation of Wg:
The isolation and characterization of porc mutations marked the beginning of our understanding of Wg protein processing. porc mutant embryos show retention of Wg protein within the wg-expressing cells, very similar to what is observed in embryos mutant for some missense alleles of wg, such as the wgts allele at restrictive temperature (van den Heuvel et al. 1993). The porc gene encodes a multiple transmembrane-spanning protein resident in the endoplasmic reticulum (Kadowaki et al. 1996), with homology to O-acyltransferases (Hofmann 2000). Heroic efforts in the Nusse laboratory pioneered the purification of active Wg (van Leeuwen et al. 1994) and vertebrate Wnt molecules (Willert et al. 2003), and demonstrated the presence of a fatty acyl group covalently attached to the protein. The mouse homolog of porc was associated with this lipid modification of vertebrate Wnts, where a monounsaturated palmitoleic acid is attached to a conserved serine residue (Takada et al. 2006). Overexpression of porc also increased the N-linked glycosylation of Wg protein passing through the secretory pathway (Tanaka et al. 2002), suggesting that lipid modification is a required precursor for subsequent modifications. However, mutation of both reported asparagines eliminated glycosylation of Wg without affecting its secretion or signaling activity in cultured cells or in transgenic flies (Herr and Basler 2012; Tang et al. 2012). By contrast, mutating the lipidation site on Wg, Ser239, blocked secretion and signaling activity (Franch-Marro et al. 2008; Herr and Basler 2012; Tang et al. 2012).
The discovery of lipid attachment explained why the Wnts had been so difficult to characterize biochemically, but was puzzling in light of observations that Wg protein was detected and could act over many cell diameters both in embryos (van den Heuvel et al. 1989; Bejsovec and Wieschaus 1995; Lawrence et al. 1996; Sanson et al. 1999) and in imaginal discs (Struhl and Basler 1993; Zecca et al. 1996; Neumann and Cohen 1997; Strigini and Cohen 2000). One possible explanation is that lipid-anchored Wg molecules are sorted into lipid rafts in the membrane (Zhai et al. 2004), where they can then associate with extracellular lipoprotein particles (Panakova et al. 2005). Indeed, the Eaton laboratory showed that RNAi knockdown of the fly apo-lipophorin, which organizes lipoprotein particles, reduces the range of Wg protein movement and signaling activity within the wing imaginal disc (Panakova et al. 2005). A second possible explanation is that Wntless might be involved not only in chaperoning Wg through the secretory pathway, but also in packaging Wg into extracellular vesicles called exosomes. The Vincent laboratory showed that Wg and Wntless can be recovered together, along with homologs of mammalian exosome proteins, from the culture medium of Wg-expressing Drosophila S2 cells (Beckett et al. 2013). However, their RNAi perturbation of exosome production did not disrupt Wg secretion and gradient formation in imaginal discs, leading them to question whether exosomes are relevant to Wg signaling in the wing disc. A third possibility is that there are dedicated chaperone molecules that bind Wg, shielding the lipid and allowing the complex to be soluble. The Nusse laboratory realized that they were losing activity of Wg during its purification from the culture medium of Wg-expressing S2 cells. They deduced that some component in the conditioned medium was essential for Wg activity, and used mass spectrometry to identify a fly lipocalin homolog that they call Swim, for Secreted Wg-interacting molecule (Mulligan et al. 2012). RNAi knockdown for Swim restricted the movement of Wg and signaling activity in the wing imaginal disc in a manner similar to what the Eaton laboratory observed for lipophorin knockdown. Thus, it is possible that any or all three of these processes may function in delivering Wg to more distant cell populations [reviewed in Langton et al. (2016) and McGough and Vincent (2016)].
Interaction with receptors and proteoglycans:
The lipid attached to Wg may also be shielded by interaction with Frizzled receptors. To solve the structure of a Wnt molecule, it was coexpressed and cocrystallized with the extracellular CRD of a Fz molecule. The resulting structure showed that the palmitoleic acid was inserted into a hydrophobic groove in the CRD, rendering the complex soluble for crystallization (Janda et al. 2012). However, as discussed earlier (in fz and fz2 transgenic flies clarified distinctions between polarity and Wg signaling), the Fz CRD was shown to be dispensable for Wnt signal transduction (Chen et al. 2004; Povelones et al. 2005), therefore the biological relevance of this particular interaction is not clear. Replacing the CRD with a Wg molecule fused to the N-terminus of Fz results in a constitutively active molecule (Povelones and Nusse 2005), suggesting that the CRD may help to concentrate Wg at the cell surface.
A similar role for sulfated glycosaminoglycans may explain why the sugarless and sulfateless genes were recovered through their wg-like mutant effects in germ line clones (Häcker et al. 1997; Lin and Perrimon 1999). Overexpressing wg can rescue these effects (Häcker et al. 1997), suggesting that proteoglycans might act to increase the local concentration of Wg and increase signaling efficiency. Dally, the fly glypican, was examined as a candidate for the proteoglycan core protein modified by these enzymes because mutant alleles had been associated with adult wing margin notching, a wg-like phenotype (Nakato et al. 1995). Indeed, dally homozygous mutants showed partial loss of naked cuticle (Tsuda et al. 1999) and strong wg-like cuticle patterns when derived from germ line clones (Lin and Perrimon 1999). Mutations in dally also suppressed the formation of ectopic wing margin bristles caused by overexpressing fz2 in the wing imaginal disc, indicating that Dally function is required for optimal Fz2 activity (Lin and Perrimon 1999). A second proteoglycan, Dally-like (Dlp), was also found to have effects on modulating Wg signaling in embryonic and imaginal disc patterning (Baeg et al. 2001). Heparan-sulfated Dlp restricted Wg protein distribution in the wing imaginal disc (Baeg et al. 2004; Kirkpatrick et al. 2004), suggesting that it can bind to extracellular Wg and modulate its interaction with Fz receptors. The dlp mutants showed genetic interactions with Notum mutations; in particular, dlp mutations suppressed the effects of overexpressing Notum (Kirkpatrick et al. 2004). Thus, Dlp can influence Notum enzymatic activity, perhaps because Notum’s interaction with glycosaminoglycans may colocalize it with Wg at the cell surface and facilitate its cleavage of the Wg lipid group (Kakugawa et al. 2015).
Interactions with both the Fz receptors and cell surface proteoglycans help to shape the graded distribution of Wg protein across the epithelium, as it moves away from the Wg-producing cells (Franch-Marro et al. 2005; Piddini et al. 2005; Hufnagel et al. 2006). In the embryo, endocytosis also plays a role in shaping the Wg distribution (Dubois et al. 2001). Blocking endocytosis, by disrupting dynamin function in a stripe of cells within each embryonic segment, causes Wg-dependent pattern defects on the far side of the disrupted domain (Moline et al. 1999). The “shadow” cast by a spatially restricted endocytosis block suggests that, in the embryo, movement of Wg protein requires internalization and transit through cells. In the wing imaginal disc, blocking endocytosis does not disrupt the extracellular distribution of Wg protein (Strigini and Cohen 2000), but instead disrupts the apical-to-basal movement of Wg, which is required for its signaling activity (Yamazaki et al. 2016). The Vincent laboratory has used new gene-editing techniques to address the functional significance of the Wg gradient, engineering the loci of several key Wg pathway components with clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 to allow replacement of the wild-type gene with tagged and/or mutated forms (Baena-Lopez et al. 2013). A membrane-tethered form of Wg containing the Neurotactin transmembrane domain (Zecca et al. 1996), in addition to the normal lipidation site, was inserted into the endogenous wg locus; the resulting molecule was able to support normal cell proliferation and patterning of the wing (Alexandre et al. 2014). Studies of Wg/Wnt trafficking are complicated by high accumulation of Wg in the Wg-producing cells when export or movement is impaired; this intense signal can obscure detection of low levels of Wg elsewhere. For example, embryos that are heterozygous for the wgts allele at restrictive temperature appear to have no detectable Wg outside of the Wg-expressing cells, even though they produce half a dose of wild-type Wg, and show completely normal patterning and viability (van den Heuvel et al. 1993). It is also difficult to spatially restrict cell surface molecules with confidence. Bride-of-sevenless, the ligand for the Sevenless receptor, is internalized in its entirety into the adjacent responding cell, in spite of being anchored with seven transmembrane domains (Kramer et al. 1991; Cagan et al. 1992). Further work, particularly with the valuable new reagents generated by the Vincent laboratory, will build a clearer understanding of the processes that move Wg from cell to cell, and of possible roles for Wg as a graded morphogen.
Genetically separable domains within the Wg protein:
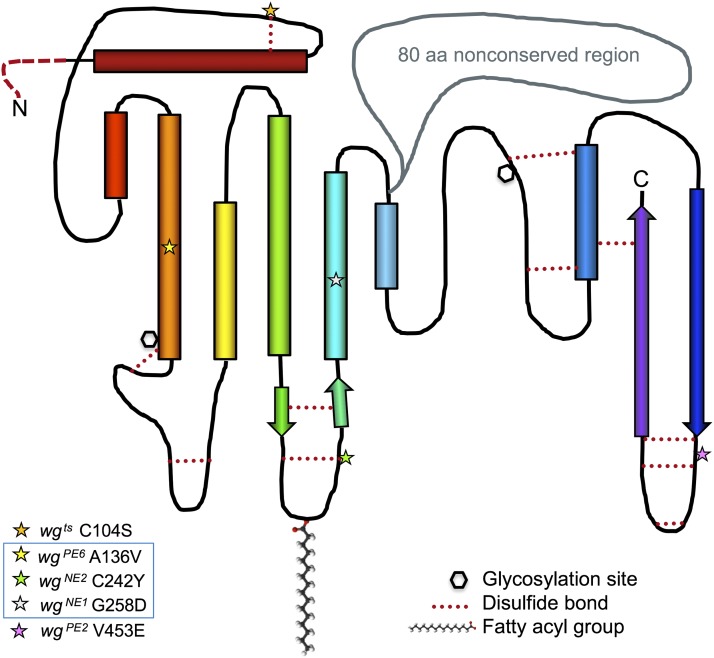
Wg differs from vertebrate Wnts in having a hydrophilic 80-amino acid region that is not conserved (Rijsewijk et al. 1987; Willert and Nusse 2012). This appears to have been the most immunogenic epitope when antibodies were raised against Wg, as an in-frame deletion of this region yields a functional molecule that does not cross-react with polyclonal Wg antibodies (Hays et al. 1997). This nonconserved region divides the molecule into two regions (Figure 6), each predicted to have internal disulfide bond pairs—five in the N-terminal half and six in the C-terminal half—suggesting that the regions fold into separate domains. The Nusse laboratory showed that the halves could be separately produced and secreted in Drosophila S2 cells, but could transduce signal only when they were expressed together (Wu and Nusse 2002). Many new mutant alleles of wg have been generated, facilitated by the wg1 allele, which is still viable in trans with lethal mutations, but with a strong, fully penetrant wingless phenotype. A mutation that truncates wg at the end of the nonconserved region produces mutant protein that showed normal distribution and endocytosis into neighboring cells, but had no detectable signaling activity (Bejsovec and Wieschaus 1995). Missense mutations that alter highly conserved amino acids in the N-terminal half (marked by a blue box in Figure 6) produced the opposite effect: the mutant proteins could signal locally, stabilizing en expression in the neighboring cells, but showed a restricted distribution in the embryo (Dierick and Bejsovec 1998). These observations suggest that the cell-to-cell movement of Wg protein is independent of its ability to signal, and confirm that the C-terminal half is essential for signaling. Indeed, a missense mutation in the C-terminal domain was found to reduce the efficiency of Wg signaling, but was rescued to wild-type activity by overexpressing fz2 or by reducing dally (Moline et al. 2000), suggesting that this portion of Wg interacts with the receptor complex.
