Although extracellular matrices function as protective barriers to many types of environmental insult, their role in sensing stress and regulating adaptive gene induction responses has not been studied carefully...
Keywords: damage sensor, collagen, detoxification, osmotic stress, antimicrobial response
Abstract
Extracellular matrix barriers and inducible cytoprotective genes form successive lines of defense against chemical and microbial environmental stressors. The barrier in nematodes is a collagenous extracellular matrix called the cuticle. In Caenorhabditis elegans, disruption of some cuticle collagen genes activates osmolyte and antimicrobial response genes. Physical damage to the epidermis also activates antimicrobial responses. Here, we assayed the effect of knocking down genes required for cuticle and epidermal integrity on diverse cellular stress responses. We found that disruption of specific bands of collagen, called annular furrows, coactivates detoxification, hyperosmotic, and antimicrobial response genes, but not other stress responses. Disruption of other cuticle structures and epidermal integrity does not have the same effect. Several transcription factors act downstream of furrow loss. SKN-1/Nrf and ELT-3/GATA are required for detoxification, SKN-1/Nrf is partially required for the osmolyte response, and STA-2/Stat and ELT-3/GATA for antimicrobial gene expression. Our results are consistent with a cuticle-associated damage sensor that coordinates detoxification, hyperosmotic, and antimicrobial responses through overlapping, but distinct, downstream signaling.
EXTRACELLULAR matrices (ECMs) are ubiquitous features of animal tissues composed of secreted fibrous proteins and polysaccharides. Although they were once considered inert mechanical scaffolds (Hay 1981), it is now clear that there is dynamic and reciprocal cross talk between cells and ECMs that regulates cell differentiation, morphogenesis, and tumorigenesis (Rozario and DeSimone 2010; Clause and Barker 2013; Samarakoon et al. 2013; Winograd-Katz et al. 2014; Gaggar and Weathington 2016). Less is known about how ECMs influence cellular responses to environmental stress.
Internal and epidermal tissues secrete ECMs as mechanical support. Epidermal ECMs also function as barriers to environmental stress. Examples include a keratin- and lipid-rich matrix in mammals (i.e., the stratum corneum of the skin) and a rigid chitinous exoskeleton in insects. Nematodes such as Caenorhabditis elegans are covered by a flexible cuticle of cross-linked collagen fibers that is secreted by underlying epidermal cells (Page and Johnstone 2007; Chisholm and Xu 2012). It provides a first line of defense against desiccation, as well as some pathogens and toxins (Alvarez et al. 2007; Partridge et al. 2008; Burns et al. 2010).
C. elegans mounts distinct cellular responses to stressors that are broadly conserved (Lamitina et al. 2006; Wheeler and Thomas 2006; Pujol et al. 2008a,b; Rohlfing et al. 2011; Choe 2013; Zugasti et al. 2014). In response to high osmolarity, C. elegans synthesizes the organic osmolyte glycerol in part by inducing gpdh-1, which encodes the rate-limiting enzyme GPDH (glycerol-3-phosphate dehydrogenase) (Lamitina et al. 2004, 2006). High osmolarity and infection with fungal pathogens that pierce the C. elegans cuticle induce several antimicrobial peptide genes including nlp-29 (Pujol et al. 2008a,b; Zugasti et al. 2014). Genetic studies have identified cuticle collagens that are required to regulate gpdh-1 and nlp-29 under basal conditions (Lamitina et al. 2006; Wheeler and Thomas 2006; Pujol et al. 2008b; Choe 2013; Zugasti et al. 2016) suggesting that the cuticle may contain a sensor for stress (Lamitina et al. 2006; Wheeler and Thomas 2006; Choe 2013; Taffoni and Pujol 2015). The nature of this putative sensor and downstream signaling mechanisms remain poorly defined and it is unclear if other stress responses are also activated.
In response to reactive small molecules, cap “n” collar (CNC) transcription factors activate antioxidant and detoxification genes in nematodes, insects, and mammals (Hu et al. 2006; Oliveira et al. 2009; Park et al. 2009; Sykiotis and Bohmann 2010; Choe et al. 2012; Blackwell et al. 2015). The single C. elegans CNC, SKN-1, promotes stress resistance, slows aging, and extends life span, while the mammalian CNC Nrf2 protects against cancer, neurodegeneration, inflammation, and fibrosis (Oliveira et al. 2009; Park et al. 2009; Sykiotis and Bohmann 2010). CNCs are regulated by a complex set of intracellular signals that influence post-translational modifications, degradation, and nuclear translocation (Bryan et al. 2013; Niture et al. 2013; Blackwell et al. 2015); regulation of CNCs via the ECM would represent a distinct mechanism.
We used RNA interference (RNAi) to test disruption of diverse aspects of cuticle and epidermal integrity for activation of six conserved stress responses. Our results show that osmolyte accumulation and detoxification responses are coactivated by disruption of a specific cuticle structure called the annular furrow, and not by general changes in body shape or epidermal integrity. Antimicrobial response genes were also activated by furrow disruption, and more generally by loss of epidermal integrity. Hyperosmolarity also induces skn-1-dependent detoxification genes. Surprisingly, we also find that in furrow mutants, skn-1 is required for full induction of genes that regulate the accumulation of osmolytes. Alternatively, activation of antimicrobial genes by furrow loss is dependent on STA-2/STAT and ELT-3/GATA transcription factors. Our results are consistent with the presence of a damage sensor residing in, or associated with, furrows in the cuticle that coregulates three different stress defense pathways.
Materials and Methods
C. elegans strains
The following strains were used vs.: wild-type N2 Bristol, VP596 vsIs33[dop-3p::DsRed2];dvIs19[gst-4p::GFP], VP604 kbIs24[gpdh-1p::DsRed2;myo-2p::GFP;unc-119 rescue], SJ4005 zcIS4[hsp-4p::GFP], SJ4100 zcIs13[hsp-6p::GFP], QV65 gpIs1[hsp-16.2p::GFP];vsls33[dop-3p::DsRed2], QV285 frIs7[nlp-29p::GFP, col-12p::DsRed], IG274 frIs7, CB128 dpy-10(e128), CB88 dpy-7(e88), BE3 sqt-2(sc3), CB61 dpy-5(e61), QV41 dpy-10(e128);vsls33;dvIs19, QV248 dpy-7(e88);dvIs19, QV261 dpy-7(e88);kbIs24, IG1689 dpy-7(e88);frIs7, IG1710 dpy-7(e88);elt-3(gk121);frIs7, IG1705 dpy-7(e88);sta-2(ok1860);frIs7, IG1685 dpy-3(e27);frIs7, G1709 dpy-3(e27)elt-3(gk121);frIs7, IG1704 dpy-3(e27);sta-2(ok1860);frIs7, IG1457 dpy-10(e128);frIs7, IG1712 dpy-10(e128);elt-3(gk121);frIs7, IG1707 dpy-10(e128);sta-2(ok1860);frIs7, TP12 kaIs12[COL-19::GFP], VP332 gpdh-1(kb24);gpdh-2(kb33), LD001 ldIs7[skn-1B/C::GFP + pRF4(rol-6(su1006))], and GR2245 skn-1(mg570). QV251, which contains a reporter for gst-10p, was generated using PCR to fuse 951-bp upstream from the start codon to GFP (Hobert 2002). The fusion PCR product was then injected at ∼20 ng/μl with myo-3p::dsRed as a comarker. Unless noted otherwise, worms were cultured at 20° using standard methods (Brenner 1974).
RNAi and screening
RNAi was performed by feeding worms strains of Escherichia coli [HT115(DE3)] that are engineered to transcribe double-stranded RNA (dsRNA) homologous to a target gene (Kamath et al. 2001). The cuticle and epidermal screen in Figure 1A was performed with dsRNA feeding constructs from the ORFeome library (Open Biosystems, Huntsville, AL) (Rual et al. 2004) and supplemented with clones for dpy-10, dpy-20, dpy-3, and mua-6 from the genomic library (Geneservice, Cambridge, UK) (Kamath et al. 2003). All clones used in Figure 1B were derived from the genomic library. Positive hit clone inserts were verified by sequencing and targets identified using Clone Mapper (Thakur et al. 2014). Bacteria with plasmid pPD129.36 expressing 202 bases of dsRNA that are not homologous to any predicted C. elegans gene and the sta-1(RNAi) clone (Zugasti et al. 2016) were used as controls for nonspecific RNAi effects. RNAi was performed as described previously (Choe et al. 2009) with minor modifications. dsRNA-producing bacteria were grown in lysogeny broth containing selective antibiotic and then transferred to agar nematode growth medium (NGM) plates containing 0.2% β-lactose, or 1 or 3 mM IPTG (Choe et al. 2009). Eggs or synchronized populations of L1 larvae were placed on RNAi plates and tested at the young and gravid adult stages.
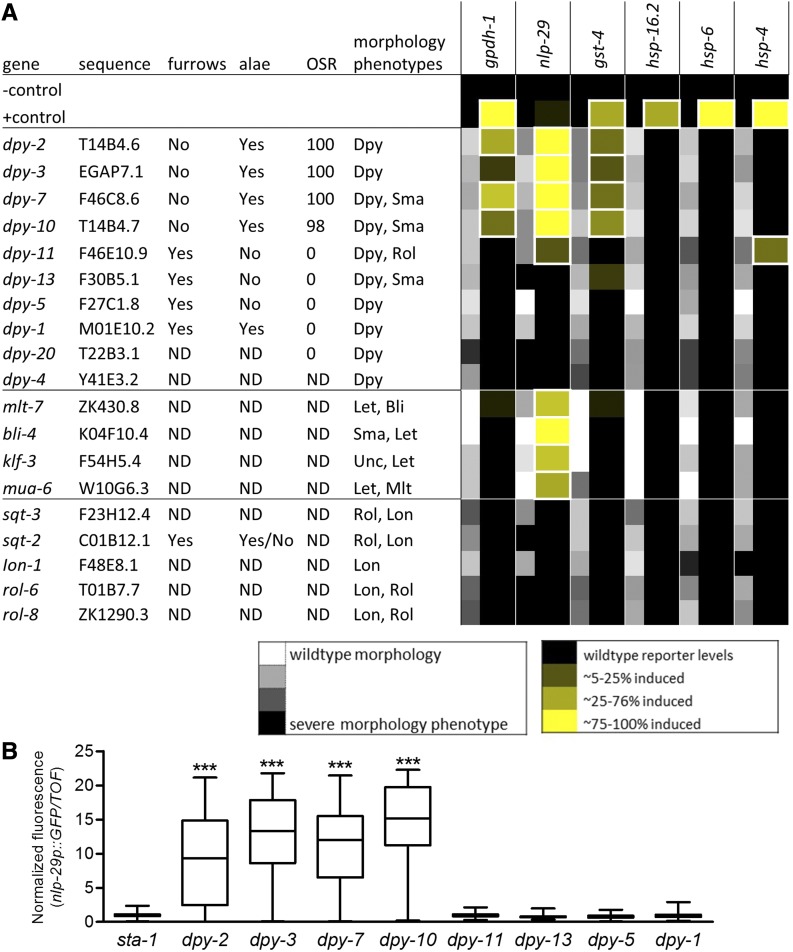
Figure 1.
