Abstract
Objective
To develop a list of non-emergent, potentially harmful interventions commonly performed in ICUs that require a clear understanding of patients’ treatment goals.
Background
A 2016 policy statement from the American Thoracic Society and American College of Critical Care Medicine calls on intensivists to engage in shared decision-making when “making major treatment decisions that may be affected by personal values, goals, and preferences.”
Methods
A three-round modified Delphi consensus process was conducted via a panel of 6 critical care physicians, 6 ICU nurses, 6 former ICU patients, and 6 family members from 6 academic and community-based medical institutions in the U.S. mid-Atlantic region.
Results
Recommendations about 8 interventions achieved consensus among respondents: 1) Permanent feeding tube (percutaneous feeding tube or “PEG” tube), 2) Permanent dialysis catheter, 3) Suprapubic urinary catheter, 4) Tracheotomy, 5) Long-term venous catheter, 6) Pulmonary artery catheter, 7) In-hospital dialysis, or 8) temporary NG feeding tube.
Conclusions
Clinical and patient/family participants in a modified Delphi consensus process were able to identify preference-sensitive decisions that should trigger clinicians to clarify patient goals and consider initiating shared decision making.
Keywords: life sustaining treatments, decision making, clinical decision making, Delphi Technique, critical care
INTRODUCTION
The Institute of Medicine defines high-quality healthcare as the degree to which “health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge.”1 In the ICU setting, establishing an individual’s desired health outcome can be complicated. Patients are often unable to communicate and rely on family members, 2,3 who are sometimes unsure what outcomes their loved ones will consider acceptable. The desired outcome or goal that patients or families initially express is also not always achievable and frequently changes as prognosis becomes more or less certain.4 As a result, determining whether a test or procedure is an appropriate way to achieve a critically ill patients’ desired health outcome is challenging.
Recent research has estimated that intensivists make an average of 9 treatment decisions per patient during bedside rounds.5 In a busy ICU this means making hundreds of decisions over a few hours. The vast majority of these decisions (e.g., electrolyte replacement) are unlikely to benefit from patient input. Patient or proxy input into other decisions is highly desirable, but real-time discussion is logistically impractical when responding to an acutely unstable patient (e.g., cardiopulmonary resuscitation). Previous work has shown that the preferred role of patients and their proxies also varies over the course of an illness and by whether the decision is technical, value-neutral, or value-laden.6–9 A 2016 policy statement from the American Thoracic Society and American College of Critical Care Medicine calls on intensivists to engage patients and proxies in shared decision-making when establishing a patient’s overall goals of care and when “making major treatment decisions that may be affected by personal values, goals, and preferences.”10,11 Given the inconsistent way shared decision making is currently practiced in the ICU, 12–16 there is likely to be substantial variability in the interpretation of this guideline.
As a first step toward identifying triggers for considering shared decision-making, we sought to develop a list of non-emergent ICU interventions whose value is highly dependent on a patient’s treatment goals. We chose to focus on non-emergent interventions because they allow time for a clinical team to locate a patient proxy, clarify patient goals, and deliberate. Emergent treatments generally must be discussed prospectively as part of advance care planning even though goals may change and the treatment may never be indicated. We used a 3-phase, modified Delphi consensus development technique that granted equal representation and full suffrage to clinical and patient-family experts.