Figure 6.
Diagram of putative Wg protein structure. The structure of Xenopus Wnt8 was solved by cocrystallizing it with the mouse Fz8 CRD, allowing determination of the positions for glycosylations, conserved cysteines involved in disulfide bonds, and the fatty acyl attachment (Janda et al. 2012). Cylinders depict α-helical regions and block arrows depict β-sheets predicted in the crystal structure. Approximate positions of wg mutations are indicated by stars (those implicated in Wg protein transport are within the blue box), and the position of the nonconserved insert region of Wg is indicated in gray (van den Heuvel et al. 1993; Bejsovec and Wieschaus 1995; Dierick and Bejsovec 1998).
Consequences of response to Wg
Cell fate specification in different tissues:
Although much of the pathway was identified through the effects on embryonic and imaginal disc patterning, many tissues throughout the fly body require Wg signaling. The nervous system requires multiple inputs from Wg signaling throughout development, starting with neurogenesis in the early embryo, where wg mutations cause loss or duplication of specific neuroblasts (Patel et al. 1989; Chu-LaGraff and Doe 1993). To study wg function in the nervous system at later stages requires either mosaic analysis or use of the wgts mutation, which can be cultured at low temperatures through the early stages of development. The latter was used to show that Wg signaling across the larval neuromuscular junction is required for proper synapse formation (Packard et al. 2002), in a process that requires Wntless for the packaging and release of Wg in exosomes (Korkut et al. 2009; Koles et al. 2012). Wg signaling in the eye-antennal disc initially blocks neuronal fate to specify the portion of the disc that will form the head capsule [reviewed in Legent and Treisman (2008)], and then later specifies neural cell fates along the edge of the retina (Tomlinson 2003). Wg signaling even persists in the adult fly brain, where it has been associated with long-term memory formation (Tan et al. 2013).
In the embryo, Wg secreted by the ectoderm signals to the developing mesoderm, establishing fates in the somatic musculature (Baylies et al. 1995) and in the heart precursor cells (Lawrence et al. 1995). Embryonic Wg signaling has also been associated with the formation of a number of tubular structures: the foregut (Pankratz and Hoch 1995), midgut [reviewed in Bienz (1994)], hindgut (San Martin and Bate 2001), Malpighian tubules (Skaer and Martinez Arias 1992), trachae (Llimargas 2000), and salivary glands (Bradley and Andrew 2001). The requirement for Wg signaling in these disparate tissues hints at a very diverse array of potential target genes that might respond to Wg activation.
Interaction with other signaling pathways during development:
Often, Wg-mediated cell fate specification events involve the modulation of other signaling pathway activities, particularly the Decapentaplegic (Dpp), Epidermal growth factor receptor (EGFR), Hedgehog (Hh), and Notch pathways. For example, Wg and Hh signaling from adjacent rows of cells in the embryonic epidermis mutually reinforces their striped expression patterns, as hh gene expression is turned on by the En transcription factor, which requires Wg signaling for its stable expression (Bejsovec and Martinez Arias 1991; Heemskerk et al. 1991; Bejsovec and Wieschaus 1993; Heemskerk and DiNardo 1994). A similar positive feedback loop between Wg and Dpp signaling, where each pathway regulates expression of the other signal, appears to function in embryonic midgut development (Yu et al. 1996). Both Wg and Dpp in turn activate the EGFR pathway in the midgut, with Dpp and EGFR signaling also showing an interdependence (Szuts et al. 1998). The EGFR pathway plays a role in shaping the segmental pattern in the embryonic epidermis as well, acting in opposition to Wg signaling to define the denticle-secreting field of cells (Szuts et al. 1997; Urban et al. 2004; Walters et al. 2005). The interplay of EGFR and Wg in the embryo requires input from the Hh and Notch signaling pathways, both of which activate the production of EGF ligand, whereas Wg signaling represses it (O’Keefe et al. 1997; Alexandre et al. 1999).
A combination of Wg, Dpp, and Hh signaling generates the overall pattern of wing and leg imaginal discs (Blair 2007; Estella et al. 2012). Spatially restricted expression of each signal organizes the anterior–posterior, dorsal–ventral, and proximal–distal axes for proper morphogenesis of the appendage (Basler and Struhl 1994; Zecca et al. 1995; Jiang and Struhl 1996; Penton and Hoffmann 1996). The Wg and Notch pathways also interact in wing patterning. Notch signaling is required for the expression of wg along the presumptive wing margin in the imaginal disc (Rulifson and Blair 1995), and both pathways are required for proper patterning of sensory neurons in the wing (Couso et al. 1994; Bray 1997). Indeed, the Wg and Notch pathway share several downstream components, such as Zw3/Shaggy and Groucho, leading to strong genetic interaction among mutations in the two pathways (Couso and Martinez Arias 1994).
The search for Wg target genes and their functions:
The complex interaction of signaling pathways, and their various effects on promoting specific cell fates, begs the question of how they do what they do. Presumably the target genes activated in response to each signal include, or control expression of, structural gene products that are physically responsible for morphogenesis of the tissue. A few Wg target genes were identified early on: engrailed in the epidermis (DiNardo et al. 1988; Martinez Arias et al. 1988), and Ultrabithorax and labial in the midgut (Immergluck et al. 1990; Thuringer and Bienz 1993; Thuringer et al. 1993), all of which encode homeobox-containing transcription factors. Some of the targets required for particular cell fate specifications in other tissues have been identified as well. For example, the sloppy-paired and even-skipped pair-rule genes are induced by Wg in heart progenitor cells, and were shown to have functional Tcf-binding sites in their regulatory regions (Lee and Frasch 2000; Knirr and Frasch 2001).
Two of the segment polarity genes associated with the all-naked cuticle phenotype, naked (nkd) (Zeng et al. 2000) and shaven-baby (svb) (Payre et al. 1999), were also shown to be targets of Wg signaling but with opposite control. Expression of nkd is induced by Wg, whereas svb is repressed by it. Notum is another example of a Wg-activated gene that, like nkd, is part of a negative feedback loop (Gerlitz and Basler 2002). The Cadigan laboratory has characterized Tcf-binding sites in the cis regulatory modules at a number of Wg target loci, particularly Notum and nkd. They have demonstrated direct binding of the transcription complex at two distinct consensus sequences: the HMG-box-binding motif (SCTTTGWWSWW; S = G/C, W = A/T) and a second “helper” motif (GCCGCCR; R = A/G) that is bound by a separate DNA-binding domain in Tcf, called the C-clamp (Chang et al. 2008; Parker et al. 2008; Archbold et al. 2014).
A number of examples of Wg-mediated repression beyond svb have been characterized: in the developing wing imaginal disc, Wg represses expression of its own Fz2 receptor, and this helps to shape the graded distribution of Wg protein within the disc (Cadigan et al. 1998); in the ventral embryonic epidermis, Wg represses the expression of SoxN (Overton et al. 2007), a gene that imposes negative control on the transcriptional response to Wg signaling (Chao et al. 2007). The Wg-mediated repression of SoxN and svb in the late embryonic epidermis is critical for the segmental pattern of denticle belts. Both genes encode transcription factors that control the expression of many structural genes required for denticle construction (Chanut-Delalande et al. 2006; Fernandes et al. 2010; Rizzo and Bejsovec 2017).
Wg-mediated repression of these genes may be indirect, through the induction of targets that encode transcriptional repressors, but it may be direct. The Cadigan laboratory had conducted a genome-wide microarray screen for transcriptional targets of Wg in the Drosophila Kc167 cell line, and characterized a number of genes that were either activated or repressed by Wg signaling. They discovered a cis regulatory sequence in Ugt36Bc, Ugt58Fa, Peroxidasin (Pxn), and Tiggrin, which is associated with direct repression of these genes by Tcf-Arm (Blauwkamp et al. 2008; Parker et al. 2008; Zhang et al. 2014). This sequence (AGAWAW, W = A/T) is distinct from sequences found in promoters of genes activated by Tcf-Arm, implying that interaction with this alternative sequence correlates with altered function of the Tcf-Arm complex. The Basler laboratory identified some of these Wg targets as well, in an RNA-sequencing-based screen in Kc cells (Franz et al. 2017). Cells exposed to Wg-conditioned medium upregulated 40 genes at least twofold, and downregulated 11 genes. These transcriptional changes were eliminated in Kc cells lines with CRISPR-generated null alleles for arm or Tcf/pan, clearly demonstrating that both activation and repression depend on Arm and Tcf function (Franz et al. 2017).
Conclusions
Current questions and controversies
Role of Dishevelled in linking receptor activation to Arm stabilization:
Dsh remains the most upstream cytosolic component of the pathway known, but even after years of study in the fly and in vertebrate systems, it is still not clear exactly what it does. Dsh can bind to the cytosolic domain of Fz, and Axin can interact with the cytosolic portion of Arrow. Thus, it seems likely that Wg-mediated clustering of Fz and Arrow brings together Dsh and Axin, in a way that antagonizes destruction complex activity and allows accumulation of Arm inside the cell. The Bienz laboratory, who showed that Dsh can recruit Axin to the membrane (Cliffe et al. 2003), postulate a model where Dsh and Axin polymerize in response to Wg signaling (Schwarz-Romond et al. 2007a,b). Mutating the Dsh interaction (DIX) domain of Axin prevents it from rescuing the axin mutant phenotype in embryos, supporting the idea that sequestering Axin in these polymers blocks its destruction complex activity (Fiedler et al. 2011). Others have proposed a variety of possible roles for Dsh: (1) direct binding between Dsh and Axin inhibits destruction complex activity (Logan and Nusse 2004; Malbon and Wang 2006), (2) recruitment of Axin to the membrane leads to its degradation and thereby inactivates the destruction complex (Mao et al. 2001; Tolwinski et al. 2003), or (3) modifications of the Arrow/LRP cytosolic domain directly inactivate GSK and block Arm/β-catenin phosphorylation (Piao et al. 2008; Wu et al. 2009). However, data from a vertebrate system suggest that the destruction complex remains intact during response to Wnt signaling (Hernandez et al. 2012; Li et al. 2012). These experiments led the Kirschner laboratory to propose a model (Hernandez et al. 2012) where Wnt signaling diminishes, but does not eliminate phosphorylation of β-catenin by the destruction complex, and that a reduced degradation rate is sufficient to explain the β-catenin accumulation observed in cells responding to Wnt. The Clevers laboratory proposes an alternative model (Li et al. 2012), where the destruction complex becomes saturated with phosphorylated β-catenin due to Wnt-mediated blockage of the ubiquitination step. This then allows newly synthesized β-catenin to accumulate, free of destruction complex activity, and drive target gene expression. However, neither of these models explains how Dsh controls this process.