Genetic disruption of specific dpy genes activates detoxification, antimicrobial, and osmotic stress responses. (A) Double-stranded RNA (dsRNA)-expressing clones for genes required for diverse aspects of cuticle and epidermal integrity were scored for cuticle and morphology phenotypes and induction of stress response reporters. Stress response reporters were scored for penetrance and averaged together across three trials. Cuticle and morphology phenotype penetrance and expressivity (Dpy only) were scored and averaged together across three trials for each reporter line. Positive controls for reporters were 200 mM NaCl for gpdh-1 and nlp-29, wdr-23(RNAi) for gst-4, 34° for 1 hr for hsp-16.2, 0.5 mM paraquat for hsp-6, and 10 µg/ml tunicamycin for hsp-4. ND, not determined. Reporter results outlined with a thick white boarder were positive in at least two of three trials. Percent acute osmotic stress resistance (OSR) from trial run in (B) and the presence (Yes) or absence (No) of intact furrows and alae (from Figure 2) are also summarized. (B) The ratio of nlp-29p::GFP to time of flight was measured in a BIOSORT and normalized to the negative control sta-1(RNAi). n = 99–228 worms. Boxes are 25% percentiles above and below the median, and whiskers are minimum and maximum.
For the screen in Figure 1A, each dsRNA clone was tested with all six stress response reporter strains in 12-well agar plates in three independent trials. Fluorescent reporter induction was scored manually as an estimate of percent penetrance (0, similar to control vector; 1, ∼5–25%; 2, ∼25–75%; and 3, ∼75% or more) and averaged together across trials for each reporter strain. The penetrance of phenotypes affecting body morphology and behavior was scored on a scale of 0–3 (0, similar to control vector; 1, ∼5–10%; 2, ∼10–50%; and 3, ∼50–100%) and averaged together across all trials with all reporter strains. In all trials except the first, Dumpy was also scored for expressivity (0, similar to control vector; 1, mild; 2, moderate; and 3, strong Dumpy), and the penetrance and expressivity scores were averaged together to calculate an average value for the strength of the morphology phenotype. Only results for clones that caused an average morphology phenotype score of ≥ 1.0 are shown.
Quantitative PCR and transgene analysis
Detoxification gene reporters (gst-4p::GFP and gst-10p::GFP) and COL-19::GFP were imaged with an Olympus BX60 microscope with UPlanFl objectives and a Zeiss Axiocam MRm camera ([Carl Zeiss], Thornwood, NY). Fluorescence of nlp-29p::GFP was quantified using a COPAS BIOSORT as previously described (Pujol et al. 2008a) and normalized to time of flight. The BIOSORT was not sensitive enough to reliably measure gst-4p::GFP and gpdh-1p::dsRed2 fluorescence in dpy-7 worms and these were instead quantified manually in individual worms from images using Image J 1.48v to calculate average whole-worm fluorescence. Hypodermal-specific gst-4p::GFP fluorescence was also scored manually in individual worms as follows: low (dim signal limited to a few spots), medium (dim signal throughout the epidermis or bright signal only in head or tail regions), and high (bright signal throughout the epidermis). In one experiment, gst-4p::GFP was quantified using a fluorescent plate reader (Synergy HT; BioTek) (Leung et al. 2011). SKN-1b/c::GFP was visualized with a Zeiss Axiovert 200M inverted microscope, and an LCM 5 Pascal Vario One confocal laser scanning system and 40× C-apochromat water objective.
Quantitative PCR (qPCR) assays were performed by isolating total RNA with a Quick-RNA MicroPrep kit from ZYMO Research. Reverse transcription and PCR were performed with the GoTaq 2-Step RT-qPCR system from Promega in an Eppendorf RealPlex2 using primers for rpl-2 or cdc-42 as internal controls. Relative mRNA levels were calculated using the ΔΔCT method adjusted with primer efficiencies calculated from standard curves. Primer sequences are provided in Supplemental Material, Table S1.
Whole-transcriptome RNA sequencing
N2 worms were synchronized at the L1 larval stage via hypochlorite treatment and grown on RNAi bacteria. For high NaCl, N2 worms were transferred to 300 mM NaCl NGM agar plates as young adults and exposed for 3 or 24 hr. Mutant dpy-7 worms were harvested 1 day after first becoming gravid, which corresponds to when osmotic and detoxification gene reporters are most active. RNA was extracted from three replicates per treatment, with ∼1000–2000 worms per replicate, using the RNAqueous-Micro Total RNA Isolation Kit (ThermoFisher Scientific). Total RNA was sent to The Yale Center for Genome Analysis for 75 nucleotide single-end sequencing in an Illumina HiSequation 2000.
Raw sequences were processed with Kallisto to quantify transcripts and Sleuth was used for differential abundance analysis (Pimentel et al. 2017), which generated an estimate for differential gene expression effect size termed “b” that is analogous to log2 fold change and an adjusted P-value termed “q.” We analyzed six pairs of conditions from our experiment and a previously published pair of raw sequence data (GSE63075) (Steinbaugh et al. 2015).
We considered genes differentially expressed if they had a q-value ≤ 0.05 and a b-value ≥ 1 or ≤ −1. All differentially expressed genes present in all conditions were clustered with Gene Cluster 3.0 using correlation (uncentered) average linkage and mapped with Java Treeview 1.1.6r4. Differentially expressed genes were tested for Gene Ontology analysis by DAVID (the Database for Annotation, Visualization, and Integrated Discovery) 6.8 for gene functional classification using a high stringency (Huang et al. 2009); classifications with a Benjamini adjusted P ≤ 0.05 are listed.
In vivo assays
Acute osmotic resistance assays were performed by counting the percentage of worms that responded to gentle touches with a worm pick after 10 min on an agar plate with 500 mM NaCl, as described previously (Wheeler and Thomas 2006). Longevity and juglone resistance assays were performed as described previously (Tang and Choe 2015), except that instead of using floxuridine (FUDR) for longevity studies on high NaCl, adults were manually transferred to fresh plates daily for the first few days to avoid mixing generations. Osmotic survival assays were performed by transferring worms from 51 to 450 mM NaCl agar plates and counting live and dead worms a day later.
Glycerol assays
Glycerol assays were conducted on populations of whole worms lysed by sonication using the PicoProbe Free Glycerol Fluorometric Assay Kit (Biovision). Values were normalized to total protein using the Pierce BCA Protein Assay Kit (ThermoFisher Scientific).
Statistical analysis
Statistical significance was determined using a Student’s t-test when two means were compared, a one-way ANOVA with a Dunnet or Tukey’s post hoc test when three or more means were compared, a log-rank test when survival curves were compared, and a χ2 test for categorical reporter data. Overall P-values of < 0.05 were taken to indicate statistical significance. Bonferroni corrections to P-values were used when more than two survival curves were compared.
Data availability
Strains are available upon request. Raw numeric data are at https://figshare.com/s/7863ed7b6e2bed9e6510 and RNAseq (RNA sequencing) raw data are at the Gene Expression Omnibus (GSE107704).
Results
RNAi screening identifies a specific cuticle structure required for regulation of detoxification, osmolyte, and antimicrobial responses
The cuticle is a complex ECM composed of over 100 distinct collagen proteins secreted from underlying epidermal cells. It forms a hydrostatic skeleton, and acts as the primary barrier and first line of defense against many environmental insults (Johnstone 2000; Alvarez et al. 2007; Burns et al. 2010). The cuticle is composed of multiple layers, some with distinct structures discernable by light and/or electron microscopy. We used dsRNA feeding to disrupt diverse aspects of cuticle or epidermal integrity and test for induction of six stress response gene reporters: osmolyte accumulation (gpdh-1), antimicrobial (nlp-29), detoxification (gst-4), heat shock (hsp-16.2), and mitochondrial and endoplasmic reticulum unfolded protein (hsp-6 and hsp-4, respectively). These stress responses are conserved and well studied in C. elegans (Jones et al. 1989; Shen et al. 2001; Yoneda et al. 2004; Lamitina et al. 2006; Pujol et al. 2008a).
Morphology phenotypes and reporter scores averaged over three trials are shown in Figure 1A, organized by morphology phenotype and strength of reporter induction. Results for the 19 of 40 dsRNA feeding clones that caused the most consistent morphology phenotypes are shown (gray shading), with variation between trials as is common with feeding clones (Zugasti et al. 2016). A test was considered positive if the reporter gene was induced in ≥ 5% of the worm population, in at least two of three trials (white outlined boxes). We report for the first time that silencing of four cuticle/epidermal integrity genes activates gst-4p::GFP, as well as gpdh-1p::DsRed2 and nlp-29p::GFP. We also observed acute osmotic stress resistance when silencing the same genes (Figure 1A, Figure S1A in File S1, and Table S2), a phenotype associated with gpdh-1 induction (Wheeler and Thomas 2006). The hsp-16.2 and hsp-6 reporters were not activated by any clones. Silencing of many other genes caused a diverse range of expected cuticle, morphology, and epidermal phenotypes (e.g., Dumpy, Blister, Roller, Long, and Molting defect), but did not consistently activate gst-4 or gpdh-1 reporters. Together, these results suggest that detoxification and osmolyte responses are activated by silencing of a specific subset of dpy collagens and not by body shape or general epidermis disorganization. Consistent with previous studies (Tong et al. 2009; Ward et al. 2014; Taffoni and Pujol 2015; Zugasti et al. 2016), the nlp-29 reporter was activated by silencing the same specific dpy genes, but was also activated by loss of genes that cause severe disruption of molting or epidermal integrity (mlt-7, bli-4, klf-3, and mua-6) and slightly by loss of dpy-11, which also activated hsp-4.
We also used a COPAS BIOSORT to quantify nlp-29p::GFP fluorescence with RNAi inactivation of 11 dpy genes (Figure 1B and Figure S1 in File S1), nine of which overlap with Figure 1A. These results confirmed strong activation of nlp-29p::GFP with dpy-2, 3, 7, and 10 and lack of strong activation with dpy-11, 13, 5, 1, and 20; they also added dpy-8 and 9 to the list of dpy clones activating nlp-29p::GFP. nlp-29p::GFP induction has also been confirmed in mutants of dpy-9 and 10 (Pujol et al. 2008b; Zugasti et al. 2014). Interestingly, mutants of dpy-2, 3, 7, 8, 9, and 10 were previously found to have no annular furrows but did have other cuticle structures including alae, which are lateral ridges in the cuticle perpendicular to furrows (Cox et al. 1980; McMahon et al. 2003; Thein et al. 2003); morphology and stress response results are summarized for dpy genes in Table S2.