THEORY
Our consensus development process was based on the Delphi method. The Delphi method is a structured technique for harnessing expert opinion originally developed in the 1950s for scientific and technology forecasting.17 Modified versions of the Delphi method have been employed in healthcare to reach consensus on issues lacking adequate empirical data including indicators of high-quality care,18,19 research priorities,20,21 disease definitions,22 prescribing indicators,23 and core outcome sets for clinical trials.24,25 Although there is no universal guideline for the conduct or reporting of studies using the Delphi technique,26 reviews of its use in healthcare have produced recommendations for best practices.18,27 Common to all Delphi variations is the recruitment of a panel of informed experts. The panel completes a series of surveys or “rounds” related to the study question. After each round individuals compare their own responses to a summary of the entire panel’s responses. A key feature of this methodology is that panel members remain anonymous so that prominent or opinionated panel members do not disproportionately influence results, and initial opinions and positions can be changed without publicly admitting error.28,29 Whenever possible we adhered to recent recommendations for reporting modified Delphi consensus studies with the goal of selecting healthcare quality indicators.18,27
MATERIALS AND METHODS
Panel objective and intervention criteria
The objective of the expert panel was to identify tests and procedures (“interventions”) which ICU clinicians, former ICU patients, and family members agree meet the following three criteria: 1) the intervention could potentially be incompatible with at least one of six previously validated treatment goals of ICU patients, 2) the intervention has the potential to cause physical, emotional, or financial harm to patients, and 3) the intervention can usually be anticipated on a non-emergent basis. These three criteria were developed as an a priori starting point by the study investigators. Panel members were given the opportunity to suggest additional criteria during Round 1 of the consensus process. Additional criteria suggested by panel members were adopted into the consensus process if supported by ≥80% of panel members participating in Round 2. The six treatment goals (evaluated within Criteria 1 from above) were: 1) To be cured, 2) To live longer, 3) To improve health, 4) To maintain health, 5) To be comfortable, and 6) To accomplish a particular personal life goal. These goals were previously validated among ICU patients 30,31 and used in studies examining the concurrence of ICU care with patient treatment goals.32
Recruitment of the expert panel
We convened a panel of ICU physicians, ICU nurses, former ICU patients, and family members of former ICU patients from 6 hospitals within the Johns Hopkins Clinical Research Network (JHCRN). The JHCRN is an integrated network of academic and community-based medical institutions in the mid-Atlantic region ranging in size from 245 to >1,000 beds in both rural and urban communities.33 Each participating hospital was represented by 1 physician, 1 nurse, 1 patient, and 1 family member. At Johns Hopkins Hospital, the principal investigator asked the Patient and Family Advisory Council to nominate representatives. At the other 5 participating sites, JHCRN staff worked with ICU directors to identify representatives. Potential representatives were screened for eligibility and the study objectives and procedure were explained using a standardized telephone screening script. Physicians and nurses had to possess a MD, DO, or RN degree respectively, and have spent at least 4 weeks performing clinical work in an ICU during the past 12 months to be eligible. Patients and family members had to be former patients, or a family member of a former patient in one of the hospital’s adult ICUs, be able to read and write in English, and have reliable internet and e-mail access. Patients and family members were not recruited together (i.e., not matched pairs) and there was no minimum or maximum severity of illness or length of stay requirement. The institutional review board of Johns Hopkins University approved the study and all expert panel members providing oral informed consent to participate.
Consensus development process
The consensus development process consisted of three rounds. Panel members received an e-mail at the beginning of each round containing a link to an individualized online survey. Surveys were developed using the Qualtrics© online survey platform. Results of each round were summarized and displayed on the study website (www.ccapg.org) with responses to open-ended questions provided on a password-protected page accessible only to panel members. All rounds were completed between January and November 2015 and anonymity of panel members was maintained throughout the process.
The overall goals of the rounds are summarized in Figure 1 and were as follows: In Round 1 both interventions and criteria for including interventions were brainstormed and clinicians cast non-binding votes on an initial expansive list of candidate interventions. In Round 2 all participants reviewed proposed amendments to criteria, patients and family members provided data on the outcomes they felt were most important for clinicians to discuss with them when developing a treatment plan, and clinicians cast votes to narrow the list of candidate interventions. In Round 3 all participants cast binding votes on interventions receiving strong support in the previous two previous rounds.
Figure 1.
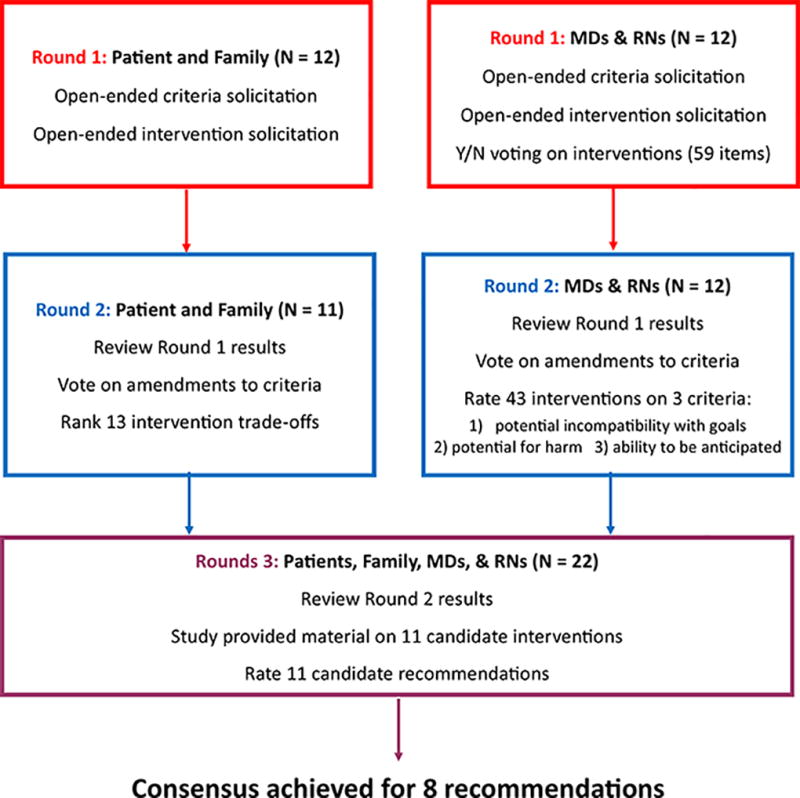
Modified Delphi flowchart. Tasks completed by expert panel members in each Delphi round are shown within boxes
Round 1
In Round 1 all panel members provided basic demographic information and answered questions about their previous experiences as ICU clinicians, patients, and family. The three criteria for identifying interventions, defined a priori by the study investigators (see panel objective and intervention criteria above) were explained, and all participants were asked to suggest other criteria that should be considered. All panel members were also asked to brainstorm interventions that might meet the three a priori criteria. Lastly, physicians and nurses were asked to review a list of 59 interventions and indicate (yes vs. no), whether each intervention fit the three criteria. This initial list of 59 interventions was derived from previous work enumerating and classifying tests and procedures commonly performed in ICUs5,34 with additional input from critical care clinicians.