Reorganization of the destruction complex as a means of controlling Arm stability:
Axin is the primary scaffolding molecule in the destruction complex, with binding sites for Arm, CK1, GSK, protein phosphatase 2A, and Apc (Ha et al. 2004). Although mutations in Apc are strongly correlated with colon cancer initiation in the general population (Kandoth et al. 2013), the role of Apc in the destruction complex has been unclear. The Bienz laboratory found that Apc competes with Dsh for Axin binding, and interferes with a default tendency for Axin polymerization at the plasma membrane (Mendoza-Topaz et al. 2011). They made point mutations in the Apc-binding region of Axin and found that these fail to rescue axin mutant phenotypes, and cause Axin to move to the plasma membrane in a Dsh-dependent fashion. The Ahmed laboratory also found that plasma membrane association of Axin in puncta was relevant to its destruction complex role (Wang et al. 2016a). The Peifer laboratory examined the interaction of Apc2 with Axin by expressing the fly molecules in SW80 cells, a human colorectal cancer cell line that lacks Apc function (Pronobis et al. 2015). They discovered that Axin forms large, complex macromolecular structures in the presence of Apc2 through two different contact points between the molecules. Both interactions are required for proper β-catenin degradation, and one is regulated by GSK phosphorylation of Apc2. They propose a model where Apc2 cross-links the Axin scaffold and forms pockets where Arm/β-catenin is presented to the kinases. Apc2 then undergoes phosphorylation itself, causing it to swing one end away from Axin and present Arm/β-catenin to the E3-ligase for ubiquitination. This model is consistent with both the Kirschner and Clevers models for Wnt regulation of the destruction complex.
Modifications of Arm downstream of stabilization:
In the simplest view of Wg pathway activation, Arm accumulation drives its interaction with the Tcf transcription factor, recruiting Legless and Pygopus to form a transcriptional activation complex. However, the high flux of Arm may be in excess of what is needed to transduce a signal. We have found that embryos mutant for weak wg alleles show substantial cuticle patterning and expression of target genes, but produce no change in Arm levels from the uniform low level typical of wg null mutants (Moline et al. 2000). This raises the possibility that only a subset of the stabilized Arm molecules observed in wild-type are required for signal transduction. The essential role of Arm/β-catenin in cell adhesion also creates a tension with Wg/Wnt signaling, where increased cadherin can pull Arm/β-catenin away from the signaling pool and diminish pathway activity (Hinck et al. 1994; Sanson et al. 1996; Gottardi and Gumbiner 2004; Wodarz et al. 2006). Thus, part of the response to Wg/Wnt signals may involve shifting the balance between these competing functions of Arm/β-catenin.
The central Arm repeat region of β-catenin provides interaction domains for a variety of proteins: α-catenin and E-cadherin for its adhesive role, and Axin, Apc, Lgs, and Tcf for its signaling role. A number of phosphorylation sites in vertebrate β-catenin have been identified that correlate with enhanced signaling activity rather than degradation [reviewed in Valenta et al. (2012)]. These phosphorylations appear to decrease affinity for adhesive binding partners and increase the recruitment of signaling partners; for example, phosphorylation of a tyrosine in the first Arm repeat decreases β-catenin binding to α-catenin and favors binding to BCL9 (Brembeck et al. 2004). However, the corresponding Tyr in Drosophila Arm does not have a similar function (Hoffmans and Basler 2007). Other phosphorylation sites might play as yet uncharacterized roles in activating Arm or changing its subcellular location. In particular, we do not know whether Arm translocation to the nucleus is regulated. Arm lacks classical nuclear import and export signals, but a small portion of the Arm repeat region is sufficient for nuclear localization; the conventional nuclear import chaperone, Importin-β, possesses similar Arm repeats, and both molecules interact with the same nuclear pore complex protein as they move through the pore (Sharma et al. 2012). It is easy to imagine that phosphorylation, or other modification, of Arm might increase or decrease its transit into the nucleus and have profound effects on the activation of target genes.
Future directions
The power of the fly system for gene discovery and functional analysis will only increase as technology evolves. Basic forward genetic screens are still important as an unbiased means to identify unexpected players in the Wg pathway, and the rich array of community resources—FlyBase, the Bloomington Drosophila Stock Center, the Gene Disruption Project, the Transgenic RNAi Project, the Vienna Drosophila Resource Center, and the Drosophila Genomic Resources Center, for example—has dramatically shortened the time it takes to go from a new mutation to a fully characterized gene function. Big questions still remain about how the Wg signal is transduced from the cell surface to the nucleus, and it may be because we are still missing some key components. Finding these is crucial to our understanding of how cell fates are specified during development, and how cell function goes awry in the many human diseases associated with deranged Wnt signaling [reviewed in Nusse and Clevers (2017)]. The essential role of Arm/β-catenin in both cell adhesion and Wg/Wnt signaling makes it particularly tricky to find suitable therapies for these disorders. For example, tumors that are caused by Wnt pathway hyperactivation could metastasize if β-catenin were targeted directly, as this would lead to loss of adherens junctions and epithelial-to-mesenchymal transition [reviewed in Heuberger and Birchmeier (2010)]. Thus, there is great interest in learning how the Wnt pathway might be downregulated in ways that do not affect cell–cell adhesion. Perhaps the best candidate for intervention is Axin, which is degraded in response to tankyrase-mediated ADP-ribosylation in flies (Wang et al. 2016b) and in human cells (Huang et al. 2009; Zhang et al. 2011). Small-molecule inhibitors of tankyrase have been shown to stabilize Axin (Chen et al. 2009; Huang et al. 2009), and to block the proliferation of colon cancer cell lines (Kulak et al. 2015) and of Apc mutant tumors in mice (Waaler et al. 2012; Lau et al. 2013). More potential targets of intervention may present themselves as we discover new components and understand the Wnt pathway in ever greater detail.
Acknowledgments
My sincere apologies to all the Wg/Wnt researchers whose work I did not have the opportunity to mention. I thank the National Institutes of Health and the National Science Foundation for supporting my work.
Footnotes
Communicating editor: J. Treisman
Literature Cited
- Adler P. N., 1992. The genetic control of tissue polarity in Drosophila. Bioessays 14: 735–741. [DOI] [PubMed] [Google Scholar]
- Ahmed Y., Hayashi S., Levine A., Wieschaus E., 1998. Regulation of Armadillo by a Drosophila APC inhibits neuronal apoptosis during retinal development. Cell 93: 1171–1182. [DOI] [PubMed] [Google Scholar]
- Ahmed Y., Nouri A., Wieschaus E., 2002. Drosophila Apc1 and Apc2 regulate Wingless transduction throughout development. Development 129: 1751–1762. [DOI] [PubMed] [Google Scholar]
- Akong K., Grevengoed E. E., Price M. H., McCartney B. M., Hayden M. A., et al. , 2002. Drosophila APC2 and APC1 play overlapping roles in Wingless signaling in the embryo and imaginal discs. Dev. Biol. 250: 91–100. [DOI] [PubMed] [Google Scholar]
- Alexandre C., Lecourtois M., Vincent J., 1999. Wingless and Hedgehog pattern Drosophila denticle belts by regulating the production of short-range signals. Development 126: 5689–5698. [DOI] [PubMed] [Google Scholar]
- Alexandre C., Baena-Lopez A., Vincent J. P., 2014. Patterning and growth control by membrane-tethered Wingless. Nature 505: 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit S., Hatzubai A., Birman Y., Andersen J. S., Ben-Shushan E., et al. , 2002. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 16: 1066–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archbold H. C., Broussard C., Chang M. V., Cadigan K. M., 2014. Bipartite recognition of DNA by TCF/Pangolin is remarkably flexible and contributes to transcriptional responsiveness and tissue specificity of Wingless signaling. PLoS Genet. 10: e1004591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M., 1989. Drosophila: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- Axelrod J. D., 2001. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 15: 1182–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod J. D., 2009. Progress and challenges in understanding planar cell polarity signaling. Semin. Cell Dev. Biol. 20: 964–971. [DOI] [PubMed] [Google Scholar]
- Axelrod J. D., Miller J. R., Shulman J. M., Moon R. T., Perrimon N., 1998. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 12: 2610–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg G. H., Lin X., Khare N., Baumgartner S., Perrimon N., 2001. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development 128: 87–94. [DOI] [PubMed] [Google Scholar]
- Baeg G. H., Selva E. M., Goodman R. M., Dasgupta R., Perrimon N., 2004. The Wingless morphogen gradient is established by the cooperative action of Frizzled and heparan sulfate proteoglycan receptors. Dev. Biol. 276: 89–100. [DOI] [PubMed] [Google Scholar]
- Baena-Lopez L. A., Alexandre C., Mitchell A., Pasakarnis L., Vincent J. P., 2013. Accelerated homologous recombination and subsequent genome modification in Drosophila. Development 140: 4818–4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N. E., 1987. Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. EMBO J. 6: 1765–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N. E., 1988a Embryonic and imaginal requirements for wingless, a segment-polarity gene in Drosophila. Dev. Biol. 125: 96–108. [DOI] [PubMed] [Google Scholar]
- Baker N. E., 1988b Transcription of the segment-polarity gene wingless in the imaginal discs of Drosophila, and the phenotype of a pupal-lethal wg mutation. Development 102: 489–497. [DOI] [PubMed] [Google Scholar]
- Banziger C., Soldini D., Schutt C., Zipperlen P., Hausmann G., et al. , 2006. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 125: 509–522. [DOI] [PubMed] [Google Scholar]
- Bartscherer K., Pelte N., Ingelfinger D., Boutros M., 2006. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell 125: 523–533. [DOI] [PubMed] [Google Scholar]
- Basler K., Struhl G., 1994. Compartment boundaries and the control of Drosophila limb pattern by Hedgehog protein. Nature 368: 208–214. [DOI] [PubMed] [Google Scholar]
- Baylies M. K., Martinez Arias A., Bate M., 1995. wingless is required for the formation of a subset of muscle founder cells during Drosophila embryogenesis. Development 121: 3829–3837. [DOI] [PubMed] [Google Scholar]
- Beckett K., Monier S., Palmer L., Alexandre C., Green H., et al. , 2013. Drosophila S2 cells secrete Wingless on exosome-like vesicles but the Wingless gradient forms independently of exosomes. Traffic 14: 82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J., von Kries J. P., Kuhl M., Bruhn L., Wedlich D., et al. , 1996. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 382: 638–642. [DOI] [PubMed] [Google Scholar]