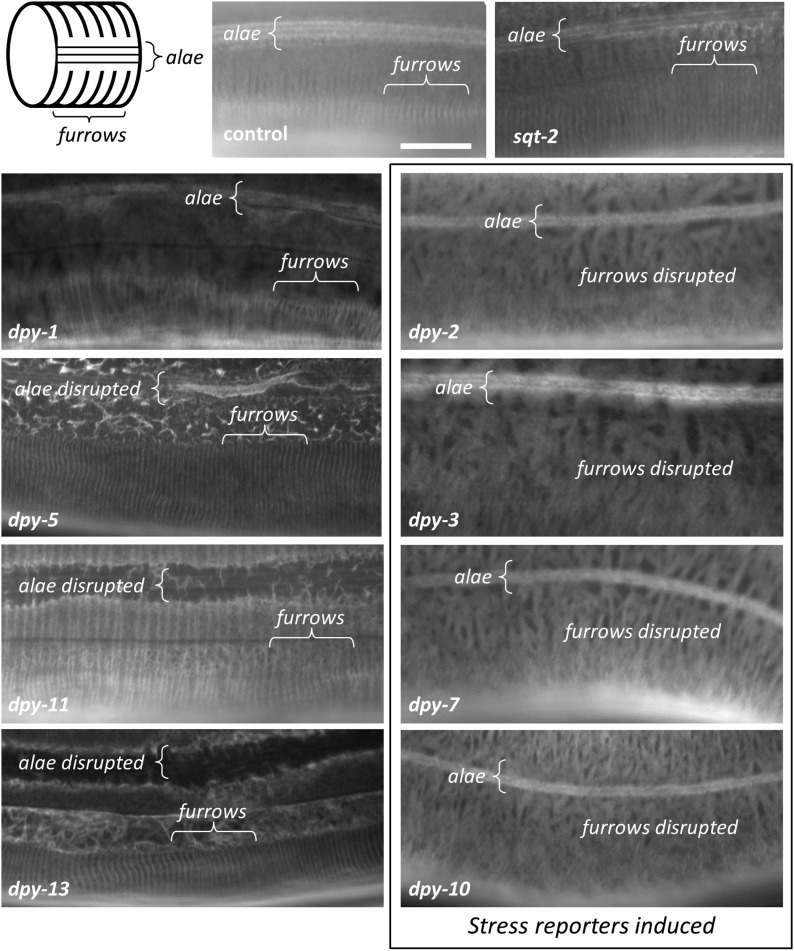
To confirm the role of alae and furrows, we silenced dpy-2, 3, 7, and 10, and five other collagens with morphology phenotypes (dpy-5, 11, 1, 13, and sqt-2), in a strain of worm expressing COL-19::GFP, a collagen marker that labels the matrix of the circumferential transverse annuli and the trilaminate lateral alae (Thein et al. 2003). Similar to prior analysis of mutants, silencing of dpy-2, 3, 7, or 10 completely disrupted the wild-type parallel pattern of furrows without eliminating alae (Figure 2). Conversely, silencing of dpy-5 or 13 eliminated alae without disrupting furrows, and silencing of dpy-1 and sqt-2 did not cause any obvious disruption to either alae or furrows.
Figure 2.
The cuticle furrow is disrupted by dpy double-stranded RNA clones that activate stress responses. A model of cortical cuticle structures and fluorescent micrographs of COL-19::GFP in worms treated with RNA interference. Images are representative of 10 worms. Bar, 10 μM.
Silencing dpy-11 caused partial irregular branching of furrows and eliminated alae. Interestingly, it was only with dpy-11 RNAi that we observed activation of the endoplasmic reticulum stress response reporter (hsp-4) together with weak activation of the antimicrobial reporter (nlp-29) (positive in two out of three visual scoring trials and a nonsignificant 1.4-fold increase in BIOSORT quantification). Unlike most of the other Dpy genes tested, dpy-11 does not encode a collagen but rather a nematode-specific protein with a thioredoxin domain (pfam00085). It is exclusively expressed in epidermal cells, where is has been suggested to be localized to the endoplasmic reticulum or Golgi apparatus (Ko and Chow 2002). It may be required for the maturation of cuticle collagens, but could also be involved in post-translational modification of other substrates, including signaling molecules linked to a distinct cellular stress pathway.
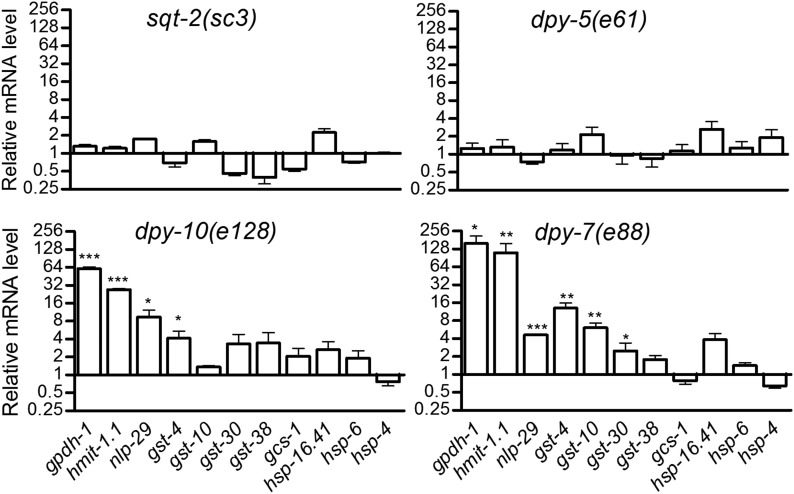
We next used qPCR with two collagen mutants with disrupted furrows [dpy-10(e128) and dpy-7(e88)], one with intact furrows [dpy-5(e61)], and one reported to have more general alae and furrow disruption [sqt-2(sc3)] (Thein et al. 2003). As shown in Figure 3, gpdh-1, gst-4, and nlp-29 were induced in dpy-10 and dpy-7 worms, but not in sqt-2 or dpy-5 worms, similar to the RNAi results (Figure 1). Another osmotic response gene (hmit-1.1) (Kage-Nakadai et al. 2011) was also induced only in dpy-10 and dpy-7 worms, and detoxification response genes (gst-10 and gst-30) were induced in dpy-7 worms. Other stress responsive genes that we tested were not induced by any of the mutations (Figure 3).
Figure 3.
Quantitative PCR verification of stress response gene expression. mRNA levels for stress-inducible genes in four collagen mutants. n = 3–9 replicates of worms combined from one or two trials. * P < 0.05, ** P < 0.01, and *** P < 0.001 relative to N2, which was normalized to a mean of 1.0.
Taken together, Figure 1, Figure 2, and Figure 3 suggest that specific loss of annular furrows, and not alteration of body shape, alae, or general epidermal integrity, initiates a signal that coactivates osmolyte accumulation, antimicrobial, and detoxification responses, but not all stress responses. The antimicrobial nlp-29 reporter also responded to detachment of the cuticle (blister phenotype, bli-4 and mlt-7), hemidesmosome disruption (mua-6), disruption of muscle/cuticle attachments (klf-3), and disruption of fatty acid metabolism (acs-3 and fasn-1) (Lee et al. 2010; Ward et al. 2014); the nlp-29 reporter also responded weakly to loss of thioredoxin domain-encoding gene dpy-11. This is consistent with nlp-29 being activated by a broad range of signals affecting epidermal integrity (Pujol et al. 2008a; Zugasti et al. 2016).
Given that furrow mutants accumulate high levels of glycerol (Lamitina et al. 2006; Wheeler and Thomas 2006), we speculated that detoxification, antimicrobial, and osmolyte accumulation stress responses could be induced in response to high internal osmolarity. As expected, dpy-7(RNAi) strongly induced glycerol accumulation in wild-type worms (Figure S1B in File S1). Deletions in both gpdh genes (gpdh-1 and 2) reduced glycerol accumulation by dpy-7(RNAi) almost completely, i.e., by 87% (Figure S1B in File S1). When measured by qPCR, dpy-7(RNAi) was able to significantly increase gst-4, nlp-29, and hmit-1.1 mRNA levels in gpdh knockout worms, albeit by a reduced relative amount compared to wild-type in part because of elevated basal levels (Figure S1C in File S1). Therefore, loss of furrows can activate stress responses without the majority of glycerol accumulation.
Role of skn-1 in gene expression during osmotic stress and in dpy-7 mutants
The transcription factor SKN-1 was previously reported to be important for detoxification responses under basal conditions and during oxidative stress (Oliveira et al. 2009; Park et al. 2009). We addressed the role of SKN-1 in dpy-7(e88) mutants and worms exposed to 300 mM NaCl for 3 or 24 hr with RNAseq. We also reanalyzed a previously published RNAseq data set for skn-1(RNAi) under basal conditions (Steinbaugh et al. 2015). Genes upregulated by dpy-7(e88) or osmotic stress were similar to those from previous studies of dpy-10 mutants and osmotic stress (Rohlfing et al. 2010), and include osmolyte accumulation, pathogen response, and detoxification response (Table S3). As expected, skn-1(RNAi) reduced expression of many phase II detoxification genes (Table S3).
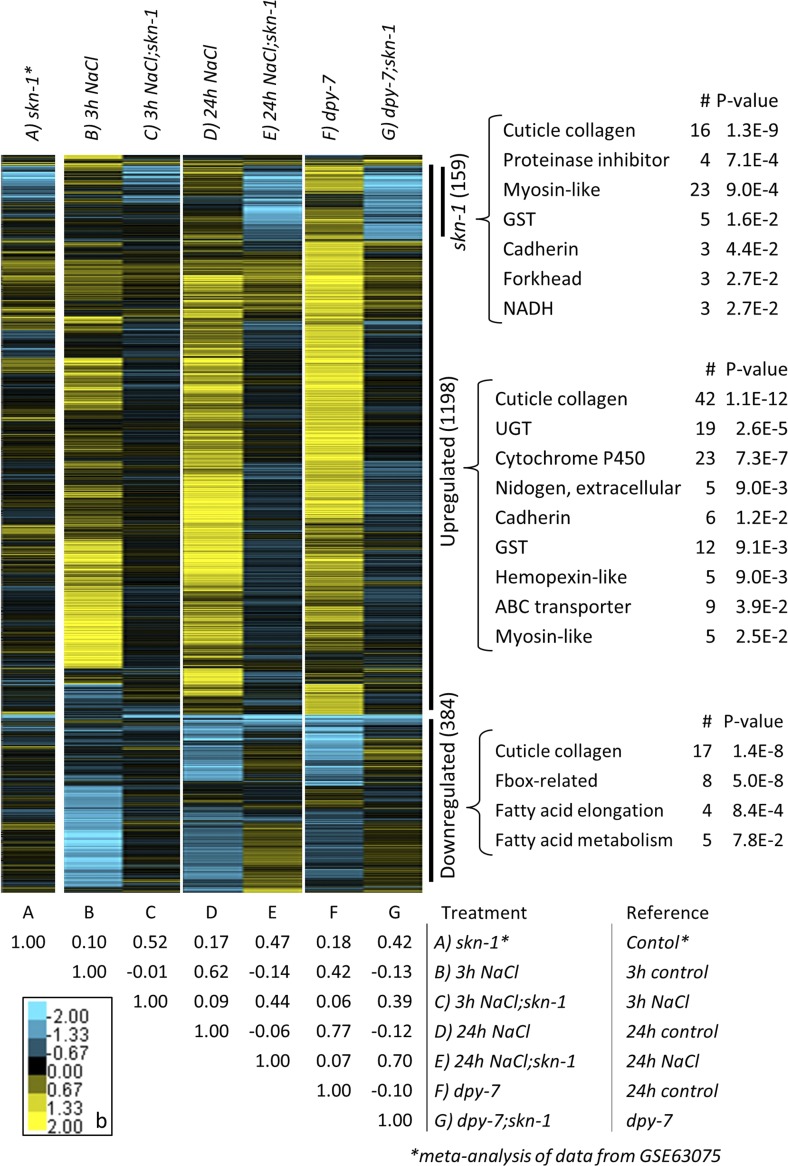
A heat map of genes differentially expressed in at least one of the seven comparisons and clustered by expression is shown in Figure 4, with a table of correlation coefficients below (values are in Table S4 and Table S5 and Gene Expression Omnibus, GSE107704). The longer 24-hr exposure to 300 mM NaCl had similar effects on expression as dpy-7(e88) (correlation coefficient of 0.77), and they both upregulated more genes than they downregulated. The shorter 3-hr exposure to 300 mM NaCl had a smaller effect. The cluster of genes upregulated by high NaCl and dpy-7(e88) is enriched for structural functions (cuticle collagen, nidogen, cadherin, and myosin-like) and detoxification [UGT (glycosyltransferase), cytochrome P450, GST (glutathione S-transferase), and ABC transporters, Figure 4]. The cluster of genes downregulated by 300 mM NaCl and dpy-7(e88) is enriched for cuticle collagen, F-box, and fatty acid metabolism. These data are consistent with gene expression responses to compensate for osmotically-induced mechanical stress and changes to metabolism.