Round 2
Panel members were requested to review the results of Round 1 on the study website before completing Round 2. All panel members voted on 3 amendments to the inclusion criteria suggested in Round 1. Only patient and family members then ranked the importance of explaining 13 negative consequences or “trade-offs” associated with ICU interventions. This list of trade-offs was developed by critical care clinicians and by volunteers from the Johns Hopkins Patient and Family Advisory Council who were not panel members. A low ranking (i.e., being ranked 1 or 2) indicated that the participant felt it was very important to discuss the trade-off with an ICU patient or their family.
Within the Round 2 survey, physicians and nurses were shown their voting results from Round 1 compared to a summary of Round 1 voting by the other clinicians. They then rated how well 43 interventions met each of the three a priori criteria using a 9-point Likert scale, ranging from Strongly Disagree to Strongly Agree. The 43 interventions in Round 2 was smaller than the original list of 59 in Round 1 because items which received the same number of votes and described similar procedures in Round 1 were combined. For example, “arterial line (radial)” and “arterial line (femoral)” were combined into a single item called “Arterial Line – radial or femoral.” We defined the total score for an intervention as the sum of its median rating on each of the three criteria. Interventions with a total score >17 and a median rating of ≥5 on all criteria were included in Round 3.
Round 3
Interventions with unanimous clinician support in Round 1 and those meeting the threshold for inclusion during Round 2 were re-formulated into recommendations in Round 3. Each recommendation was worded as: “Do not offer [name of intervention] unless it will help achieve the patient’s treatment goal.” To ensure all panel members were able to make informed decisions regarding the interventions, we provided educational material on each intervention via a dedicated page of the study website. Each page contained at least one image of the intervention and responses to the following questions: 1) what is it? 2) what does it do? 3) how might this intervention cause physical, emotional or financial harm to a patient? 4) how quickly does this decision need to be made? 5) why might some people choose this intervention? and 6) why might some people choose not to have this intervention? Responses to these questions were written for patients and families, reviewed for clarity by volunteers from the Johns Hopkins Patient and Family Advisory Council, and reviewed for accuracy by physician specialists in critical care medicine, nephrology and gastroenterology. Page content is freely available on the study website www.ccapg.org.
Prior to voting in Round 3, all panel members were requested to review the educational material on the 11 intervention provided on the study website. Each patient or family member was provided an individualized report showing how they ranked the 13 trade-offs from Round 2 compared to the rest of the patients and family members on the panel. A summary of the patient and family ranking of trade-offs was provided to each clinician for review within their Round 3 survey. This summary was provided to clinicians to help illustrate the variability in the outcomes that patients and families consider important to discuss. Clinicians were also shown their own Round 2 ratings of interventions compared to the median ratings of the other clinician panel members. Finally, all panel members rated the 11 recommendations using a 9-point Likert scale, as previously described. Recommendations receiving a rating of ≥5 from at least 80% of panel members were defined as achieving consensus.
RESULTS
All panel members completed Round 1. All clinician panel members and 11 of 12 patient-family representatives completed Round 2, and 22 (92%) panel members completed Round 3 (Figure 1). The median number of years in practice was 12 for physicians and 25 for nurses (Table 1). Patient representatives ranged in age from 52 to 72, and half of family representatives had been a decision-maker for a family member who died while in the ICU. Among clinicians, 2 (17%) had themselves been adult ICU patients, and 5 (42%) reported having been a decision-maker for an adult family member in the ICU.