- Bejsovec A., Martinez Arias A., 1991. Roles of wingless in patterning the larval epidermis of Drosophila. Development 113: 471–485. [DOI] [PubMed] [Google Scholar]
- Bejsovec A., Wieschaus E., 1993. Segment polarity gene interactions modulate epidermal patterning in Drosophila embryos. Development 119: 501–517. [DOI] [PubMed] [Google Scholar]
- Bejsovec A., Wieschaus E., 1995. Signaling activities of the Drosophila wingless gene are separately mutable and appear to be transduced at the cell surface. Genetics 139: 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen H. J., Levis R. W., He Y., Carlson J. W., Evans-Holm M., et al. , 2011. The Drosophila gene disruption project: progress using transposons with distinctive site specificities. Genetics 188: 731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanot P., Brink M., Samos C. H., Hsieh J. C., Wang Y., et al. , 1996. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 382: 225–230. [DOI] [PubMed] [Google Scholar]
- Bhanot P., Fish M., Jemison J. A., Nusse R., Nathans J., et al. , 1999. Frizzled and Dfrizzled-2 function as redundant receptors for Wingless during Drosophila embryonic development. Development 126: 4175–4186. [DOI] [PubMed] [Google Scholar]
- Bienz M., 1994. Homeotic genes and positional signalling in the Drosophila viscera. Trends Genet. 10: 22–26. [DOI] [PubMed] [Google Scholar]
- Blair S. S., 2007. Wing vein patterning in Drosophila and the analysis of intercellular signaling. Annu. Rev. Cell Dev. Biol. 23: 293–319. [DOI] [PubMed] [Google Scholar]
- Blauwkamp T. A., Chang M. V., Cadigan K. M., 2008. Novel TCF-binding sites specify transcriptional repression by Wnt signalling. EMBO J. 27: 1436–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros M., Mihaly J., Bouwmeester T., Mlodzik M., 2000. Signaling specificity by Frizzled receptors in Drosophila. Science 288: 1825–1828. [DOI] [PubMed] [Google Scholar]
- Boutros M., Kiger A. A., Armknecht S., Kerr K., Hild M., et al. , 2004. Genome-wide RNAi analysis of growth and viability in Drosophila cells. Science 303: 832–835. [DOI] [PubMed] [Google Scholar]
- Bradley P. L., Andrew D. J., 2001. ribbon encodes a novel BTB/POZ protein required for directed cell migration in Drosophila melanogaster. Development 128: 3001–3015. [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N., 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Bray S. J., 1997. Expression and function of enhancer of split bHLH proteins during Drosophila neurogenesis. Perspect. Dev. Neurobiol. 4: 313–323. [PubMed] [Google Scholar]
- Brembeck F. H., Schwarz-Romond T., Bakkers J., Wilhelm S., Hammerschmidt M., et al. , 2004. Essential role of BCL9–2 in the switch between beta-catenin’s adhesive and transcriptional functions. Genes Dev. 18: 2225–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges C. B., Brehme K. S., 1944. The Mutants of Drosophila melanogaster. Carnegie Institution of Washington, Washington, DC [Google Scholar]
- Brown A. M., Papkoff J., Fung Y. K., Shackleford G. M., Varmus H. E., 1987. Identification of protein products encoded by the proto-oncogene int-1. Mol. Cell. Biol. 7: 3971–3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner E., Peter O., Schweizer L., Basler K., 1997. pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature 385: 829–833. [DOI] [PubMed] [Google Scholar]
- Brunner E., Brunner D., Fu W., Hafen E., Basler K., 1999. The dominant mutation Glazed is a gain-of-function allele of wingless that, similar to loss of APC, interferes with normal eye development. Dev. Biol. 206: 178–188. [DOI] [PubMed] [Google Scholar]
- Cadigan K. M., Nusse R., 1996. wingless signaling in the Drosophila eye and embryonic epidermis. Development 122: 2801–2812. [DOI] [PubMed] [Google Scholar]
- Cadigan K. M., Fish M. P., Rulifson E. J., Nusse R., 1998. Wingless repression of Drosophila frizzled 2 expression shapes the Wingless morphogen gradient in the wing. Cell 93: 767–777. [DOI] [PubMed] [Google Scholar]
- Cagan R. L., Kramer H., Hart A. C., Zipursky S. L., 1992. The bride of sevenless and sevenless interaction: internalization of a transmembrane ligand. Cell 69: 393–399. [DOI] [PubMed] [Google Scholar]
- Cavallo R. A., Cox R. T., Moline M. M., Roose J., Polevoy G. A., et al. , 1998. Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature 395: 604–608. [DOI] [PubMed] [Google Scholar]
- Chan C. C., Zhang S., Rousset R., Wharton K. A., Jr., 2008. Drosophila naked cuticle (Nkd) engages the nuclear import adaptor Importin-alpha3 to antagonize Wnt/beta-catenin signaling. Dev. Biol. 318: 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M. V., Chang J. L., Gangopadhyay A., Shearer A., Cadigan K. M., 2008. Activation of Wingless targets requires bipartite recognition of DNA by TCF. Curr. Biol. 18: 1877–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanut-Delalande H., Fernandes I., Roch F., Payre F., Plaza S., 2006. Shavenbaby couples patterning to epidermal cell shape control. PLoS Biol. 4: e290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A. T., Jones W. M., Bejsovec A., 2007. The HMG-box transcription factor SoxNeuro acts with Tcf to control Wg/Wnt signaling activity. Development 134: 989–997. [DOI] [PubMed] [Google Scholar]
- Chen B., Dodge M. E., Tang W., Lu J., Ma Z., et al. , 2009. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 5: 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. M., Struhl G., 1999. Wingless transduction by the Frizzled and Frizzled2 proteins of Drosophila. Development 126: 5441–5452. [DOI] [PubMed] [Google Scholar]
- Chen C. M., Strapps W., Tomlinson A., Struhl G., 2004. Evidence that the cysteine-rich domain of Drosophila Frizzled family receptors is dispensable for transducing Wingless. Proc. Natl. Acad. Sci. USA 101: 15961–15966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodaparambil J. V., Pate K. T., Hepler M. R., Tsai B. P., Muthurajan U. M., et al. , 2014. Molecular functions of the TLE tetramerization domain in Wnt target gene repression. EMBO J. 33: 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K. W., Benzer S., 1994. Rotation of photoreceptor clusters in the developing Drosophila eye requires the nemo gene. Cell 78: 125–136. [DOI] [PubMed] [Google Scholar]
- Chou T.-B., Perrimon N., 1992. Use of a yeast site-specific recombinase to produce female germline chimeras in Drosophila. Genetics 131: 643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T.-B., Noll E., Perrimon N., 1993. Autosomal P[ovoD1] dominant female-sterile insertions in Drosophila and their use in generating female germ-line chimeras. Development 119: 1359–1369. [DOI] [PubMed] [Google Scholar]
- Chu-LaGraff Q., Doe C. Q., 1993. Neuroblast specification and formation regulated by wingless in the Drosophila CNS. Science 261: 1594–1597. [DOI] [PubMed] [Google Scholar]
- Cliffe A., Hamada F., Bienz M., 2003. A role of Dishevelled in relocating Axin to the plasma membrane during Wingless signaling. Curr. Biol. 13: 960–966. [DOI] [PubMed] [Google Scholar]
- Cong F., Schweizer L., Varmus H., 2004. Wnt signals across the plasma membrane to activate the beta-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development 131: 5103–5115. [DOI] [PubMed] [Google Scholar]
- Cooley L., Verheyen E., Ayers K., 1992. chickadee encodes a profilin required for intracellular cytoplasm transport during Drosophila oogenesis. Cell 69: 173–184. [DOI] [PubMed] [Google Scholar]
- Couso J. P., Martinez Arias A., 1994. Notch is required for wingless signaling in the epidermis of Drosophila. Cell 79: 259–272. [DOI] [PubMed] [Google Scholar]
- Couso J. P., Bishop S. A., Martinez Arias A., 1994. The wingless signalling pathway and the patterning of the wing margin in Drosophila. Development 120: 621–636. [DOI] [PubMed] [Google Scholar]
- Cox R. T., Pai L. M., Kirkpatrick C., Stein J., Peifer M., 1999. Roles of the C terminus of Armadillo in Wingless signaling in Drosophila. Genetics 153: 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly C. S., Shaw P., Ordonez L. D., Williams G. T., Quist J., et al. , 2017. Functional redundancy between Apc and Apc2 regulates tissue homeostasis and prevents tumorigenesis in murine mammary epithelium. Oncogene 36: 1793–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels D. L., Weis W. I., 2005. Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat. Struct. Mol. Biol. 12: 364–371. [DOI] [PubMed] [Google Scholar]
- Dierick H. A., Bejsovec A., 1998. Functional analysis of Wingless reveals a link between intercellular ligand transport and dorsal-cell-specific signaling. Development 125: 4729–4738. [DOI] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K. C., Barinova Y., et al. , 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156. [DOI] [PubMed] [Google Scholar]
- DiNardo S., Kuner J. M., Theis J., O’Farrell P. H., 1985. Development of embryonic pattern in D. melanogaster as revealed by accumulation of the nuclear engrailed protein. Cell 43: 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S., Sher E., Heemskerk-Jongens J., Kassis J. A., O’Farrell P., 1988. Two-tiered regulation of spatially patterned engrailed gene expression during Drosophila embryogenesis. Nature 322: 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois L., Lecourtois M., Alexandre C., Hirst E., Vincent J. P., 2001. Regulated endocytic routing modulates Wingless signaling in Drosophila embryos. Cell 105: 613–624. [DOI] [PubMed] [Google Scholar]
- Estella C., Voutev R., Mann R. S., 2012. A dynamic network of morphogens and transcription factors patterns the fly leg. Curr. Top. Dev. Biol. 98: 173–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M., Li J., Blauwkamp T., Bhambhani C., Campbell N., et al. , 2006. C-terminal-binding protein directly activates and represses Wnt transcriptional targets in Drosophila. EMBO J. 25: 2735–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes I., Chanut-Delalande H., Ferrer P., Latapie Y., Waltzer L., et al. , 2010. Zona pellucida domain proteins remodel the apical compartment for localized cell shape changes. Dev. Cell 18: 64–76. [DOI] [PubMed] [Google Scholar]
- Fiedler M., Mendoza-Topaz C., Rutherford T. J., Mieszczanek J., Bienz M., 2011. Dishevelled interacts with the DIX domain polymerization interface of Axin to interfere with its function in down-regulating beta-catenin. Proc. Natl. Acad. Sci. USA 108: 1937–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franch-Marro X., Marchand O., Piddini E., Ricardo S., Alexandre C., et al. , 2005. Glypicans shunt the Wingless signal between local signalling and further transport. Development 132: 659–666. [DOI] [PubMed] [Google Scholar]
- Franch-Marro X., Wendler F., Griffith J., Maurice M. M., Vincent J. P., 2008. In vivo role of lipid adducts on Wingless. J. Cell Sci. 121: 1587–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz A., Shlyueva D., Brunner E., Stark A., Basler K., 2017. Probing the canonicity of the Wnt/Wingless signaling pathway. PLoS Genet. 13: e1006700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlitz O., Basler K., 2002. Wingful, an extracellular feedback inhibitor of Wingless. Genes Dev. 16: 1055–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlitz O., Nellen D., Ottiger M., Basler K., 2002. A screen for genes expressed in Drosophila imaginal discs. Int. J. Dev. Biol. 46: 173–176. [PubMed] [Google Scholar]
- Giraldez A. J., Copley R. R., Cohen S. M., 2002. HSPG modification by the secreted enzyme Notum shapes the Wingless morphogen gradient. Dev. Cell 2: 667–676. [DOI] [PubMed] [Google Scholar]