Figure 4.
Heat map of differentially expressed genes clustered by relative expression change. All 1594 genes that were differentially expressed in at least one of the seven comparisons and present in all conditions tested are included. Average sleuth analysis estimates of expression effect size for each comparison (“b”) are provided on a log2 scale from decreased (blue) to increased (yellow). Correlation coefficients are provided below the heat map. GSE63075 data from N2 with and without skn-1(RNAi) (Steinbaugh et al. 2015) are included. Gene functional category enrichments are from the Database for Annotation, Visualization, and Integrated Discovery (DAVID) 6.8. n = 3 replicates of worms per treatment.
Using our analysis pipeline applied to a previously published data set, skn-1(RNAi) only downregulated 20 genes under basal conditions; skn-1(RNAi) only downregulated 1.2% of the genes upregulated by 3 hr of 300 mM NaCl (Table S3). Alternatively, skn-1(RNAi) downregulated 4.1 and 12.1% of the genes upregulated by 24 hr NaCl and dpy-7(e88), respectively. These data indicate that SKN-1 plays a larger role in gene regulation during chronic high NaCl and furrow loss than under basal conditions and short-term high-NaCl exposure. The cluster of genes most strongly downregulated by skn-1(RNAi) in the heat map are enriched for detoxification and structural functions (top of Figure 4), which is similar to enrichment within all genes upregulated by 24-hr 300 mM NaCl and dpy-7(e88). It is also clear in the heat map that many genes are induced independently of skn-1.
In Figure S2 in File S1, we present Sleuth b and q-values for core genes of the six stress responses screened in Figure 1. Similar to the reporter data, dpy-7 significantly activated genes of the osmotic, antimicrobial, and detoxification responses without activating other stress responses. Exposure to 300 mM NaCl had similar effects, particularly at 24 hr. As expected, skn-1(RNAi) reduced induction of many phase II detoxification genes, particularly within the gst gene class. Surprisingly, expression of gpdh-1 and hmit-1.1 osmolyte accumulation genes were partially decreased by skn-1(RNAi) in dpy-7(e88). Upregulation of some antimicrobial genes was actually slightly enhanced by skn-1(RNAi) at 24-hr NaCl and in dpy-7(e88). Lastly, there were also many ugt and a few gst genes that were induced regardless of skn-1(RNAi), suggesting independent or compensatory mechanisms of regulation.
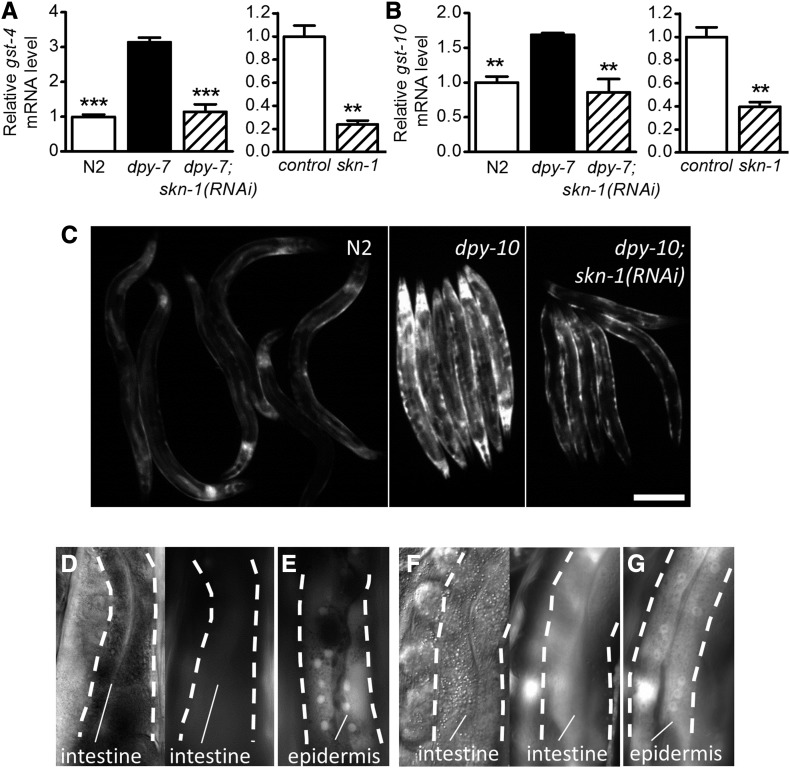
Annular furrow defects activate detoxification genes in the epidermis via SKN-1
Members of the gst gene class are well-established targets of SKN-1, and qPCR confirmed the requirement of skn-1 for expression of gst-4 and gst-10 in dpy-7(e88) worms (Figure 5, A and B); these two direct targets are also under the control of skn-1 in wild-type worms (Figure 5, A and B). As shown in Figure 5C, a gst-4p::GFP reporter was activated in dpy-10(e128) worms in a skn-1-dependent manner. We next used the gst-4p::GFP reporter and another SKN-1 target reporter, gst-10p::GFP, to identify the tissues in which detoxification genes were induced. In dpy-10(e128) and dpy-7(RNAi) worms, gst-4p::GFP and gst-10p::GFP fluorescence was predominantly visible in epidermal cells (Figure 5, D–G). This contrasts with the robust SKN-1 activation observed in the intestine during oxidative stress (An and Blackwell 2003; Kell et al. 2007; Kahn et al. 2008; Choe et al. 2009). Exposure of dpy-7(e88) worms to acrylamide, a strong SKN-1 inducer, activated gst-4p::GFP strongly in the intestine (Figure S3B in File S1), indicating that the intestinal detoxification response is still intact when furrows are disrupted.
Figure 5.
Disruption of cuticle annular furrows activates skn-1-dependent detoxification genes in the epidermis. (A and B) mRNA levels for gst-4 and gst-10 with and without skn-1(RNAi) in dpy-7(e88) worms (left graphs); results for skn-1(RNAi) in N2 worms are included for reference (right graphs). ** P < 0.001 and *** P < 0.001 relative to dpy-7 or control. (C) gst-4p::GFP fluorescence images in N2, dpy-10(e128), and dpy-10(e128); skn-1(RNAi) worms. (D and E) gst-4p::GFP in dpy-10(e128) worms; images taken at the focal plane of the intestine are paired with a differential interference contrast micrograph. (F and G) gst-10p::GFP in dpy-7(RNAi) worms; images taken at the focal plane of the intestine are paired with a differential interference contrast micrograph. (D–G) Broken lines mark the boundaries for the intestine (left) or epidermis (right). RNAi, RNA interference.
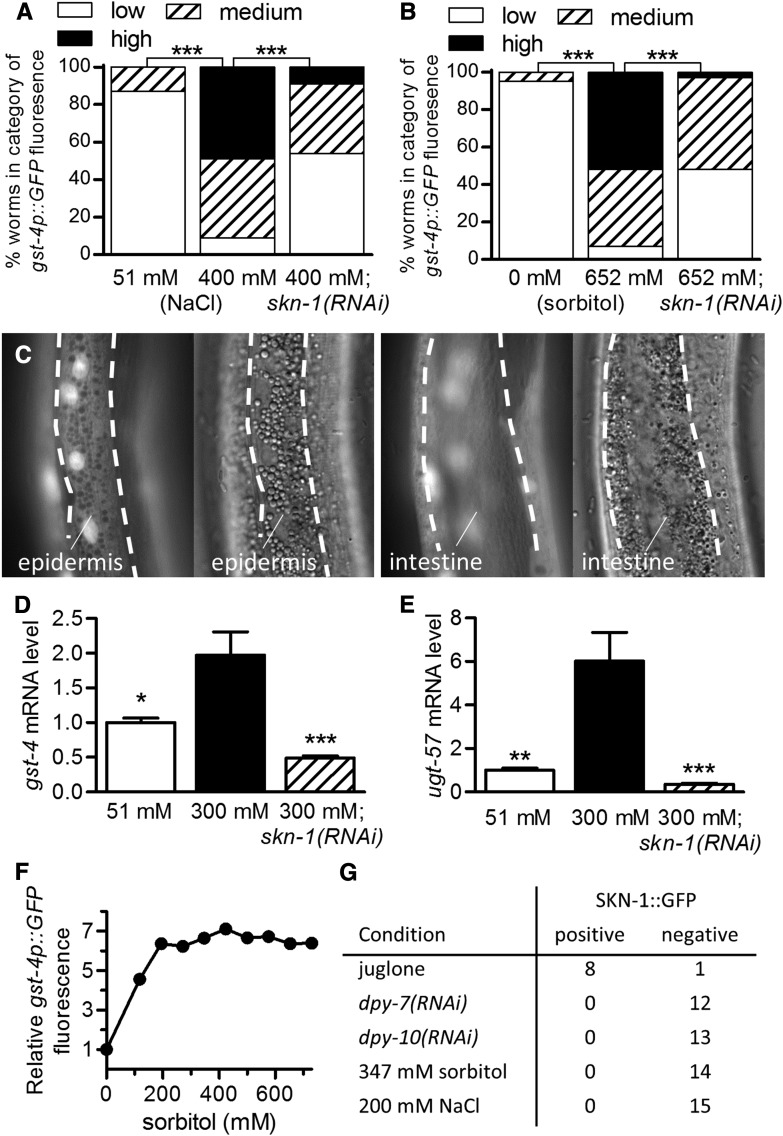
High external osmolarity activates skn-1-dependent detoxification genes in the epidermis
Given the similar transcriptional profiles observed with high NaCl and dpy-7(e88) (Figure 4), we next addressed the role of SKN-1 in gene regulation upon osmotic stress. As shown in Figure 6, A and B, high concentrations of NaCl or sorbitol induced gst-4p::GFP by a skn-1-dependent mechanism. Similar to dpy-7 and dpy-10 worms, the reporter was predominantly induced in the epidermis (Figure 6C). qPCR confirmed induction of gst-4 and another detoxification gene, ugt-57, via skn-1 in worms exposed to 300 mM NaCl for 3 hr (Figure 6, D and E). Using a fluorescent plate reader, we observed induction of gst-4p::GFP by as little as 118 mM sorbitol (Figure 6F), a level that has no obvious effects on worm health in our hands.
Figure 6.
High osmolarity activates skn-1-dependent detoxification genes in the epidermis. gst-4p::GFP is induced in worms exposed to 400 mM NaCl (A) or 652 mM sorbitol (B), and suppressed by skn-1(RNAi) (*** P < 0.001); low (dim signal limited to a few spots), medium (dim signal throughout the epidermis or bright signal only in head or tail regions), and high (bright signal throughout the epidermis). (C) Paired fluorescent and differential interference contrast images of the intestine (left) and epidermis (right) of a worm exposed to 400 mM NaCl. (D and E) Relative gst-4 and ugt-57 mRNA levels in worms exposed to 300 mM NaCl for 3 hr with and without skn-1(RNAi) (* P < 0.05, ** P < 0.01, and *** P < 0.001 vs. 300 mM, n = 4–5 populations of worms). (F) gst-4p::GFP fluorescence levels in worms exposed to a range of sorbitol concentrations overnight. n = 16 microplate wells. (G) Number of worms with and without nuclear SKN-1::GFP in the head region observed with confocal microscopy. No SKN-1::GFP was observed in the intestine under any conditions.