Table 1.
Demographic characteristics of expert panel
| Patients (N = 6) |
Family (N = 6) |
Physicians (N = 6) |
Nurses (N = 6) |
|
|---|---|---|---|---|
| Male, n (%) | 2 (33%) | 1 (17%) | 5 (83%) | 1 (17%) |
| Age, median (range) | 62 (52 – 72) | 61 (49 – 73) | 41 (38 – 49) | 44 (28 – 56) |
| Self-reported race, n (%) | ||||
| White | 6 (100%) | 4 (67%) | 4 (67%) | 6 (100%) |
| African-American | 0 (0%) | 2 (33%) | 0 (0%) | 0 (0%) |
| Asian | 0 (0%) | 0 (0%) | 2 (33%) | 0 (0%) |
| Years in practice, median (range) | NA | NA | 12 (8 – 25) | 25 (3 – 32) |
| Have you ever been a patient in an ICU as an adult? (Yes) | 6 (100%) | 0 (0%) | 0 (0%) | 2 (33%) |
| Have you ever been a decision-maker for a family member or friend who was admitted to the ICU? (Yes) | 2 (33%) | 6 (100%) | 3 (50%) | 2 (33%) |
| Have you ever been a decision-maker for a family member or friend who died while in the ICU? (Yes) | 0 (0%) | 3 (50%) | 1 (17%) | 1 (17%) |
In Round 1 there were suggestions to amend the criteria to reflect a broader range of possible patient goals in the ICU. Two additional patient goals received broad support in the second round of voting: 1) continue to interact with others in a meaningful way (92% approval), and 2) maintain autonomy (88% approval).
Table 2 shows the substantial variability in ranked importance of potential trade-offs associated with interventions among patient and family members in Round 2. For instance, some trade-offs (e.g. The procedure or test is very painful) were ranked as first or second by some panel members while other panel members ranked them last. The median rank of trade-offs was similar between patients and family members with 3 exceptions. Family members placed greater importance on explaining that a procedure or test would be painful (Family median rank = 7, Patient median rank = 10.5), while patients placed greater importance on explaining that a procedure or test would be expensive and not covered by insurance (Family median rank = 9, Patient median rank = 5), and on explaining that an intervention will need to be discontinued to allow a natural death (Family median rank = 9, Patient median rank = 5).
Table 2.
Median rank1 of 13 intervention trade-offs according to patients and family panel members in Round 2
|
In your opinion, how important is it for a doctor to explain the trade-offs below and consider your opinion before performing a procedure? Please rank the trade-offs below by how important they are to explain to an ICU patient or their family. |
Median (range) N = 11 |
|---|---|
| The patient might not be able to chew, swallow, or put food in their mouth for the rest of their life. | 3 (1 – 9) |
| The procedure could permanently effect the patient's ability to think clearly or remember people's names. | 3 (1 – 10) |
| The procedure might permanently limit a patient's ability to talk or communicate. | 4 (1 – 6) |
| The patient will require nursing care in a residential facility (nursing home) for the rest of their lives. | 4 (1 – 11) |
| The procedure or test is very expensive and may not be covered by insurance.2 | 5 (3 – 13) |
| The procedure will need to be "stopped" or "turned off" before the patient can die naturally (i.e. A decision to stop treatment will need to be made.)2 | 6 (1 – 13) |
| The patient might not be able to use the bathroom alone for the rest of life (may need diapers). | 6 (2 – 10) |
| The procedure or test is very painful.2 | 7 (1 – 13) |
| The procedure will require the patient to visit a treatment center multiple times per week for rest of life. | 8 (4 – 11) |
| The patient will need to be connected to a machine at home each night for the rest of life. | 9 (4 – 13) |
| The patient will look very different after the procedure (for example a large scar in a clearly visible place). | 10 (6 – 13) |
| The patient will require weekly or monthly blood tests for the rest of life. | 11 (2 – 13) |
| The patient must be sedated or asleep for days during treatment. | 11 (6 – 13) |
Half of expert panel members ranked each potential tradeoff at or above the median rank. The trade-off “The procedure could permanently effect the patient’s ability to think clearly or remember people’s names” has a median rank of 3, meaning that half of all patient and family panel members ranked it 3 or higher.