- Golic K. G., Lindquist S., 1989. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell 59: 499–509. [DOI] [PubMed] [Google Scholar]
- Goodman R. M., Thombre S., Firtina Z., Gray D., Betts D., et al. , 2006. Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development 133: 4901–4911. [DOI] [PubMed] [Google Scholar]
- Goodrich L. V., Strutt D., 2011. Principles of planar polarity in animal development. Development 138: 1877–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi C. J., Gumbiner B. M., 2004. Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes. J. Cell Biol. 167: 339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubb D., Garcia-Bellido A., 1982. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J. Embryol. Exp. Morphol. 68: 37–57. [PubMed] [Google Scholar]
- Ha N. C., Tonozuka T., Stamos J. L., Choi H. J., Weis W. I., 2004. Mechanism of phosphorylation-dependent binding of APC to beta-catenin and its role in beta-catenin degradation. Mol. Cell 15: 511–521. [DOI] [PubMed] [Google Scholar]
- Häcker U., Lin X., Perrimon N., 1997. The Drosophila sugarless gene modulates Wingless signaling and encodes an enzyme involved in polysaccharide biosynthesis. Development 124: 3565–3573. [DOI] [PubMed] [Google Scholar]
- Hamada F., Bienz M., 2004. The APC tumor suppressor binds to C-terminal binding protein to divert nuclear beta-catenin from TCF. Dev. Cell 7: 677–685. [DOI] [PubMed] [Google Scholar]
- Hamada F., Murata Y., Nishida A., Fujita F., Tomoyasu Y., et al. , 1999a Identification and characterization of E-APC, a novel Drosophila homologue of the tumour suppressor APC. Genes Cells 4: 465–474. [DOI] [PubMed] [Google Scholar]
- Hamada F., Tomoyasu Y., Takatsu Y., Nakamura M., Nagai S., et al. , 1999b Negative regulation of Wingless signaling by D-Axin, a Drosophila homolog of Axin. Science 283: 1739–1742. [DOI] [PubMed] [Google Scholar]
- Hanson A. J., Wallace H. A., Freeman T. J., Beauchamp R. D., Lee L. A., et al. , 2012. XIAP monoubiquitylates Groucho/TLE to promote canonical Wnt signaling. Mol. Cell 45: 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Rubinfeld B., Souza B., Polakis P., Wieschaus E., et al. , 1997. A Drosophila homolog of the tumor suppressor gene adenomatous polyposis coli down-regulates β-catenin but its zygotic expression is not essential for the regulation of Armadillo. Proc. Natl. Acad. Sci. USA 94: 242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays R., Gibori G. B., Bejsovec A., 1997. Wingless signaling generates pattern through two distinct mechanisms. Development 124: 3727–3736. [DOI] [PubMed] [Google Scholar]
- Heemskerk J., DiNardo S., 1994. Drosophila hedgehog acts as a morphogen in cellular patterning. Cell 76: 449–460. [DOI] [PubMed] [Google Scholar]
- Heemskerk J., DiNardo S., Kostriken R., O’Farrell P. H., 1991. Multiple modes of engrailed regulation in the progression towards cell fate determination. Nature 352: 404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held L. I., Jr., Duarte C. M., Derakhshanian K., 1986. Extra tarsal joints and abnormal cuticular polarities in various mutants of Drosophila melanogaster. Rouxs Arch. Dev. Biol. 195: 145–157. [DOI] [PubMed] [Google Scholar]
- Hernandez A. R., Klein A. M., Kirschner M. W., 2012. Kinetic responses of beta-catenin specify the sites of Wnt control. Science 338: 1337–1340. [DOI] [PubMed] [Google Scholar]
- Herr P., Basler K., 2012. Porcupine-mediated lipidation is required for Wnt recognition by Wls. Dev. Biol. 361: 392–402. [DOI] [PubMed] [Google Scholar]
- Heuberger J., Birchmeier W., 2010. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb. Perspect. Biol. 2: a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikasa H., Ezan J., Itoh K., Li X., Klymkowsky M. W., et al. , 2010. Regulation of TCF3 by Wnt-dependent phosphorylation during vertebrate axis specification. Dev. Cell 19: 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck L., Nelson W. J., Papkoff J., 1994. Wnt-1 modulates cell-cell adhesion in mammalian cells by stabilizing beta-catenin binding to the cell adhesion protein cadherin. J. Cell Biol. 124: 729–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmans R., Basler K., 2007. BCL9–2 binds Arm/beta-catenin in a Tyr142-independent manner and requires Pygopus for its function in Wg/Wnt signaling. Mech. Dev. 124: 59–67. [DOI] [PubMed] [Google Scholar]
- Hoffmans R., Stadeli R., Basler K., 2005. Pygopus and Legless provide essential transcriptional coactivator functions to Armadillo/beta-catenin. Curr. Biol. 15: 1207–1211. [DOI] [PubMed] [Google Scholar]
- Hofmann K., 2000. A superfamily of membrane-bound O-acyltransferases with implications for Wnt signaling. Trends Biochem. Sci. 25: 111–112. [DOI] [PubMed] [Google Scholar]
- Hu T., Li C., Cao Z., Van Raay T. J., Smith J. G., et al. , 2010. Myristoylated Naked2 antagonizes Wnt-beta-catenin activity by degrading Dishevelled-1 at the plasma membrane. J. Biol. Chem. 285: 13561–13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. M., Mishina Y. M., Liu S., Cheung A., Stegmeier F., et al. , 2009. Tankyrase inhibition stabilizes Axin and antagonizes Wnt signalling. Nature 461: 614–620. [DOI] [PubMed] [Google Scholar]
- Huber A. H., Nelson W. J., Weis W. I., 1997. Three-dimensional structure of the Armadillo repeat region of beta-catenin. Cell 90: 871–882. [DOI] [PubMed] [Google Scholar]
- Hufnagel L., Kreuger J., Cohen S. M., Shraiman B. I., 2006. On the role of glypicans in the process of morphogen gradient formation. Dev. Biol. 300: 512–522. [DOI] [PubMed] [Google Scholar]
- Immergluck K., Lawrence P. A., Bienz M., 1990. Induction across germ layers in Drosophila mediated by a genetic cascade. Cell 62: 261–268. [DOI] [PubMed] [Google Scholar]
- Janda C. Y., Waghray D., Levin A. M., Thomas C., Garcia K. C., 2012. Structural basis of Wnt recognition by Frizzled. Science 337: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda C. Y., Dang L. T., You C., Chang J., de Lau W., et al. , 2017. Surrogate Wnt agonists that phenocopy canonical Wnt and beta-catenin signalling. Nature 545: 234–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Struhl G., 1996. Complementary and mutually exclusive activities of decapentaplegic and wingless organize axial patterning during Drosophila leg development. Cell 86: 401–409. [DOI] [PubMed] [Google Scholar]
- Jiang J., Struhl G., 1998. Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein slimb. Nature 391: 493–496. [DOI] [PubMed] [Google Scholar]
- Jürgens G., Wieschaus E., Nüsslein-Volhard C., Kluding H., 1984. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster: II. Zygotic loci on the third chromosome. Wihelm Roux Arch. Dev. Biol. 193: 283–295. [DOI] [PubMed] [Google Scholar]
- Jursnich V. A., Fraser S. E., Held L. I., Jr., Ryerse J., Bryant P. J., 1990. Defective gap-junctional communication associated with imaginal disc overgrowth and degeneration caused by mutations of the dco gene in Drosophila. Dev. Biol. 140: 413–429. [DOI] [PubMed] [Google Scholar]
- Kadowaki T., Wilder E., Klingensmith J., Zachary K., Perrimon N., 1996. The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev. 10: 3116–3128. [DOI] [PubMed] [Google Scholar]
- Kakugawa S., Langton P. F., Zebisch M., Howell S., Chang T. H., et al. , 2015. Notum deacylates Wnt proteins to suppress signalling activity. Nature 519: 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandoth C., McLellan M. D., Vandin F., Ye K., Niu B., et al. , 2013. Mutational landscape and significance across 12 major cancer types. Nature 502: 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King N., Westbrook M. J., Young S. L., Kuo A., Abedin M., et al. , 2008. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature 451: 783–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. A., Dimitroff B. D., Rawson J. M., Selleck S. B., 2004. Spatial regulation of Wingless morphogen distribution and signaling by dally-like protein. Dev. Cell 7: 513–523. [DOI] [PubMed] [Google Scholar]
- Klein T. J., Jenny A., Djiane A., Mlodzik M., 2006. CKIE/discs overgrown promotes both Wnt-Fz/β-catenin and Fz/PCP signaling in Drosophila. Curr. Biol. 16: 1337–1343. [DOI] [PubMed] [Google Scholar]
- Klingensmith J., Nusse R., Perrimon N., 1994. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev. 8: 118–130. [DOI] [PubMed] [Google Scholar]
- Kloss B., Price J. L., Saez L., Blau J., Rothenfluh A., et al. , 1998. The Drosophila clock gene double-time encodes a protein closely related to human casein kinase IE. Cell 94: 97–107. [DOI] [PubMed] [Google Scholar]
- Knirr S., Frasch M., 2001. Molecular integration of inductive and mesoderm-intrinsic inputs governs even-skipped enhancer activity in a subset of pericardial and dorsal muscle progenitors. Dev. Biol. 238: 13–26. [DOI] [PubMed] [Google Scholar]
- Koles K., Nunnari J., Korkut C., Barria R., Brewer C., et al. , 2012. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J. Biol. Chem. 287: 16820–16834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkut C., Ataman B., Ramachandran P., Ashley J., Barria R., et al. , 2009. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell 139: 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostriken R., Weisblat D. A., 1992. Expression of a Wnt gene in embryonic epithelium of the leech. Dev. Biol. 151: 225–241. [DOI] [PubMed] [Google Scholar]
- Kramer H., Cagan R. L., Zipursky S. L., 1991. Interaction of bride of sevenless membrane-bound ligand and the sevenless tyrosine-kinase receptor. Nature 352: 207–212. [DOI] [PubMed] [Google Scholar]
- Kramps T., Peter O., Brunner E., Nellen D., Froesch B., et al. , 2002. Wnt/Wingless signaling requires BCL9/Legless-mediated recruitment of Pygopus to the nuclear beta-catenin-TCF complex. Cell 109: 47–60. [DOI] [PubMed] [Google Scholar]
- Kulak O., Chen H., Holohan B., Wu X., He H., et al. , 2015. Disruption of Wnt/beta-catenin signaling and telomeric shortening are inextricable consequences of tankyrase inhibition in human cells. Mol. Cell. Biol. 35: 2425–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton P. F., Kakugawa S., Vincent J. P., 2016. Making, exporting, and modulating Wnts. Trends Cell Biol. 26: 756–765. [DOI] [PubMed] [Google Scholar]
- Lau T., Chan E., Callow M., Waaler J., Boggs J., et al. , 2013. A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth. Cancer Res. 73: 3132–3144. [DOI] [PubMed] [Google Scholar]
- Lawrence P. A., Bodmer R., Vincent J. P., 1995. Segmental patterning of heart precursors in Drosophila. Development 121: 4303–4308. [DOI] [PubMed] [Google Scholar]
- Lawrence P. A., Sanson B., Vincent J. P., 1996. Compartments, wingless and engrailed: patterning the ventral epidermis of Drosophila embryos. Development 122: 4095–4103. [DOI] [PubMed] [Google Scholar]
- Lee H. H., Frasch M., 2000. Wingless effects mesoderm patterning and ectoderm segmentation events via induction of its downstream target sloppy paired. Development 127: 5497–5508. [DOI] [PubMed] [Google Scholar]
- Legent K., Treisman J. E., 2008. Wingless signaling in Drosophila eye development. Methods Mol. Biol. 469: 141–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legent K., Steinhauer J., Richard M., Treisman J. E., 2012. A screen for X-linked mutations affecting Drosophila photoreceptor differentiation identifies Casein kinase 1α as an essential negative regulator of Wingless signaling. Genetics 190: 601–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Sutter C., Parker D. S., Blauwkamp T., Fang M., et al. , 2007. CBP/p300 are bimodal regulators of Wnt signaling. EMBO J. 26: 2284–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li V. S., Ng S. S., Boersema P. J., Low T. Y., Karthaus W. R., et al. , 2012. Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell 149: 1245–1256. [DOI] [PubMed] [Google Scholar]