The skn gene generates three different transcripts (skn-1a, b, and c) that share a common C-terminus but with alternative start sites, and our dsRNA clone targets all three. gst-4 mRNA was fully induced by 300 mM NaCl and dpy-7(RNAi) in a recently engineered strain with a stop codon introduced into the first skn-1a-specific exon (Figure S4A in File S1), consistent with either skn-1b or c functioning in these contexts. Although some prooxidants and genetic manipulations that induce the expression of skn-1-dependent genes provoke nuclear accumulation of SKN-1b/c::GFP, there are also many conditions in which skn-1-dependent genes are induced without visible nuclear accumulation (An and Blackwell 2003; Kahn et al. 2008; Wu et al. 2016). These results are consistent with mechanisms that can increase gene expression via SKN-1 without increasing nuclear levels above what already exists under basal conditions. When accumulation does occur, it is most easily observed in the intestine and epidermis (Wu et al. 2016). We used worms expressing a SKN-1b/c::GFP fusion protein to determine if furrow disruption or high osmolarity cause nuclear accumulation of the transcription factor. We counted the number of worms with visible SKN-1b/c::GFP fluorescence when treated with dpy-7(RNAi), dpy-10(RNAi), 347 mM sorbitol, or 200 mM NaCl (Figure 6G). We focused on the head epidermis, where gst-4p::GFP fluorescence is highest in furrow mutants (Figure 5). As shown in Figure 6G, we observed accumulation of SKN-1b/c::GFP only with the positive control prooxidant juglone.
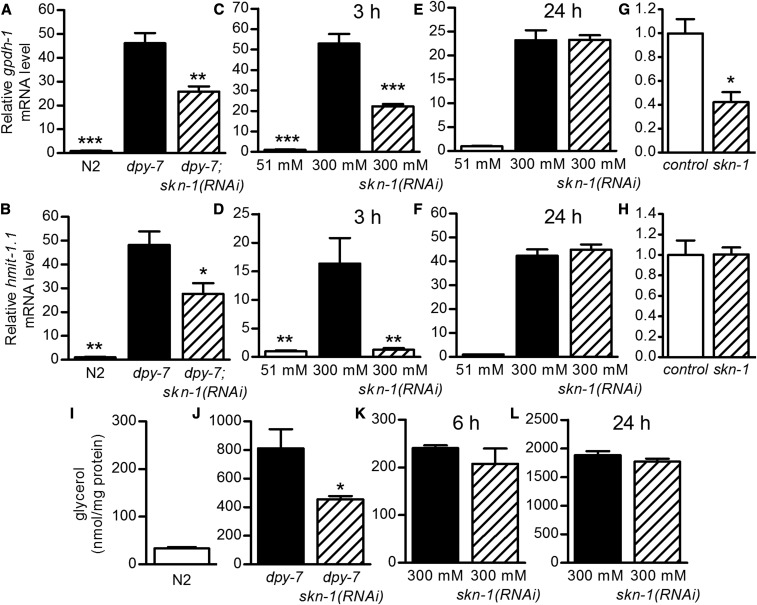
Osmolyte accumulation gene induction is partially dependent on skn-1
As mentioned above, our transcriptional analyses show that skn-1(RNAi) reduced the expression of some osmolyte accumulation genes after exposure to high concentrations of NaCl and in dpy-7 worms (Figure 4). This represents a novel function for SKN-1. To confirm these effects, we used qPCR and found that gpdh-1 and hmit-1.1 expression was partially, or fully, dependent on skn-1 in worms exposed to 300 mM NaCl for 3 hr and in dpy-7 worms (Figure 7, A–D). On the other hand, skn-1(RNAi) had no effect on gpdh-1 or hmit-1.1 expression after 24 hr on 300 mM NaCl corresponding to a time when glycerol levels approach a steady state (Figure 7, E and F) (Lamitina et al. 2004). Under basal conditions in wild-type worms, gpdh-1, but not hmit-1.1, was partially dependent on skn-1 (Figure 7, G and H).
Figure 7.
Induction of osmolyte synthesis genes partially requires skn-1. (A and B) Relative gpdh-1 and hmit-1.1 mRNA levels in dpy-7(e88) worms with and without skn-1(RNAi). (C–F) Relative gpdh-1 and hmit-1.1 mRNA levels in worms exposed to 300 mM NaCl for 3 hr (C and D) or 24 hr (E and F) with and without skn-1(RNAi). (G and H) Relative gpdh-1 and hmit-1.1 mRNA levels in N2 with and without skn-1(RNAi) [* P < 0.05 vs. dpy-7 (A and B), 300 mM NaCl (C and D), or control (G and H); n = 3–4 replicates of worms]. (I and J) Whole-worm glycerol levels in N2 and dpy-7(e88) worms with and without skn-1(RNAi). n = 7–9 replicates from two trials. * P < 0.05. (K and L) Whole-worm glycerol levels in worms exposed to 300 mM NaCl for 6 (K) or 24 hr (L) with and without skn-1(RNAi). n = 3–5 replicates of worms from one trial.
We next measured whole-worm glycerol levels in dpy-7 worms and worms exposed to 300 mM NaCl for 6 or 24 hr, because previous studies reported high glycerol accumulation rates at 6 hr and an elevated steady-state glycerol level at 24 hr (Lamitina et al. 2004). As expected, glycerol was dramatically elevated by dpy-7 mutation and high osmolarity (Figure 7, I–L). Loss of skn-1 partially reduced glycerol levels in dpy-7 worms (Figure 7J), but not in worms exposed to high osmolarity (Figure 7, K and L). Taken together, these data indicate that in worms with disrupted annular furrows, skn-1 is partially required for the expression of osmotic-responsive genes and accumulation of glycerol. skn-1 also plays a role in the initial induction of osmolyte accumulation genes by high osmolarity, but without a measurable effect on total glycerol levels.
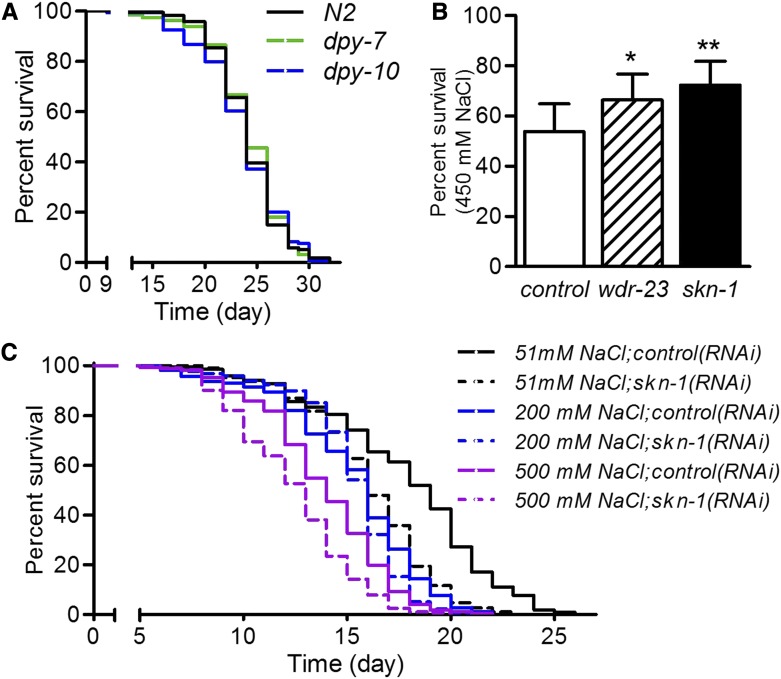
Physiological assays with skn-1
We next tested if longevity or resistance to a prooxidant was altered in dpy-10 or dpy-7 worms as might be expected with constitutive activation of detoxification genes. As shown in Figure 8A, dpy-7 and dpy-10 worms had life spans that were very similar to wild-type, and both were actually hypersensitive to the naturally occurring reactive small molecule juglone compared to wild-type worms (Figure S4B in File S1). The cuticle is the nematode’s primary barrier and its disruption is known to increase sensitivity to diverse small molecules (Partridge et al. 2008). The sensitivity of dpy-7 and dpy-10 worms is consistent with barrier disruption, and this was also observed for dpy-5 mutants (Figure S4B in File S1) that do not exhibit an elevated level of gst gene expression (Figure 3). Inactivation of skn-1 further decreased survival (Figure S4B in File S1), consistent with SKN-1 contributing generally to protection against oxidants.
Figure 8.
Physiological assays for skn-1. (A) Survival curves for longevity. P > 0.7095 for dpy-7 and dpy-10 relative to N2. n > 150 worms from two trials combined. (B) Survival, after 24 hr, of skn-1 and wdr-23(RNAi) worms transferred directly from 51 to 450 mM NaCl agar. ** P < 0.05 and ** P < 0.01 relative to control(RNAi), n = 4 trials of 30–581 worms. (C) Survival of chronic exposure to high NaCl. All worms were treated from L1 larval stage except for 500 mM NaCl; these worms were grown on 200 mM NaCl until young adults and then transferred to 500 mM NaCl. n = 234–342 worms from three trials. P < 0.001 for skn-1(RNAi) at 51 and 500 mM NaCl, and P = 0.0254 for skn-1(RNAi) at 200 mM NaCl.
We next conducted experiments to explore the influence of SKN-1 on survival in the presence of high NaCl. We first tested the effects of SKN-1 loss and activation using skn-1 and wdr-23(RNAi), respectively, on the ability of young adult worms to survive for a day after direct transfer from standard growth media containing 51 mM NaCl to media containing 450 mM NaCl. WDR-23 is a direct and robust repressor of SKN-1 (Choe et al. 2009). Both skn-1 and wdr-23 RNAi increased survival under these conditions, although the effects were small (Figure 8B). These effects suggest that SKN-1 manipulation has complex effects in survival upon acute hyperosmotic exposure that might include compensatory responses.
We also tested the effect of skn-1(RNAi) on longevity under conditions of chronic high NaCl. It was recently demonstrated that high NaCl can increase longevity of C. elegans, but only in the presence of the DNA synthesis inhibitor 5-fluorodeoxyuridine, which is commonly used to prevent growth of progeny (Anderson et al. 2016). To circumvent this complication, we avoided progeny in these experiments by manually transferring adult worms to new plates. Life span was measured at 51 mM NaCl, 200 mM NaCl, and 500 mM NaCl. Worms had to first be grown on 200 mM NaCl before transferring to 500 mM at the early adult stage to allow larval development. Loss of skn-1 decreased life span at 500 mM, but this was similar to the effect on standard 51 mM media (Figure 8C), consistent with SKN-1 promoting life span in many contexts.