The absolute difference in the median rank of family members versus patients was >3 for these trade-offs. Patients ranked “The procedure or test is very expensive and may not be covered by insurance” and “The procedure will need to be ‘stopped’ or ‘turned off’ before the patient can die naturally” as having greater importance. Family members ranked the item “The procedure or test is very painful” as having greater importance.
Round 2 voting by clinicians is summarized in Table 3. Eight interventions met the inclusion criteria for rating by the full panel in Round 3: 1) renal replacement therapy, 2) nasogastric (NG) tube, 3) tracheotomy, 4) subcutaneous venous port (e.g., portacath), 5) rectal tube/fecal management system, 6) gastrointestinal endoscopy (upper or lower), 7) lumbar puncture, and 8) pulmonary artery catheter. Interventions rated as being potentially harmful and incompatible with a patient’s goals, but not consistently foreseeable, included: extracorporeal life support, endotracheal intubation, mechanical ventilation, prone positioning during mechanical ventilation, chest tube, intraaortic balloon pump, Sengstaken Blakemore or Minnesota tube, intracranial pressure monitoring, and defibrillation/cardioversion.
Table 3.
Intervention rating by clinical panel members (N = 12) in Round 2
| Intervention | Median Incompatibility1 |
Median Harm2 |
Median Foreseeability3 |
Total score4 |
|---|---|---|---|---|
| Renal replacement therapy or CVVH | 7 | 8 | 5 | 20 |
| Nasogastric tube | 7 | 7 | 6 | 20 |
| Tracheotomy | 6 | 7 | 6.5 | 19.5 |
| Subcutaneous venous port (portacath) | 5 | 7 | 7 | 19 |
| Rectal tube fecal management system | 6 | 6 | 7 | 19 |
| Endoscopy- upper or lower | 6 | 7 | 5.5 | 18.5 |
| Extracorporeal life support (ECLS)6 | 7 | 9 | 2 | 18 |
| Endotrachael intubation6 | 7 | 8 | 3 | 18 |
| Lumbar puncture | 6 | 7 | 5 | 18 |
| Mechanical ventilation via endotracheal tube or tracheostomy6 | 7 | 8 | 2.5 | 17.5 |
| Intraaortic balloon pump6 | 7 | 7.5 | 3 | 17.5 |
| Chest tube6 | 7 | 7 | 3.5 | 17.5 |
| Pulmonary artery catheter | 5 | 7 | 5.5 | 17.5 |
| Epidural catheter | 4 | 7 | 6.5 | 17.5 |
| Bronchoscopy - rigid or fiberoptic6 | 5 | 7 | 5 | 17 |
| Paracentesis6 | 5 | 6 | 6 | 17 |
| Echocardiography Transthoracic6 | 5 | 5.5 | 6.5 | 17 |
| Arthrocentesis6 | 5 | 5 | 7 | 17 |
| Positron emission tomography (PET scan) | 4 | 5 | 8 | 17 |
| Prone positioning during mechanical ventilation6 | 6 | 7.5 | 3 | 16.5 |
| Abdominal drain gallbladder or other | 4 | 6.5 | 6 | 16.5 |
| Sengstaken Blakemore or Minnesota tube6 | 7 | 8 | 1 | 16 |
| Intracranial pressure monitoring subdural or intraventricular6 | 6 | 7 | 3 | 16 |
| Intraosseous or intravenous IO or IV access6 | 6 | 6 | 4 | 16 |
| Thoracocentesis | 4 | 6 | 6 | 16 |
| Nuclear medicine scan (ventilation-perfusion scan) | 4 | 5.5 | 6.5 | 16 |
| Foley catheter6 | 5 | 5 | 6 | 16 |
| Jugular bulb oximetry6 | 5 | 6.5 | 4 | 15.5 |
| Defibrillation cardioversion6 | 6 | 7 | 2 | 15 |
| Invasive cardiac procedures5,6 | 4.5 | 7 | 3.5 | 15 |
| Arterial Line - radial or femoral6 | 5 | 6.5 | 3.5 | 15 |
| Cricothyrotomy6 | 5 | 8 | 1.5 | 14.5 |
| Dialysis catheter temporary | 3 | 7 | 4.5 | 14.5 |
| Noninvasive ventilation CPAP or BiPAP6 | 5 | 6 | 3 | 14 |
| Peripherally inserted central catheter (PICC line) | 3 | 4 | 7 | 14 |
| Isolation | 3.5 | 3.5 | 7 | 14 |
| Central Venous Access Tunneled or Non | 4 | 6.5 | 3 | 13.5 |
| Magnetic Resonance Imaging (MRI) | 3 | 4 | 6.5 | 13.5 |
| Cardiac pacemaker temporary | 4 | 6 | 3 | 13 |
| Computed tomography (CT scan) | 4 | 3 | 5.5 | 12.5 |
| X-ray | 3 | 2.5 | 7 | 12.5 |
| Electroencephalography (EEG) | 3 | 3 | 6 | 12 |
| Skin suturing | 3 | 3 | 3 | 9 |
Abbreviations: BiPAP, Bilevel positive airway pressure; CPAP, continuous positive airway pressure; CVVH, continuous venovenous hemofiltration.