- Lin X., Perrimon N., 1999. Dally cooperates with Drosophila Frizzled 2 to transduce Wingless signalling. Nature 400: 281–284. [DOI] [PubMed] [Google Scholar]
- Lindsley D. L., Grell E. H., 1968. The Genetic Variations of Drosophila melanogaster. Carnegie Institution of Washington, Washington, DC [Google Scholar]
- Liu C., Li Y., Semenov M., Han C., Baeg G. H., et al. , 2002. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108: 837–847. [DOI] [PubMed] [Google Scholar]
- Llimargas M., 2000. Wingless and its signalling pathway have common and separable functions during tracheal development. Development 127: 4407–4417. [DOI] [PubMed] [Google Scholar]
- Logan C. Y., Nusse R., 2004. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20: 781–810. [DOI] [PubMed] [Google Scholar]
- Loh K. M., van Amerongen R., Nusse R., 2016. Generating cellular diversity and spatial form: Wnt signaling and the evolution of multicellular animals. Dev. Cell 38: 643–655. [DOI] [PubMed] [Google Scholar]
- Malbon C. C., Wang H. Y., 2006. Dishevelled: a mobile scaffold catalyzing development. Curr. Top. Dev. Biol. 72: 153–166. [DOI] [PubMed] [Google Scholar]
- Mao J., Wang J., Liu B., Pan W., Farr G. H., III, et al. , 2001. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol. Cell 7: 801–809. [DOI] [PubMed] [Google Scholar]
- Martinez Arias A., Baker N., Ingham P., 1988. Role of the segment polarity genes in the definition and maintenance of cell states in the Drosophila embryo. Development 103: 157–170. [DOI] [PubMed] [Google Scholar]
- McCartney B. M., Dierick H. A., Kirkpatrick C., Moline M. M., Baas A., et al. , 1999. Drosophila APC2 is a cytoskeletally-associated protein that regulates Wingless signaling in the embryonic epidermis. J. Cell Biol. 146: 1303–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough I. J., Vincent J. P., 2016. Exosomes in developmental signalling. Development 143: 2482–2493. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Moon R. T., 1989. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell 58: 1075–1084. [DOI] [PubMed] [Google Scholar]
- Mendoza-Topaz C., Mieszczanek J., Bienz M., 2011. The Adenomatous polyposis coli tumour suppressor is essential for Axin complex assembly and function and opposes Axin’s interaction with Dishevelled. Open Biol. 1: 110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar M., van de Wetering M., Oosterwegel M., Petersonmaduro J., Godsave S., et al. , 1996. XTcf-3 transcription factor mediates beta-catenin induced axis formation in Xenopus embryos. Cell 86: 391–399. [DOI] [PubMed] [Google Scholar]
- Moline M. M., Southern C., Bejsovec A., 1999. Directionality of Wingless protein transport influences epidermal patterning in the Drosophila embryo. Development 126: 4375–4384. [DOI] [PubMed] [Google Scholar]
- Moline M. M., Dierick H. A., Southern C., Bejsovec A., 2000. Non-equivalent roles of Drosophila Frizzled and Dfrizzled2 in embryonic Wingless signal transduction. Curr. Biol. 10: 1127–1130. [DOI] [PubMed] [Google Scholar]
- Morata G., Lawrence P. A., 1977. The development of wingless, a homeotic mutation of Drosophila. Dev. Biol. 56: 227–240. [DOI] [PubMed] [Google Scholar]
- Morgan T. H., Bridges C. B., Schultz J., 1936. Constitution of the germinal material in relation to heredity. Year B.- Carnegie Inst. Wash. 35: 289–297. [Google Scholar]
- Muller H., Samanta R., Wieschaus E., 1999. Wingless signaling in the Drosophila embryo: zygotic requirements and the role of the frizzled genes. Development 126: 577–586. [DOI] [PubMed] [Google Scholar]
- Mulligan K. A., Fuerer C., Ching W., Fish M., Willert K., et al. , 2012. Secreted Wingless-interacting molecule (Swim) promotes long-range signaling by maintaining Wingless solubility. Proc. Natl. Acad. Sci. USA 109: 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Hamada F., Ishidate T., Anai K., Kawahara K., et al. , 1998. Axin, an inhibitor of the Wnt signalling pathway, interacts with beta-catenin, GSK-3beta and APC and reduces the beta-catenin level. Genes Cells 3: 395–403. [DOI] [PubMed] [Google Scholar]
- Nakato H., Futch T. A., Selleck S. B., 1995. The division abnormally delayed (dally) gene: a putative integral membrane proteoglycan required for cell division patterning during postembryonic development of the nervous system in Drosophila. Development 121: 3687–3702. [DOI] [PubMed] [Google Scholar]
- Neumann C. J., Cohen S. M., 1997. Long-range action of Wingless organizes the dorsal-ventral axis of the Drosophila wing. Development 124: 871–880. [DOI] [PubMed] [Google Scholar]
- Nichols S. A., Dirks W., Pearse J. S., King N., 2006. Early evolution of animal cell signaling and adhesion genes. Proc. Natl. Acad. Sci. USA 103: 12451–12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordermeer J., Johnston P., Rijsewijk F., Nusse R., Lawrence P. A., 1992. The consequences of ubiquitous expression of the wingless gene in the Drosophila embryo. Development 116: 711–719. [DOI] [PubMed] [Google Scholar]
- Noordermeer J., Klingensmith J., Perrimon N., Nusse R., 1994. dishevelled and armadillo act in the Wingless signalling pathway in Drosophila. Nature 367: 80–83. [DOI] [PubMed] [Google Scholar]
- Nusse R., 2005. Wnt signaling in disease and in development. Cell Res. 15: 28–32. [DOI] [PubMed] [Google Scholar]
- Nusse R., Clevers H., 2017. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell 169: 985–999. [DOI] [PubMed] [Google Scholar]
- Nusse R., Varmus H., 2012. Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J. 31: 2670–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E., 1982. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31: 99–109. [DOI] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E., 1992. Wnt genes. Cell 69: 1073–1087. [DOI] [PubMed] [Google Scholar]
- Nusse R., van Ooyen A., Cox D., Fung Y. K., Varmus H., 1984. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature 307: 131–136. [DOI] [PubMed] [Google Scholar]
- Nusse R., Brown A., Papkoff J., Scambler P., Shackleford G., et al. , 1991. A new nomenclature for int-1 and related genes: the Wnt gene family. Cell 64: 231. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Wieschaus E., 1980. Mutations affecting segment number and polarity in Drosophila. Nature 287: 795–801. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Wieschaus E., Kluding H., 1984. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster: I. Zygotic loci on the second chromosome. Wihelm Roux Arch. Dev. Biol. 193: 267–282. [DOI] [PubMed] [Google Scholar]
- O’Keefe L., Dougan S. T., Gabay L., Raz E., Shilo B. Z., et al. , 1997. Spitz and Wingless, emanating from distinct borders, cooperate to establish cell fate across the Engrailed domain in the Drosophila epidermis. Development 124: 4837–4845. [DOI] [PubMed] [Google Scholar]
- Orsulic S., Peifer M., 1996. An in vivo structure-function study of Armadillo, the beta-catenin homologue, reveals both separate and overlapping regions of the protein required for cell adhesion and for Wingless signaling. J. Cell Biol. 134: 1283–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton P. M., Chia W., Buescher M., 2007. The Drosophila HMG-domain proteins SoxNeuro and Dichaete direct trichome formation via the activation of shavenbaby and the restriction of Wingless pathway activity. Development 134: 2807–2813. [DOI] [PubMed] [Google Scholar]
- Oving I. M., Clevers H. C., 2002. Molecular causes of colon cancer. Eur. J. Clin. Invest. 32: 448–457. [DOI] [PubMed] [Google Scholar]
- Packard M., Koo E. S., Gorczyca M., Sharpe J., Cumberledge S., et al. , 2002. The Drosophila Wnt, Wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell 111: 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai L. M., Orsulic S., Bejsovec A., Peifer M., 1997. Negative regulation of Armadillo, a Wingless effector in Drosophila. Development 124: 2255–2266. [DOI] [PubMed] [Google Scholar]
- Panakova D., Sprong H., Marois E., Thiele C., Eaton S., 2005. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature 435: 58–65. [DOI] [PubMed] [Google Scholar]
- Pankratz M. J., Hoch M., 1995. Control of epithelial morphogenesis by cell signaling and integrin molecules in the Drosophila foregut. Development 121: 1885–1898. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Brown A. M. C., Varmus H. E., 1987. The int-1 proto-oncogene products are glycoproteins that appear to enter the secretory pathway. Mol. Cell. Biol. 7: 3978–3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D. S., Jemison J., Cadigan K. M., 2002. Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development 129: 2565–2576. [DOI] [PubMed] [Google Scholar]
- Parker D. S., Ni Y. Y., Chang J. L., Li J., Cadigan K. M., 2008. Wingless signaling induces widespread chromatin remodeling of target loci. Mol. Cell. Biol. 28: 1815–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N. H., Schafer B., Goodman C. S., Holmgren R., 1989. The role of segment polarity genes during Drosophila neurogenesis. Genes Dev. 3: 890–904. [DOI] [PubMed] [Google Scholar]
- Payre F., Vincent A., Carreno S., 1999. ovo/svb integrates Wingless and DER pathways to control epidermis differentiation. Nature 400: 271–275. [DOI] [PubMed] [Google Scholar]
- Peifer M., Wieschaus E., 1990. The segment polarity gene armadillo encodes a functionally modular protein that is the Drosophila homolog of human plakoglobin. Cell 63: 1167–1178. [DOI] [PubMed] [Google Scholar]
- Peifer M., Rauskolb C., Williams M., Riggleman B., Wieschaus E., 1991. The segment polarity gene armadillo interacts with the wingless signaling pathway in both embryonic and adult pattern formation. Development 111: 1029–1043. [DOI] [PubMed] [Google Scholar]
- Peifer M., McCrea P. D., Green K. J., Wieschaus E., Gumbiner B. M., 1992. The vertebrate adhesive junction proteins beta-catenin and plakoglobin and the Drosophila segment polarity gene armadillo form a multigene family with similar properties. J. Cell Biol. 118: 681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M., Orsulic S., Sweeton D., Wieschaus E., 1993. A role for the Drosophila segment polarity gene armadillo in cell adhesion and cytoskeletal integrity during oogenesis. Development 118: 1191–1207. [DOI] [PubMed] [Google Scholar]
- Peifer M., Pai L. M., Casey M., 1994a Phosphorylation of the Drosophila adherens junction protein Armadillo: roles for wingless signal and zeste-white 3 kinase. Dev. Biol. 166: 543–556. [DOI] [PubMed] [Google Scholar]
- Peifer M., Sweeton D., Casey M., Wieschaus E., 1994b wingless signal and Zeste-white 3 kinase trigger opposing changes in the intracellular distribution of Armadillo. Development 120: 369–380. [DOI] [PubMed] [Google Scholar]
- Penton A., Hoffmann F. M., 1996. Decapentaplegic restricts the domain of wingless during Drosophila limb patterning. Nature 382: 162–164. [DOI] [PubMed] [Google Scholar]
- Perkins L. A., Holderbaum L., Tao R., Hu Y., Sopko R., et al. , 2015. The transgenic RNAi project at Harvard Medical School: resources and validation. Genetics 201: 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon N., 1984. Clonal analysis of dominant female-sterile, germline-dependent mutations in Drosophila melanogaster. Genetics 108: 927–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon N., Mahowald A. P., 1987. Multiple functions of segment polarity genes in Drosophila. Dev. Biol. 119: 587–600. [DOI] [PubMed] [Google Scholar]