Distinct, but overlapping, transcription factors function downstream from furrow disruption
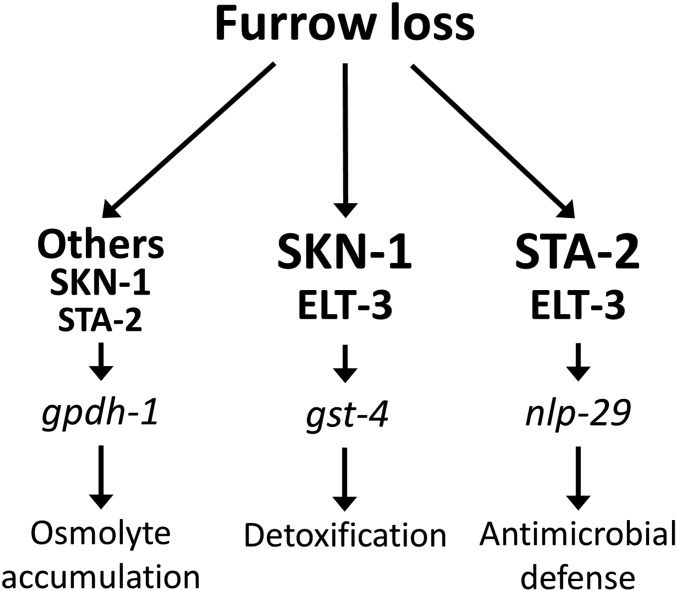
Transcriptional control of gpdh-1 and nlp-29 upon osmotic challenge in the C. elegans epidermis has been reported to be dependent on the ELT-3 GATA transcription factor (Pujol et al. 2008b; Rohlfing et al. 2010). nlp-29 also depends on the STAT transcription factor-like protein STA-2 during infection and wounding, but not high NaCl (Dierking et al. 2011). ELT-3 has been postulated to cooperate with a number of transcription factors, including SKN-1 and STA-2, to permit stress responses in the epidermis (Block and Shapira 2015). We tested the effects of RNAi for elt-3, sta-2, and skn-1 on gpdh-1, gst-4, and nlp-29 reporter induction in dpy-7 furrow mutants (Figure 9). Note that while gst-4p::GFP and nlp-29p::GFP are primarily active in the epidermis in this context, gpdh-1p::DsRed2 is active in the intestine and epidermis, and fluorescence levels are from whole worms. RNAi of skn-1 decreased gpdh-1p::DsRed2 and gst-4p::GFP confirming a shared requirement; skn-1(RNAi) actually slightly increased nlp-29p::GFP (Figure 9), which was also observed for nlp-29 mRNA in RNAseq analysis (Figure S2 in File S1). RNAi of elt-3 decreased gst-4p::GFP and nlp-29p::GFP significantly, but did not have a significant effect on total gpdh-1p::DsRed2 fluorescence (Figure 9). RNAi of sta-2 decreased gpdh-1p::DsRed2 and nlp-29p::GFP, but not gst-4p::GFP. Using mutants in dpy-3, dpy-7, and dpy-10 worms, we confirmed the requirements of sta-2 and elt-3 for nlp-19p::GFP (Figure S4C in File S1). Collectively, the results in Figure 9 and Figure S4 in File S1 suggest that disruption of furrows in the cuticle activates osmotic, detoxification, and antimicrobial transcriptional responses via distinct, but overlapping, downstream transcription factors (Figure 10).
Figure 9.
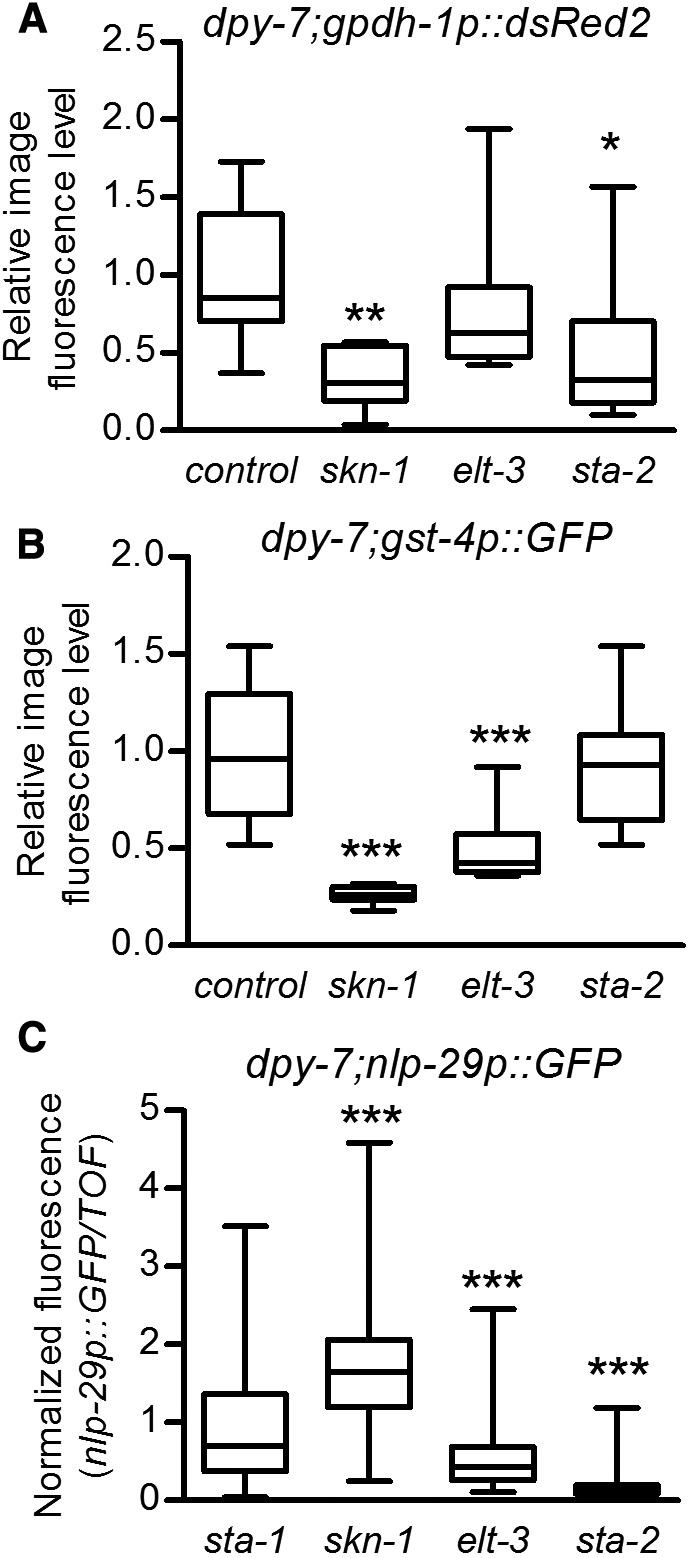
Transcription factor requirements. (A and B) Worms grown to the gravid adult stage were mounted on slides and imaged. Image J was used to measure pixel intensity of individual worms on the GFP (A) or red fluorescent protein RFP (B) filter sets and values were normalized to the mean of control(RNAi). n = 10 worms. (C) The ratio of nlp-29p::GFP to time of flight was measured in a BIOSORT and normalized to the negative control sta-1(RNAi). n = 95–259. Boxes are 25% percentiles above and below the median and whiskers are minimum and maximum. * P < 0.05, ** P < 0.01, and *** P < 0.001 vs. control(RNAi).
Figure 10.
Working model for cuticle annular furrow regulation of stress responses. Disruption of annular furrows in the cuticle initiates signals that are transduced to nlp-29, gpdh-1, and gst-4 via different sets of transcription factors.
Discussion
Coregulation of three stress responses via cuticle furrows
The cortical layer of the adult C. elegans cuticle contains lateral ridges of collagen called alae and circumferential bands called annular furrows. Annular furrows were previously implicated in regulation of gpdh-1 from a genome-wide RNAi screen (Lamitina et al. 2006; Wheeler and Thomas 2006; Choe 2013). Two collagen mutants with furrow defects were also previously shown to have high nlp-29 expression (Pujol et al. 2008b), but it was unknown if other cuticle/epidermal characteristics and stress responses were involved.
Mutation or silencing of six collagen genes (dpy-2, 3, 7, 8, 9, and 10) severely disrupts furrows without eliminating alae (Cox et al. 1980; McMahon et al. 2003; Thein et al. 2003) (Figure 2 and Table S2). We demonstrate that genetic manipulations that specifically disrupt furrows coactivate osmolyte accumulation, nlp-29, and skn-1-dependent detoxification genes, but do not activate responses to heat shock, mitochondrial unfolded proteins, or endoplasmic reticulum unfolded proteins (Figure 1, Figure 2, Figure 3, and Figure S2 in File S1). Therefore, our results are consistent with a requirement for annular furrows in regulating osmolyte accumulation, antimicrobial, and skn-1-dependent detoxification genes, but not all stress responses.
The cuticle and underlying epidermis form the primary barrier between tissues and the environment, and are therefore well positioned to detect and respond to stress. Recent atomic force microscopy analyses revealed high biomechanical stiffness at furrows relative to annuli (Essmann et al. 2017). Nematode body morphology is supported by turgor pressure against the body wall. Hypertonicity-induced water loss depletes turgor pressure in C. elegans, and the epidermis and cuticle become physically distorted (Lamitina et al. 2004; Choe 2013). Given that furrows are circumferential bands of collagen in the cuticle, we speculate that mechanical strain on these structures likely changes with turgor pressure against the body wall. Physical distortion of furrows or changes in associated extracellular ligand availability could signal via membrane proteins such as integrins, G protein-coupled receptors, phospholipases, enzyme-linked receptors, or ion channels (Clause and Barker 2013; Ross et al. 2013; Schiller and Fassler 2013; Gasparski and Beningo 2015). Interestingly, loss of a protein secreted by the epidermis named OSM-11 coactivates osmolyte synthesis, detoxification, and antimicrobial genes (Wheeler and Thomas 2006; Pujol et al. 2008b; Dresen et al. 2015) without causing any obvious cuticle defects. OSM-11 is thought to act as a ligand for Notch receptors to regulate vulva development and behavior (Komatsu et al. 2008; Singh et al. 2011). It remains to be seen if Notch signaling plays a role in transmitting signals from the cuticle. It will also be interesting to examine whether hypoosmotic-induced increases in turgor pressure are also able to initiate signals to stress responses.
A G protein-coupled receptor named DCAR-1 is partially required for induction of nlp-29 in the furrow mutants dpy-9 and dpy-10, but was not required for transcriptional activation of nlp-29 upon osmotic stress (Zugasti et al. 2014). These results suggest that different receptors might be activated downstream from a common furrow-associated sensor.
Distinct transcription factor requirements downstream of furrow disruption
In yeast, at least four transcription factors downstream from an osmosensor-associated protein named HOG1 (hyperosmolarity glycerol response 1) activate downstream genes (Hohmann et al. 2007). In mammalian cells, the hypertonicity enhancer-binding protein (TonEBP), also known as NFAT5, regulates a battery of genes responsible for organic osmolyte accumulation and cytoprotection (Lee et al. 2011). C. elegans lacks a close homolog of TonEBP, and instead GATA transcription factor elt-3 is at least partially required for gpdh-1 and nlp-29 induction by high NaCl in the epidermis (Pujol et al. 2008b; Rohlfing et al. 2010).
Our results are consistent with distinct, but partially overlapping, transcriptional pathways functioning downstream from furrow loss to activate distinct stress responses (Figure 10). Interestingly, elt-3, in addition to skn-1, is required for the detoxification response to furrow loss (Figure 9). Similarly, elt-3 and skn-1 were recently shown to coregulate detoxification genes in a stress-sensitive mutant (Hu et al. 2017). Conversely, sta-2 is completely required and elt-3 is partially required for the antimicrobial response in the epidermis (Figure 9 and Figure S4B in File S1). We found evidence of a novel role for skn-1 in induction of gpdh-1 (Figure 7 and Figure 9). SKN-1-binding elements were previously defined (Blackwell et al. 1994; Rupert et al. 1998), but we found none within 3-kb upstream of gpdh-1 nor hmit-1.1 start codons, suggesting indirect regulation.
SKN-1-dependent gene induction
Regulation of intracellular SKN-1/Nrf signaling in response to reactive small molecules is well studied in mammalian cells and C. elegans (Taguchi et al. 2011; Niture et al. 2013; Blackwell et al. 2015; Wu et al. 2016, 2017). Conversely, very little is known about regulation of SKN-1/Nrf pathways via the ECM. A lack of obvious increases in nuclear accumulation (Figure 6G) is consistent with regulation by post-translational modification or by changes in interaction with DNA or cofactors.