Response to the statement: “The intervention is potentially incompatible with ≥1 common patient goals.”
Response to the statement: “The intervention is potentially physically, emotionally, or financially harmful.”
Response to the statement: “The intervention can be anticipated on a non-emergent basis.”
Agreement with each criteria rated using a 9-point Likert scale (1, Strongly Disagree; 9, Strongly Agree). Total score is the sum of the median rating for 3 criteria. Interventions highlighted in blue met the standard for inclusion in Round 3: Total score>17 and median rating ≥5 on all criteria.
Includes pericariocentesis and angiography
These interventions received a median rating ≥5 on incompatibility and harm but not foreseeability.
The three interventions with unanimous support in Round 1 and the eight interventions meeting the criteria for inclusion in Round 2 were combined to create a list of 11 candidate recommendations in Round 3. Eight (73%) of the 11 recommendations achieved consensus (Table 4). For all 8 recommendations, consensus was achieved both among both clinicians and patient/family members of the panel. The panel recommended that the following interventions should not be offered unless they will help achieve a patient’s treatment goal: 1) Permanent feeding tube (percutaneous feeding tube or “PEG” tube), 2) Permanent dialysis catheter, 3) Suprapubic urinary catheter, 4) Tracheotomy, 5) Long-term venous catheter, 6) Pulmonary artery catheter, 7) In-hospital dialysis, or 8) temporary NG feeding tube.
Table 4.
Percent of expert panel rating each recommendation ≥5 on a 9-point Likert scale in Round 3
| Patients and Family (N = 11) |
Physicians and Nurses (N = 11) |
All (N = 22) |
|
|---|---|---|---|
| Do not offer a permanent feeding tube unless it will help achieve the patient’s treatment goal | 100% | 100% | 100% |
| Do not offer a permanent dialysis catheter unless it will help achieve the patient’s treatment goal | 91% | 91% | 91% |
| Do not offer a suprapubic urinary catheter unless it will help achieve the patient’s treatment goal | 91% | 91% | 91% |
| Do not offer a tracheotomy unless it will help achieve the patient’s treatment goal | 91% | 82% | 86% |
| Do not offer a long-term venous catheter unless it will help achieve the patient’s treatment goal | 82% | 91% | 86% |
| Do not offer a pulmonary artery catheter unless it will help achieve the patient’s treatment goal | 91% | 82% | 86% |
| Do not offer in-hospital dialysis unless it will help achieve the patient’s treatment goal | 82% | 82% | 82% |
| Do not offer a temporary nasogastric feeding tube unless it will help achieve the patient’s treatment goal | 82% | 82% | 82% |
| Do not offer endoscopy unless it will help achieve the patient’s treatment goal | 64% | 73% | 68% |
| Do not offer a spinal tap unless it will help achieve the patient’s treatment goal | 64% | 73% | 68% |
| Do not offer a rectal fecal management system unless it will help achieve the patient’s treatment goal | 64% | 55% | 59% |
Agreement with each recommendation was rated using a 9-point Likert scale (1, Strongly Disagree; 9, Strongly Agree). Highlighted recommendations achieved consensus defined as >80% of expert panel members rating the recommendation ≥5.
DISCUSSION
We recruited 24 ICU physicians, nurses, former patients, and family members from 6 hospitals in the mid-Atlantic region of USA to participate in a 3-round, modified Delphi consensus development process for identifying non-emergent, ICU interventions whose value is highly dependent on a patient’s treatment goals. There was strong agreement between the clinician and patient-family participants completing the final round that 8 interventions can potentially cause physical, emotional, or financial harm, were potentially incompatible with common ICU patient goals, and can usually be anticipated on a non-emergent basis. Hence, consensus indicated that these 8 interventions should not be offered unless they will help achieve a patient’s treatment goal.