- Perrimon N., Smouse D., 1989. Multiple functions of a Drosophila homeotic gene zeste-white 3, during segmentation and neurogenesis. Dev. Biol. 135: 287–305. [DOI] [PubMed] [Google Scholar]
- Perrimon N., Mohler D., Engstrom L., Mahowald A. P., 1986. X-linked female-sterile loci in Drosophila melanogaster. Genetics 113: 695–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon N., Engstrom L., Mahowald A. P., 1989. Zygotic lethals with specific maternal effect phenotypes in Drosophila melanogaster. I. Loci on the X chromosome. Genetics 121: 333–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon N., Lanjuin A., Arnold C., Noll E., 1996. Zygotic lethal mutations with maternal effect phenotypes in Drosophila melanogaster. II. Loci on the second and third chromosomes identified by P-element-induced mutations. Genetics 144: 1681–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao S., Lee S. H., Kim H., Yum S., Stamos J. L., et al. , 2008. Direct inhibition of GSK3beta by the phosphorylated cytoplasmic domain of LRP6 in Wnt/beta-catenin signaling. PLoS One 3: e4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piddini E., Marshall F., Dubois L., Hirst E., Vincent J. P., 2005. Arrow (LRP6) and Frizzled2 cooperate to degrade Wingless in Drosophila imaginal discs. Development 132: 5479–5489. [DOI] [PubMed] [Google Scholar]
- Polakis P., 1997. The adenomatous polyposis coli (APC) tumor suppressor. Biochim. Biophys. Acta 1332: F127–F147. [DOI] [PubMed] [Google Scholar]
- Polakis P., 2007. The many ways of Wnt in cancer. Curr. Opin. Genet. Dev. 17: 45–51. [DOI] [PubMed] [Google Scholar]
- Povelones M., Nusse R., 2005. The role of the cysteine-rich domain of Frizzled in Wingless-Armadillo signaling. EMBO J. 24: 3493–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povelones M., Howes R., Fish M., Nusse R., 2005. Genetic evidence that Drosophila frizzled controls planar cell polarity and Armadillo signaling by a common mechanism. Genetics 171: 1643–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronobis M. I., Rusan N. M., Peifer M., 2015. A novel GSK3-regulated APC:Axin interaction regulates Wnt signaling by driving a catalytic cycle of efficient beta-catenin destruction. Elife 4: e08022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reim G., Hruzova M., Goetze S., Basler K., 2014. Protection of Armadillo/beta-catenin by armless, a novel positive regulator of Wingless signaling. PLoS Biol. 12: e1001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese J., Yu X., Munnerlyn A., Eresh S., Hsu S. C., et al. , 1997. LEF-1, a nuclear factor coordinating signaling inputs from wingless and decapentaplegic. Cell 88: 777–787. [DOI] [PubMed] [Google Scholar]
- Riggleman B., Wieschaus E., Schedl P., 1989. Molecular analysis of the armadillo locus: uniformly distributed transcripts and a protein with novel internal repeats are associated with a Drosophila segment polarity gene. Genes Dev. 3: 96–113. [DOI] [PubMed] [Google Scholar]
- Riggleman B., Schedl P., Wieschaus E., 1990. Spatial expression of the Drosophila segment polarity gene armadillo is posttranscriptionally regulated by wingless. Cell 63: 549–560. [DOI] [PubMed] [Google Scholar]
- Rijsewijk F., Schuermann M., Wagenaar E., Parren P., Weigel D., et al. , 1987. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell 50: 649–657. [DOI] [PubMed] [Google Scholar]
- Rizzo N. P., Bejsovec A., 2017. SoxNeuro and Shavenbaby act cooperatively to shape denticles in the embryonic epidermis of Drosophila. Development 144: 2248–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogge R. D., Karlovich C. A., Banerjee U., 1991. Genetic dissection of a neurodevelopmental pathway: son of evenless functions downstream of the sevenless and EGF receptor tyrosine kinases. Cell 64: 39–48. [DOI] [PubMed] [Google Scholar]
- Roose J., Molenaar M., Peterson J., Hurenkamp J., Brantjes H., et al. , 1998. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature 395: 608–612. [DOI] [PubMed] [Google Scholar]
- Rorth P., 1996. A modular misexpression screen in Drosophila detecting tissue-specific phenotypes. Proc. Natl. Acad. Sci. USA 93: 12418–12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset R., Mack J. A., Wharton K. A., Jr, Axelrod J. D., Cadigan K. M., et al. , 2001. Naked cuticle targets dishevelled to antagonize Wnt signal transduction. Genes Dev. 15: 658–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C., 1982. Genetic transformation of Drosophila with transposable element vectors. Science 218: 348–353. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Kidwell M. G., Bingham P. M., 1982. The molecular basis of P-M hybrid dysgenesis: the nature of induced mutations. Cell 29: 987–994. [DOI] [PubMed] [Google Scholar]
- Rulifson E. J., Blair S. S., 1995. Notch regulates wingless expression and is not required for reception of the paracrine wingless signal during wing margin neurogenesis in Drosophila. Development 121: 2813–2824. [DOI] [PubMed] [Google Scholar]
- Rulifson E. J., Wu C. H., Nusse R., 2000. Pathway specificity by the bifunctional receptor Frizzled is determined by affinity for Wingless. Mol. Cell 6: 117–126. [PubMed] [Google Scholar]
- San Martin B., Bate M., 2001. Hindgut visceral mesoderm requires an ectodermal template for normal development in Drosophila. Development 128: 233–242. [DOI] [PubMed] [Google Scholar]
- Sanson B., White P., Vincent J. P., 1996. Uncoupling cadherin-based adhesion from wingless signalling in Drosophila. Nature 383: 627–630. [DOI] [PubMed] [Google Scholar]
- Sanson B., Alexandre C., Fascetti N., Vincent J. P., 1999. Engrailed and Hedgehog make the range of Wingless asymmetric in Drosophila embryos. Cell 98: 207–216. [DOI] [PubMed] [Google Scholar]
- Schneider I., Schneider P. N., Derry S. W., Lin S., Barton L. J., et al. , 2010. Zebrafish Nkd1 promotes Dvl degradation and is required for left-right patterning. Dev. Biol. 348: 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubiger M., Sustar A., Schubiger G., 2010. Regeneration and transdetermination: the role of wingless and its regulation. Dev. Biol. 347: 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupbach T., Wieschaus E., 1986. Maternal-effect mutations altering the anterior-posterior pattern of the Drosophila embryo. Rouxs Arch. Dev. Biol. 195: 302–317. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T., Fiedler M., Shibata N., Butler P. J., Kikuchi A., et al. , 2007a The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat. Struct. Mol. Biol. 14: 484–492. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T., Metcalfe C., Bienz M., 2007b Dynamic recruitment of axin by Dishevelled protein assemblies. J. Cell Sci. 120: 2402–2412. [DOI] [PubMed] [Google Scholar]
- Shackleford G. M., Varmus H. E., 1987. Expression of the proto-oncogene int-1 is restricted to postmeiotic male germ cells and the neural tube of mid-gestational embryos. Cell 50: 89–95. [DOI] [PubMed] [Google Scholar]
- Sharma M., Jamieson C., Johnson M., Molloy M. P., Henderson B. R., 2012. Specific armadillo repeat sequences facilitate beta-catenin nuclear transport in live cells via direct binding to nucleoporins Nup62, Nup153, and RanBP2/Nup358. J. Biol. Chem. 287: 819–831. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sharma R. P., 1973. Wingless - a new mutant in Drosophila melanogaster. Drosoph. Inf. Serv. 50: 134. [Google Scholar]
- Sidow A., 1992. Diversification of the Wnt gene family on the ancestral lineage of vertebrates. Proc. Natl. Acad. Sci. USA 89: 5098–5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D., Jemal A., 2017. Cancer Statistics 2017, CA Cancer J. Clin. 67: 7–30. [DOI] [PubMed] [Google Scholar]
- Siegfried E., Perkins L., Capaci T., Perrimon N., 1990. Putative protein kinase product of the Drosophila segment polarity gene zeste-white 3. Nature 345: 825–829. [DOI] [PubMed] [Google Scholar]
- Siegfried E., Chou T. B., Perrimon N., 1992. wingless signaling acts through zeste-white 3, the Drosophila homolog of glycogen synthase kinase-3, to regulate engrailed and establish cell fate. Cell 71: 1167–1179. [DOI] [PubMed] [Google Scholar]
- Siegfried E., Wilder E. L., Perrimon N., 1994. Components of wingless signalling in Drosophila. Nature 367: 76–80. [DOI] [PubMed] [Google Scholar]
- Simon M. A., Bowtell D. D., Dodson G. S., Laverty T. R., Rubin G. M., 1991. Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell 67: 701–716. [DOI] [PubMed] [Google Scholar]
- Skaer H., Martinez Arias A., 1992. The wingless product is required for cell proliferation in the Malpighian tubule anlage of Drosophila melanogaster. Development 116: 745–754. [Google Scholar]
- Spradling A. C., Rubin G. M., 1982. Transposition of cloned P elements into Drosophila germ line chromosomes. Science 218: 341–347. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Stern D., Beaton A., Rhem E. J., Laverty T., et al. , 1999. The Berkeley Drosophila Genome Project gene disruption project: single P-element insertions mutating 25% of vital Drosophila genes. Genetics 153: 135–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamos J. L., Weis W. I., 2013. The beta-catenin destruction complex. Cold Spring Harb. Perspect. Biol. 5: a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strapps W. R., Tomlinson A., 2001. Transducing properties of Drosophila Frizzled proteins. Development 128: 4829–4835. [DOI] [PubMed] [Google Scholar]
- Strigini M., Cohen S. M., 2000. Wingless gradient formation in the Drosophila wing. Curr. Biol. 10: 293–300. [DOI] [PubMed] [Google Scholar]
- Struhl G., Basler K., 1993. Organizing activity of wingless protein in Drosophila. Cell 72: 527–540. [DOI] [PubMed] [Google Scholar]
- Swarup S., Pradhan-Sundd T., Verheyen E. M., 2015. Genome-wide identification of phospho-regulators of Wnt signaling in Drosophila. Development 142: 1502–1515. [DOI] [PubMed] [Google Scholar]
- Szuts D., Freeman M., Bienz M., 1997. Antagonism between EGFR and Wingless signalling in the larval cuticle of Drosophila. Development 124: 3209–3219. [DOI] [PubMed] [Google Scholar]
- Szuts D., Eresh S., Bienz M., 1998. Functional intertwining of Dpp and EGFR signaling during Drosophila endoderm induction. Genes Dev. 12: 2022–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada R., Satomi Y., Kurata T., Ueno N., Norioka S., et al. , 2006. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev. Cell 11: 791–801. [DOI] [PubMed] [Google Scholar]
- Tamai K., Semenov M., Kato Y., Spokony R., Liu C., et al. , 2000. LDL-receptor-related proteins in Wnt signal transduction. Nature 407: 530–535. [DOI] [PubMed] [Google Scholar]
- Tan Y., Yu D., Busto G. U., Wilson C., Davis R. L., 2013. Wnt signaling is required for long-term memory formation. Cell Reports 4: 1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Kitagawa Y., Kadowaki T., 2002. Drosophila segment polarity gene product porcupine stimulates the posttranslational N-glycosylation of wingless in the endoplasmic reticulum. J. Biol. Chem. 277: 12816–12823. [DOI] [PubMed] [Google Scholar]