Antioxidant/detoxification pathways protect tissues from inflammation and fibrosis, making coordination of Nrf2 signaling and the ECM important to disease pathogenesis (Wu et al. 2015; Ahmed et al. 2017; Xu et al. 2017). In cultured mammalian vascular cells, Nrf2-dependent responses have been shown to be activated by mechanical shear stress that models turbulent blood flow, with intracellular reactive oxygen species (ROS) being implicated as a downstream signal (Jones et al. 2007; Warabi et al. 2007; Hsieh et al. 2014). It is not known how the extracellular mechanical stimulus is detected and transduced into the cell where ROS are generated. It was recently shown that a skn-1-dependent detoxification response could be activated by an increase in ROS at the ER via sulfenylation of the kinase IRE-1 (Hourihan et al. 2016). It remains to be seen if ROS are increased in furrow mutants through the action of an endogenous enzyme. Signaling mechanisms that act downstream of annular furrow disruption to activate SKN-1-mediated transcriptional responses will define a novel mode of signaling for this conserved family of stress and longevity factors.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.118.300827/-/DC1.
Acknowledgments
We thank Jonathan Ewbank for comments on the manuscript, Jerome Belougne for Biosort analyses, and Gary Ruvkun for sharing the skn-1a(mg570) mutant. C. elegans strains were provided by the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN) supported by the National Institutes of Health Office of Research Infrastructure Programs (P40 OD-010440). This study was supported by National Science Foundation grants IOS-1120130 and IOS-1452948 to K.P.C., an NSERC postdoctoral fellowship to C.-W.W., and by institutional grants from the Aix-Marseille university, the Institut national de la santé et de la recherche médicale and the Centre national de la recherche scientifique and grants ANR-11-LABX-0054 (Investissements d’Avenir–Labex INFORM) and ANR-11-IDEX-0001-02 (Investissements d’Avenir–A*MIDEX) to N.P. All authors participated in conducting experiments and analyzing and interpreting data. K.P.C. and N.P. wrote the manuscript. All authors approved the final version of the manuscript.
Footnotes
Communicating editor: B. Goldstein
Literature Cited
- Ahmed S. M., Luo L., Namani A., Wang X. J., Tang X., 2017. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim. Biophys. Acta 1863: 585–597. 10.1016/j.bbadis.2016.11.005 [DOI] [PubMed] [Google Scholar]
- Alvarez L. I., Mottier M. L., Lanusse C. E., 2007. Drug transfer into target helminth parasites. Trends Parasitol. 23: 97–104. 10.1016/j.pt.2007.01.003 [DOI] [PubMed] [Google Scholar]
- An J. H., Blackwell T. K., 2003. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 17: 1882–1893. 10.1101/gad.1107803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E. N., Corkins M. E., Li J. C., Singh K., Parsons S., et al. , 2016. C. elegans lifespan extension by osmotic stress requires FUdR, base excision repair, FOXO, and sirtuins. Mech. Ageing Dev. 154: 30–42. 10.1016/j.mad.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T. K., Bowerman B., Priess J. R., Weintraub H., 1994. Formation of a monomeric DNA binding domain by SKN-1 bZIP and homeodomain elements. Science 266: 621–628. 10.1126/science.7939715 [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Steinbaugh M. J., Hourihan J. M., Ewald C. Y., Isik M., 2015. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic. Biol. Med. 88: 290–301. 10.1016/j.freeradbiomed.2015.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block D. H., Shapira M., 2012. GATA transcription factors as tissue-specific master regulators for induced responses. Worm. 4(4):e1118607 10.1080/21624054.2015.1118607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan H. K., Olayanju A., Goldring C. E., Park B. K., 2013. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 85: 705–717. 10.1016/j.bcp.2012.11.016 [DOI] [PubMed] [Google Scholar]
- Burns A. R., Wallace I. M., Wildenhain J., Tyers M., Giaever G., et al. , 2010. A predictive model for drug bioaccumulation and bioactivity in Caenorhabditis elegans. Nat. Chem. Biol. 6: 549–557. 10.1038/nchembio.380 [DOI] [PubMed] [Google Scholar]
- Chisholm A. D., Xu S., 2012. The Caenorhabditis elegans epidermis as a model skin. II: differentiation and physiological roles. Wiley Interdiscip. Rev. Dev. Biol. 1: 879–902. 10.1002/wdev.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe K. P., 2013. Physiological and molecular mechanisms of salt and water homeostasis in the nematode Caenorhabditis elegans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305: R175–R186. 10.1152/ajpregu.00109.2013 [DOI] [PubMed] [Google Scholar]
- Choe K. P., Przybysz A. J., Strange K., 2009. The WD40 repeat protein WDR-23 functions with the CUL4/DDB1 ubiquitin ligase to regulate nuclear abundance and activity of SKN-1 in Caenorhabditis elegans. Mol. Cell. Biol. 29: 2704–2715. 10.1128/MCB.01811-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe K. P., Leung C. K., Miyamoto M. M., 2012. Unique structure and regulation of the nematode detoxification gene regulator, SKN-1: implications to understanding and controlling drug resistance. Drug Metab. Rev. 44: 209–223. 10.3109/03602532.2012.684799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clause K. C., Barker T. H., 2013. Extracellular matrix signaling in morphogenesis and repair. Curr. Opin. Biotechnol. 24: 830–833. 10.1016/j.copbio.2013.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. N., Laufer J. S., Kusch M., Edgar R. S., 1980. Genetic and phenotypic characterization of roller mutants of Caenorhabditis elegans. Genetics 95: 317–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierking K., Polanowska J., Omi S., Engelmann I., Gut M., et al. , 2011. Unusual regulation of a STAT protein by an SLC6 family transporter in C. elegans epidermal innate immunity. Cell Host Microbe 9: 425–435. 10.1016/j.chom.2011.04.011 [DOI] [PubMed] [Google Scholar]
- Dresen A., Finkbeiner S., Dottermusch M., Beume J. S., Li Y., et al. , 2015. Caenorhabditis elegans OSM-11 signaling regulates SKN-1/Nrf during embryonic development and adult longevity and stress response. Dev. Biol. 400: 118–131. 10.1016/j.ydbio.2015.01.021 [DOI] [PubMed] [Google Scholar]
- Essmann C. L., Elmi M., Shaw M., Anand G. M., Pawar V. M., et al. , 2017. In-vivo high resolution AFM topographic imaging of Caenorhabditis elegans reveals previously unreported surface structures of cuticle mutants. Nanomedicine 13: 183–189. 10.1016/j.nano.2016.09.006 [DOI] [PubMed] [Google Scholar]
- Gaggar A., Weathington N., 2016. Bioactive extracellular matrix fragments in lung health and disease. J. Clin. Invest. 126: 3176–3184. 10.1172/JCI83147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparski A. N., Beningo K. A., 2015. Mechanoreception at the cell membrane: more than the integrins. Arch. Biochem. Biophys. 586: 20–26. 10.1016/j.abb.2015.07.017 [DOI] [PubMed] [Google Scholar]
- Hay E. D., 1981. Extracellular matrix. J. Cell Biol. 91: 205s–223s. 10.1083/jcb.91.3.205s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O., 2002. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques 32: 728–730. [DOI] [PubMed] [Google Scholar]
- Hohmann S., Krantz M., Nordlander B., 2007. Yeast osmoregulation. Methods Enzymol. 428: 29–45. 10.1016/S0076-6879(07)28002-4 [DOI] [PubMed] [Google Scholar]
- Hourihan J. M., Moronetti Mazzeo L. E., Fernandez-Cardenas L. P., Blackwell T. K., 2016. Cysteine sulfenylation directs IRE-1 to activate the SKN-1/Nrf2 antioxidant response. Mol. Cell 63: 553–566. 10.1016/j.molcel.2016.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H. J., Liu C. A., Huang B., Tseng A. H., Wang D. L., 2014. Shear-induced endothelial mechanotransduction: the interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. J. Biomed. Sci. 21: 3 10.1186/1423-0127-21-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q., D’Amora D. R., MacNeil L. T., Walhout A. J. M., Kubiseski T. J., 2017. The oxidative stress response in Caenorhabditis elegans requires the GATA transcription factor ELT-3 and SKN-1/Nrf2. Genetics 206: 1909–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R., Xu C., Shen G., Jain M. R., Khor T. O., et al. , 2006. Identification of Nrf2-regulated genes induced by chemopreventive isothiocyanate PEITC by oligonucleotide microarray. Life Sci. 79: 1944–1955. 10.1016/j.lfs.2006.06.019 [DOI] [PubMed] [Google Scholar]
- Huang D. W., Sherman B. T., Lempicki R. A., 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4: 44–57. 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- Johnstone I. L., 2000. Cuticle collagen genes. Expression in Caenorhabditis elegans. Trends Genet. 16: 21–27. 10.1016/S0168-9525(99)01857-0 [DOI] [PubMed] [Google Scholar]
- Jones C. I., Zhu H., Martin S. F., Han Z., Li Y., et al. , 2007. Regulation of antioxidants and phase 2 enzymes by shear-induced reactive oxygen species in endothelial cells. Ann. Biomed. Eng. 35: 683–693. 10.1007/s10439-007-9279-9 [DOI] [PubMed] [Google Scholar]
- Jones D., Dixon D. K., Graham R. W., Candido E. P., 1989. Differential regulation of closely related members of the hsp16 gene family in Caenorhabditis elegans. DNA 8: 481–490. 10.1089/dna.1.1989.8.481 [DOI] [PubMed] [Google Scholar]
- Kage-Nakadai E., Uehara T., Mitani S., 2011. H+/myo-inositol transporter genes, hmit-1.1 and hmit-1.2, have roles in the osmoprotective response in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 410: 471–477. 10.1016/j.bbrc.2011.06.001 [DOI] [PubMed] [Google Scholar]
- Kahn N. W., Rea S. L., Moyle S., Kell A., Johnson T. E., 2008. Proteasomal dysfunction activates the transcription factor SKN-1 and produces a selective oxidative-stress response in Caenorhabditis elegans. Biochem. J. 409: 205–213. 10.1042/BJ20070521 [DOI] [PubMed] [Google Scholar]
- Kamath R. S., Martinez-Campos M., Zipperlen P., Fraser A. G., Ahringer J., 2001. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2: RESEARCH0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath R. S., Fraser A. G., Dong Y., Poulin G., Durbin R., et al. , 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421: 231–237. 10.1038/nature01278 [DOI] [PubMed] [Google Scholar]
- Kell A., Ventura N., Kahn N., Johnson T. E., 2007. Activation of SKN-1 by novel kinases in Caenorhabditis elegans. Free Radic. Biol. Med. 43: 1560–1566. 10.1016/j.freeradbiomed.2007.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko F. C., Chow K. L., 2002. A novel thioredoxin-like protein encoded by the C. elegans dpy-11 gene is required for body and sensory organ morphogenesis. Development 129: 1185–1194. [DOI] [PubMed] [Google Scholar]
- Komatsu H., Chao M. Y., Larkins-Ford J., Corkins M. E., Somers G. A., et al. , 2008. OSM-11 facilitates LIN-12 Notch signaling during Caenorhabditis elegans vulval development. PLoS Biol. 6: e196 10.1371/journal.pbio.0060196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamitina S. T., Morrison R., Moeckel G. W., Strange K., 2004. Adaptation of the nematode Caenorhabditis elegans to extreme osmotic stress. Am. J. Physiol. Cell Physiol. 286: C785–C791. 10.1152/ajpcell.00381.2003 [DOI] [PubMed] [Google Scholar]