Many of the interventions with consensus support in this Delphi process, including permanent PEG feeding tube placement, tracheotomy, and initiation of renal replacement therapy, have previously been identified as criteria for palliative care assessment during a hospital stay.35,36 Our findings support these interventions as indicative of transitional junctures in a patient’s care. While patients may benefit from the involvement of palliative care specialists, all intensivists should be proficient in “primary” palliative care skills including initiating a discussion about a patient or family member’s preferred role in decision making, goals of care, expected functional prognosis, and the potential benefits and harms of common interventions.35,37 On the other hand, temporary NG feeding tubes have generally not been treated as potentially harmful or preference-sensitive in the ICU setting. Although the indications for nasogastric feeding tubes are different in critically ill patients, their inclusion in this list may have been influenced by the American Geriatrics Society’s position statement against the use of percutaneous feeding tubes for patients with advanced dementia,38 as well as editorials on medical blogs39 and in the popular press describing the misuse of both interventions in hospitalized patients with dementia and in prisoners.40–42
The range in rankings of potential trade-offs associated with interventions in Round 2 reinforces that there is substantial variability in the outcomes and health states that patients and families consider important. The comparatively high rank given to being able to think clearly or remember people’s names is consistent with previous findings that patients place great importance on remaining mentally aware at the end of life.43 The high value assigned by patients to knowing that an intervention is expensive or potentially not covered by insurance likely stems from a desire not to be a burden on family members.43
Framing the recommendations of this research as “Do not offer [intervention] unless” was intentional. Prior literature regarding the use of life-sustaining technologies indicates that patients and their families are heavily influenced by physician recommendations,44,45 choice architecture,46,47 and default treatment options.48 Discussing an intervention inherently suggests there is a chance it will achieve a desired outcome. Once discussed or offered, a proxy may feel they have abandoned or failed their loved one if they say no. This perceived failure may contribute to psychologic distress 49,50 and the elevated rates of depression and symptoms of post-traumatic stress 51–54 experienced by ICU proxies. When facing a preference-sensitive treatment decision, it is incumbent on clinicians to attempt to understand what role patients and proxies wish to play in decision-making and thoroughly explore their goals of care before offering possible interventions.55 When a patient’s goal or an intervention’s efficacy are uncertain, it is ethically appropriate to offer the intervention in a way that guides a patient or proxy toward a preferred option while simultaneously preserving choice.56 For example: “Given what you’ve told me about your mom, I don’t think a permanent dialysis catheter is a good idea. It won’t get her on the transplant list and she’d need another invasive procedure. Do you still want to hear about it?”
This study is a step toward ensuring patients and their proxies are routinely given the opportunity to engage in shared decision-making about value-sensitive treatment decisions. The study’s limitations include recruiting a small expert panel from hospitals in a single region of the country. The panel was comprised of volunteer patients and family over the age of 49, and volunteer clinicians whose views may differ from those of their peers and colleagues. Without larger-scale replication, it is not possible to know how well the panel’s recommendations translate to other regions, or to populations in specialty ICUs.
Delphi studies have been used to develop quality criteria for shared decision-making,57 and scales assessing organizational readiness to implement widespread patient engagement.58 However, patients and family members rarely constitute >20% of these panels giving them minimal voting power. Recruiting diverse expert panels for Delphi studies with sufficient representation from all relevant stakeholder groups is both essential and difficult.59 One of this study’s strengths is the equal representation of physicians, nurses, patients, and families, and the panel’s excellent retention rate over all survey rounds. Our desire to fully integrate patient and family representatives into the panel required modifying the traditional Delphi format so that clinical and patient/family members were separately surveyed regarding their unique expertise in Round 2. It also required creating extensive educational materials aimed at patient/family members prior to Round 3. We did not ask patient and family participants to review educational materials for all 59 interventions in Round 1 because this would have required an extraordinary time commitment that could have contributed to drop-out among participants. Notably, lay panel members generally chose less extreme ratings (i.e., less often choosing values at the extremes of the 9-point Likert scale) than clinical panel members in Round 3. One hypothesis for this finding is that patients and families had less confidence in their ability to make informed decisions about interventions. If true, this supports the essential role that clinicians play is helping patients and families with complex decision-making in the critical care setting.
CLINICAL AND RESEARCH IMPLICATIONS
In addition to establishing goals of care at the time of ICU admission, clinicians should recognize common treatment decisions that are highly preference sensitive. We recommend that clinicians pause and consider whether these treatments are likely to help achieve a patient’s goals, recognizing that patients’ goals often change over the course of an ICU stay. The answer is likely to be yes for patients whose preferences have been well articulated and stable. However when patients or proxies have struggled to identify achievable goals, or when a patient’s clinical trajectory has changed substantially, these treatment decisions can act as reminders to re-initiate the shared decision-making process. We encourage both large-scale replication of this study and informal discussions between stakeholders to identify the treatment decisions that best represent junctures when it is vital to re-assess the alignment between a patient’s goals and their treatment plan.