- Tang X., Wu Y., Belenkaya T. Y., Huang Q., Ray L., et al. , 2012. Roles of N-glycosylation and lipidation in Wg secretion and signaling. Dev. Biol. 364: 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosiou N. A., Zhang S., Wang W. Y., Xu T., 1998. slimb coordinates wg and dpp expression in the dorsal-ventral and anterior-posterior axes during limb development. Development 125: 3411–3416. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R., 1990. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature 346: 847–850. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Musci T. S., Neumann P. E., Capecchi M. R., 1991. swaying is a mutant allele of the proto-oncogene Wnt-1. Cell 67: 969–976. [DOI] [PubMed] [Google Scholar]
- Thuringer F., Bienz M., 1993. Indirect autoregulation of a homeotic Drosophila gene mediated by extracellular signaling. Proc. Natl. Acad. Sci. USA 90: 3899–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuringer F., Cohen S. M., Bienz M., 1993. Dissection of an indirect autoregulatory response of a homeotic Drosophila gene. EMBO J. 12: 2419–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolwinski N. S., Wehrli M., Rives A., Erdeniz N., DiNardo S., et al. , 2003. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev. Cell 4: 407–418. [DOI] [PubMed] [Google Scholar]
- Tomlinson A., 2003. Patterning the peripheral retina of the fly: decoding a gradient. Dev. Cell 5: 799–809. [DOI] [PubMed] [Google Scholar]
- Tsuda M., Kamimura K., Nakato H., Archer M., Staatz W., et al. , 1999. The cell-surface proteoglycan dally regulates Wingless signalling in Drosophila. Nature 400: 276–280. [DOI] [PubMed] [Google Scholar]
- Urban S., Brown G., Freeman M., 2004. EGF receptor signalling protects smooth-cuticle cells from apoptosis during Drosophila ventral epidermis development. Development 131: 1835–1845. [DOI] [PubMed] [Google Scholar]
- Valenta T., Hausmann G., Basler K., 2012. The many faces and functions of beta-catenin. EMBO J. 31: 2714–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M., Nusse R., Johnston P., Lawrence P. A., 1989. Distribution of the wingless gene product in Drosophila embryos: a protein involved in cell-cell communication. Cell 59: 739–749. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M., Harryman-Samos C., Klingensmith J., Perrimon N., Nusse R., 1993. Mutations in the segment polarity genes wingless and porcupine impair secretion of the Wingless protein. EMBO J. 12: 5293–5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M., Cavallo R., Dooijes D., van Beest M., van Es J., et al. , 1997. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88: 789–799. [DOI] [PubMed] [Google Scholar]
- van Leeuwen F., Samos C. H., Nusse R., 1994. Biological activity of soluble wingless protein in cultured Drosophila imaginal disc cells. Nature 368: 342–344. [DOI] [PubMed] [Google Scholar]
- van Tienen L. M., Mieszczanek J., Fiedler M., Rutherford T. J., Bienz M., 2017. Constitutive scaffolding of multiple Wnt enhanceosome components by Legless/BCL9. Elife 6: e20882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van ’t Veer L. J., van Kessel A. G., van Heerikhuizen H., van Ooyen A., Nusse R., 1984. Molecular cloning and chromosomal assignment of the human homolog of int-1, a mouse gene implicated in mammary tumorigenesis. Mol. Cell. Biol. 4: 2532–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., 1984. The molecular genetics of cellular oncogenes. Annu. Rev. Genet. 18: 553–612. [DOI] [PubMed] [Google Scholar]
- Verheyen E. M., Purcell K. J., Fortini M. E., Artavanis-Tsakonas S., 1996. Analysis of dominant enhancers and suppressors of activated Notch in Drosophila. Genetics 144: 1127–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheyen E. M., Mirkovic I., MacLean S. J., Langmann C., Andrews B. C., et al. , 2001. The tissue polarity gene nemo carries out multiple roles in patterning during Drosophila development. Mech. Dev. 101: 119–132. [DOI] [PubMed] [Google Scholar]
- Vinson C. R., Adler P. N., 1987. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature 329: 549–551. [DOI] [PubMed] [Google Scholar]
- Vinson C. R., Conover S., Adler P. N., 1989. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature 338: 263–264. [DOI] [PubMed] [Google Scholar]
- Waaler J., Machon O., Tumova L., Dinh H., Korinek V., et al. , 2012. A novel tankyrase inhibitor decreases canonical Wnt signaling in colon carcinoma cells and reduces tumor growth in conditional APC mutant mice. Cancer Res. 72: 2822–2832. [DOI] [PubMed] [Google Scholar]
- Waldrop S., Chan C. C., Cagatay T., Zhang S., Rousset R., et al. , 2006. An unconventional nuclear localization motif is crucial for function of the Drosophila Wnt/wingless antagonist Naked cuticle. Genetics 174: 331–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters J. W., Munoz C., Paaby A. B., DiNardo S., 2005. Serrate-Notch signaling defines the scope of the initial denticle field by modulating EGFR activation. Dev. Biol. 286: 415–426. [DOI] [PubMed] [Google Scholar]
- Waltzer L., Bienz M., 1998. Drosophila CBP represses the transcription factor TCF to antagonize Wingless signalling. Nature 395: 521–525. [DOI] [PubMed] [Google Scholar]
- Wang Y., Macke J. P., Abella B. S., Andreasson K., Worley P., et al. , 1996. A large family of putative transmembrane receptors homologous to the product of the Drosophila tissue polarity gene frizzled. J. Biol. Chem. 271: 4468–4476. [DOI] [PubMed] [Google Scholar]
- Wang Z., Tacchelly-Benites O., Yang E., Ahmed Y., 2016a Dual roles for membrane association of Drosophila Axin in Wnt signaling. PLoS Genet. 12: e1006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Tacchelly-Benites O., Yang E., Thorne C. A., Nojima H., et al. , 2016b Wnt/Wingless pathway activation is promoted by a critical threshold of Axin maintained by the tumor suppressor APC and the ADP-ribose polymerase Tankyrase. Genetics 203: 269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli M., Dougan S. T., Caldwell K., O’Keefe L., Schwartz S., et al. , 2000. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature 407: 527–530. [DOI] [PubMed] [Google Scholar]
- Wieschaus E., Noell E., 1986. Specificity of embryonic lethal mutations in Drosophila analyzed in germline clones. Wihelm Roux Arch. Dev. Biol. 195: 63–73. [DOI] [PubMed] [Google Scholar]
- Wieschaus E., Riggleman R., 1987. Autonomous requirements for the segment polarity gene armadillo during Drosophila embryogenesis. Cell 49: 177–184. [DOI] [PubMed] [Google Scholar]
- Wieschaus E., Nüsslein-Volhard C., Jurgens G., 1984. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster: zygotic loci on the X-chromosome and the fourth chromosome. Wihelm Roux Arch. Dev. Biol. 193: 296–307. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bailes J. A., McMahon A. P., 1987. Expression of the proto-oncogene int-1 is restricted to specific neural cells in the developing mouse embryo. Cell 50: 79–88. [DOI] [PubMed] [Google Scholar]
- Willert K., Nusse R., 2012. Wnt proteins. Cold Spring Harb. Perspect. Biol. 4: a007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K., Logan C. Y., Arora A., Fish M., Nusse R., 1999. A Drosophila Axin homolog, Daxin, inhibits Wnt signaling. Development 126: 4165–4173. [DOI] [PubMed] [Google Scholar]
- Willert K., Brown J. D., Danenberg E., Duncan A. W., Weissman I. L., et al. , 2003. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423: 448–452. [DOI] [PubMed] [Google Scholar]
- Wodarz A., Nusse R., 1998. Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 14: 59–88. [DOI] [PubMed] [Google Scholar]
- Wodarz A., Stewart D. B., Nelson W. J., Nusse R., 2006. Wingless signaling modulates cadherin-mediated cell adhesion in Drosophila imaginal disc cells. J. Cell Sci. 119: 2425–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H. C., Bourdelas A., Krauss A., Lee H. J., Shao Y., et al. , 2003. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol. Cell 12: 1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. H., Nusse R., 2002. Ligand receptor interactions in the Wnt signaling pathway in Drosophila. J. Biol. Chem. 277: 41762–41769. [DOI] [PubMed] [Google Scholar]
- Wu G., Huang H., Garcia Abreu J., He X., 2009. Inhibition of GSK3 phosphorylation of beta-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS One 4: e4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Mlodzik M., 2008. The Frizzled extracellular domain is a ligand for Van Gogh/Stbm during nonautonomous planar cell polarity signaling. Dev. Cell 15: 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Klein T. J., Mlodzik M., 2004. Subcellular localization of Frizzled receptors, mediated by their cytoplasmic tails, regulates signaling pathway specificity. PLoS Biol. 2: E158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Roman A. C., Carvajal-Gonzalez J. M., Mlodzik M., 2013. Wg and Wnt4 provide long-range directional input to planar cell polarity orientation in Drosophila. Nat. Cell Biol. 15: 1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Wang W., Zhang S., Stewart R. A., Yu W., 1995. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development 121: 1053–1063. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y., Palmer L., Alexandre C., Kakugawa S., Beckett K., et al. , 2016. Godzilla-dependent transcytosis promotes Wingless signalling in Drosophila wing imaginal discs. Nat. Cell Biol. 18: 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa S., Matsuda Y., Lee J. S., Matsubayashi H., Sese S., et al. , 2002. Casein kinase I phosphorylates the Armadillo protein and induces its degradation in Drosophila. EMBO J. 21: 1733–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Hoppler S., Eresh S., Bienz M., 1996. decapentaplegic, a target gene of the wingless signalling pathway in the Drosophila midgut. Development 122: 849–858. [DOI] [PubMed] [Google Scholar]
- Yu X., Waltzer L., Bienz M., 1999. A new Drosophila APC homologue associated with adhesive zones of epithelial cells. Nat. Cell Biol. 1: 144–151. [DOI] [PubMed] [Google Scholar]
- Zecca M., Basler K., Struhl G., 1995. Sequential organizing activities of engrailed, hedgehog and decapentaplegic in the Drosophila wing. Development 121: 2265–2278. [DOI] [PubMed] [Google Scholar]
- Zecca M., Basler K., Struhl G., 1996. Direct and long-range action of a Wingless morphogen gradient. Cell 87: 833–844. [DOI] [PubMed] [Google Scholar]
- Zeng W., Wharton K. A., Jr, Mack J. A., Wang K., Gadbaw M., et al. , 2000. naked cuticle encodes an inducible antagonist of Wnt signalling. Nature 403: 789–795. [DOI] [PubMed] [Google Scholar]
- Zhai L., Chaturvedi D., Cumberledge S., 2004. Drosophila Wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. J. Biol. Chem. 279: 33220–33227. [DOI] [PubMed] [Google Scholar]
- Zhang C. U., Blauwkamp T. A., Burby P. E., Cadigan K. M., 2014. Wnt-mediated repression via bipartite DNA recognition by TCF in the Drosophila hematopoietic system. PLoS Genet. 10: e1004509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Jia J., Wang B., Amanai K., Wharton K. A., Jr., et al. , 2006. Regulation of wingless signaling by the CKI family in Drosophila limb development. Dev. Biol. 299: 221–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu S., Mickanin C., Feng Y., Charlat O., et al. , 2011. RNF146 is a poly(ADP-ribose)-directed E3 ligase that regulates Axin degradation and Wnt signalling. Nat. Cell Biol. 13: 623–629. [DOI] [PubMed] [Google Scholar]
- Zilian O., Frei E., Burke R., Brentrup D., Gutjahr T., et al. , 1999. double-time is identical to discs overgrown, which is required for cell survival, proliferation and growth arrest in Drosophila imaginal discs. Development 126: 5409–5420. [DOI] [PubMed] [Google Scholar]