- Lamitina T., Huang C. G., Strange K., 2006. Genome-wide RNAi screening identifies protein damage as a regulator of osmoprotective gene expression. Proc. Natl. Acad. Sci. USA 103: 12173–12178. 10.1073/pnas.0602987103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. Z., Kniazeva M., Han M., Pujol N., Ewbank J. J., 2010. The fatty acid synthase fasn-1 acts upstream of WNK and Ste20/GCK-VI kinases to modulate antimicrobial peptide expression in C. elegans epidermis. Virulence 1: 113–122. 10.4161/viru.1.3.10974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. D., Choi S. Y., Lim S. W., Lamitina S. T., Ho S. N., et al. , 2011. TonEBP stimulates multiple cellular pathways for adaptation to hypertonic stress: organic osmolyte-dependent and -independent pathways. Am. J. Physiol. Renal Physiol. 300: F707–F715. 10.1152/ajprenal.00227.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C. K., Deonarine A., Strange K., Choe K. P., 2011. High-throughput screening and biosensing with fluorescent C. elegans strains. J. Vis. Exp. 51: 2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon L., Muriel J. M., Roberts B., Quinn M., Johnstone I. L., 2003. Two sets of interacting collagens form functionally distinct substructures within a Caenorhabditis elegans extracellular matrix. Mol. Biol. Cell 14: 1366–1378. 10.1091/mbc.E02-08-0479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niture S. K., Khatri R., Jaiswal A. K., 2014. Regulation of Nrf2-an update. Free Radic. Biol. Med. 66: 36–44. 10.1016/j.freeradbiomed.2013.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira R. P., Abate J. P., Dilks K., Landis J., Ashraf J., et al. , 2009. Condition-adapted stress and longevity gene regulation by Caenorhabditis elegans SKN-1/Nrf. Aging Cell 8: 524–541. 10.1111/j.1474-9726.2009.00501.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, A. P., and I. L. Johnstone, 2007 The cuticle (March 19, 2007), WormBook, ed. The. C. elegans Research Community, WormBook, /10.1895/wormbook.1.138.1, http://www.wormbook.org. 10.1895/wormbook.1.138.1 [DOI] [Google Scholar]
- Park S.-K., Tedesco P. M., Johnson T. E., 2009. Oxidative stress and longevity in Caenorhabditis elegans as mediated by SKN-1. Aging Cell 8: 258–269. 10.1111/j.1474-9726.2009.00473.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge F. A., Tearle A. W., Gravato-Nobre M. J., Schafer W. R., Hodgkin J., 2008. The C. elegans glycosyltransferase BUS-8 has two distinct and essential roles in epidermal morphogenesis. Dev. Biol. 317: 549–559. 10.1016/j.ydbio.2008.02.060 [DOI] [PubMed] [Google Scholar]
- Pimentel H., Bray N. L., Puente S., Melsted P., Pachter L., 2017. Differential analysis of RNA-seq incorporating quantification uncertainty. Nat. Methods 14: 687–690. 10.1038/nmeth.4324 [DOI] [PubMed] [Google Scholar]
- Pujol N., Cypowyj S., Ziegler K., Millet A., Astrain A., et al. , 2008a Distinct innate immune responses to infection and wounding in the C. elegans epidermis. Curr. Biol. 18: 481–489. 10.1016/j.cub.2008.02.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol N., Zugasti O., Wong D., Couillault C., Kurz C. L., et al. , 2008b Anti-fungal innate immunity in C. elegans is enhanced by evolutionary diversification of antimicrobial peptides. PLoS Pathog. 4: e1000105 10.1371/journal.ppat.1000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing A. K., Miteva Y., Hannenhalli S., Lamitina T., 2010. Genetic and physiological activation of osmosensitive gene expression mimics transcriptional signatures of pathogen infection in C. elegans. PLoS One 5: e9010 10.1371/journal.pone.0009010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing A. K., Miteva Y., Moronetti L., He L., Lamitina T., 2011. The Caenorhabditis elegans mucin-like protein OSM-8 negatively regulates osmosensitive physiology via the transmembrane protein PTR-23. PLoS Genet. 7: e1001267 10.1371/journal.pgen.1001267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross T. D., Coon B. G., Yun S., Baeyens N., Tanaka K., et al. , 2013. Integrins in mechanotransduction. Curr. Opin. Cell Biol. 25: 613–618. 10.1016/j.ceb.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozario T., DeSimone D. W., 2010. The extracellular matrix in development and morphogenesis: a dynamic view. Dev. Biol. 341: 126–140. 10.1016/j.ydbio.2009.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rual J. F., Ceron J., Koreth J., Hao T., Nicot A. S., et al. , 2004. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 14: 2162–2168. 10.1101/gr.2505604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupert P. B., Daughdrill G. W., Bowerman B., Matthews B. W., 1998. A new DNA-binding motif in the Skn-1 binding domain-DNA complex. Nat. Struct. Biol. 5: 484–491. 10.1038/nsb0698-484 [DOI] [PubMed] [Google Scholar]
- Samarakoon R., Overstreet J. M., Higgins P. J., 2013. TGF-beta signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell. Signal. 25: 264–268. 10.1016/j.cellsig.2012.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller H. B., Fassler R., 2013. Mechanosensitivity and compositional dynamics of cell-matrix adhesions. EMBO Rep. 14: 509–519. 10.1038/embor.2013.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Ellis R. E., Lee K., Liu C. Y., Yang K., et al. , 2001. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell 107: 893–903. 10.1016/S0092-8674(01)00612-2 [DOI] [PubMed] [Google Scholar]
- Singh K., Chao M. Y., Somers G. A., Komatsu H., Corkins M. E., et al. , 2011. C. elegans Notch signaling regulates adult chemosensory response and larval molting quiescence. Curr. Biol. 21: 825–834. 10.1016/j.cub.2011.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbaugh M. J., Narasimhan S. D., Robida-Stubbs S., Moronetti Mazzeo L. E., Dreyfuss J. M., et al. , 2015. Lipid-mediated regulation of SKN-1/Nrf in response to germ cell absence. Elife 4: 07836. 10.7554/eLife.07836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis G., Bohmann D., 2010. Stress-activated cap’n’collar transcription factors in aging and human disease. Sci. Signal. 3: re3 10.1126/scisignal.3112re3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffoni C., Pujol N., 2015. Mechanisms of innate immunity in C. elegans epidermis. Tissue Barriers 3: e1078432 10.1080/21688370.2015.1078432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi K., Motohashi H., Yamamoto M., 2011. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 16: 123–140. 10.1111/j.1365-2443.2010.01473.x [DOI] [PubMed] [Google Scholar]
- Tang L., Choe K. P., 2015. Characterization of skn-1/wdr-23 phenotypes in Caenorhabditis elegans; pleiotrophy, aging, glutathione, and interactions with other longevity pathways. Mech. Ageing Dev. 149: 88–98. 10.1016/j.mad.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Thakur N., Pujol N., Tichit L., Ewbank J. J., 2014. Clone mapper: an online suite of tools for RNAi experiments in Caenorhabditis elegans. G3 (Bethesda) 4: 2137–2145. 10.1534/g3.114.013052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thein M. C., McCormack G., Winter A. D., Johnstone I. L., Shoemaker C. B., et al. , 2003. Caenorhabditis elegans exoskeleton collagen COL-19: an adult-specific marker for collagen modification and assembly, and the analysis of organismal morphology. Dev. Dyn. 226: 523–539. 10.1002/dvdy.10259 [DOI] [PubMed] [Google Scholar]
- Tong A., Lynn G., Ngo V., Wong D., Moseley S. L., et al. , 2009. Negative regulation of Caenorhabditis elegans epidermal damage responses by death-associated protein kinase. Proc. Natl. Acad. Sci. USA 106: 1457–1461. 10.1073/pnas.0809339106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warabi E., Takabe W., Minami T., Inoue K., Itoh K., et al. , 2007. Shear stress stabilizes NF-E2-related factor 2 and induces antioxidant genes in endothelial cells: role of reactive oxygen/nitrogen species. Free Radic. Biol. Med. 42: 260–269. 10.1016/j.freeradbiomed.2006.10.043 [DOI] [PubMed] [Google Scholar]
- Ward J. D., Mullaney B., Schiller B. J., He L. D., Petnic S. E., et al. , 2014. Defects in the C. elegans acyl-CoA synthase, acs-3, and nuclear hormone receptor, nhr-25, cause sensitivity to distinct, but overlapping stresses. PLoS One 9: e92552 10.1371/journal.pone.0092552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler J. M., Thomas J. H., 2006. Identification of a novel gene family involved in osmotic stress response in Caenorhabditis elegans. Genetics 174: 1327–1336. 10.1534/genetics.106.059089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winograd-Katz S. E., Fassler R., Geiger B., Legate K. R., 2014. The integrin adhesome: from genes and proteins to human disease. Nat. Rev. Mol. Cell Biol. 15: 273–288. 10.1038/nrm3769 [DOI] [PubMed] [Google Scholar]
- Wu C. W., Deonarine A., Przybysz A., Strange K., Choe K. P., 2016. The Skp1 homologs SKR-1/2 are required for the Caenorhabditis elegans SKN-1 antioxidant/detoxification response independently of p38 MAPK. PLoS Genet. 12: e1006361 10.1371/journal.pgen.1006361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. W., Wang Y., Choe K. P., 2017. F-box protein XREP-4 Is a new regulator of the oxidative stress response in Caenorhabditis elegans. Genetics 206: 859–871. 10.1534/genetics.117.200592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Kong L., Cheng Y., Zhang Z., Wang Y., et al. , 2015. Metallothionein plays a prominent role in the prevention of diabetic nephropathy by sulforaphane via up-regulation of Nrf2. Free Radic. Biol. Med. 89: 431–442 (corrigenda: Free Radic. Biol. Med. 97: 621). 10.1016/j.freeradbiomed.2015.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Tai W., Qu X., Wu W., Li Z., et al. , 2017. Rapamycin protects against paraquat-induced pulmonary fibrosis: activation of Nrf2 signaling pathway. Biochem. Biophys. Res. Commun. 490: 535–540. 10.1016/j.bbrc.2017.06.074 [DOI] [PubMed] [Google Scholar]
- Yoneda T., Benedetti C., Urano F., Clark S. G., Harding H. P., et al. , 2004. Compartment-specific perturbation of protein handling activates genes encoding mitochondrial chaperones. J. Cell Sci. 117: 4055–4066. 10.1242/jcs.01275 [DOI] [PubMed] [Google Scholar]
- Zugasti O., Bose N., Squiban B., Belougne J., Kurz C. L., et al. , 2014. Activation of a G protein-coupled receptor by its endogenous ligand triggers the innate immune response of Caenorhabditis elegans. Nat. Immunol. 15: 833–838. 10.1038/ni.2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zugasti O., Thakur N., Belougne J., Squiban B., Kurz C. L., et al. , 2016. A quantitative genome-wide RNAi screen in C. elegans for antifungal innate immunity genes. BMC Biol. 14: 35 10.1186/s12915-016-0256-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Strains are available upon request. Raw numeric data are at https://figshare.com/s/7863ed7b6e2bed9e6510 and RNAseq (RNA sequencing) raw data are at the Gene Expression Omnibus (GSE107704).