CONCLUSIONS
As the proportion of patients treated in an ICU during their last month of life continues to climb, 60 critical care clinicians will increasingly need to engage patients and their families to ensure they provide medically appropriate treatment consistent with patients’ goals and preferences. Within this panel of critical care physicians, nurses, patients, and families from 6 hospitals in the U.S. mid-Atlantic region, there was strong consensus that 8 common ICU interventions are foreseeable, potentially harmful, and appropriate as triggers for informed discussions regarding the alignment between interventions and patient’s goals. This list of 8 ICU interventions represents a first step toward ensuring that shared decision making is consistently practiced when making important preference-sensitive treatment decisions for critically ill patients.
Acknowledgments
Funding Support: This research was supported by the Gordon and Betty Moore Foundation.
We wish to acknowledge and thank the expert panel members, the Johns Hopkins Clinical Research Network coordinators, and the Johns Hopkins Patient and Family Advisory Council for their contributions to this study. Additional thanks to clinical informationist Carrie Price, MLS, Mohamed D. Hashem, MD, Margaret M. Hayes, MD, and Ruben Hernaez, MD, MPH, PhD
Abbreviations
- ICU
Intensive care unit
- JHCRN
Johns Hopkins Clinical Research Network
Footnotes
All authors contributed to the conception and/or design of this study. AET, SKS, and DMN contributed to the acquisition of data. All authors contributed to the analysis and interpretation of data. AET drafted the manuscript, and all authors critically revised it for important intellectual content and approved the final version to be submitted.
References
- 1. [Accessed August 30, 2015];Crossing the Quality Chasm: The IOM Health Care Quality Initiative. http://iom.nationalacademies.org/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx.
- 2.Prendergast TJ, Claessens MT, Luce JM. A national survey of end-of-life care for critically ill patients. Am J Respir Crit Care Med. 1998;158(4):1163–1167. doi: 10.1164/ajrccm.158.4.9801108. [DOI] [PubMed] [Google Scholar]
- 3.Torke AM, Sachs GA, Helft PR, et al. Scope and outcomes of surrogate decision making among hospitalized older adults. JAMA Intern Med. 2014;174(3):370–377. doi: 10.1001/jamainternmed.2013.13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwarze ML, Campbell TC, Cunningham TV, White DB, Arnold RM. You Can’t Get What You Want: Innovation for End-Of-Life Communication in the ICU. Am J Respir Crit Care Med. 2016;193(1):14–16. doi: 10.1164/rccm.201508-1592OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKenzie MS, Auriemma CL, Olenik J, Cooney E, Gabler NB, Halpern SD. An Observational Study of Decision Making by Medical Intensivists. Crit Care Med. 2015;43(8):1660–1668. doi: 10.1097/CCM.0000000000001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heyland DK, Cook DJ, Rocker GM, et al. Decision-making in the ICU: perspectives of the substitute decision-maker. Intensive Care Med. 2003;29(1):75–82. doi: 10.1007/s00134-002-1569-y. [DOI] [PubMed] [Google Scholar]
- 7.Anderson WG, Arnold RM, Angus DC, Bryce CL. Passive decision-making preference is associated with anxiety and depression in relatives of patients in the intensive care unit. J Crit Care. 2009;24(2):249–254. doi: 10.1016/j.jcrc.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Johnson SK, Bautista CA, Hong SY, Weissfeld L, White DB. An empirical study of surrogates’ preferred level of control over value-laden life support decisions in intensive care units. Am J Respir Crit Care Med. 2011;183(7):915–921. doi: 10.1164/rccm.201008-1214OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madrigal VN, Carroll KW, Hexem KR, Faerber JA, Morrison WE, Feudtner C. Parental decision-making preferences in the pediatric intensive care unit*. Crit Care Med. 2012;40(10):2876–2882. doi: 10.1097/CCM.0b013e31825b9151. [DOI] [PubMed] [Google Scholar]
- 10.Kon AA, Davidson JE, Morrison W, Danis M, White DB. Shared Decision Making in ICUs: An American College of Critical Care Medicine and American Thoracic Society Policy Statement. Crit Care Med. 2016;44(1):188–201. doi: 10.1097/CCM.0000000000001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kon AA, Davidson JE, Morrison W, Danis M, White DB. Shared Decision Making in Intensive Care Units: Executive Summary of the American College of Critical Care Medicine and American Thoracic Society Policy Statement. Am J Respir Crit Care Med. 2016 Apr; doi: 10.1164/rccm.201602-0269ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnato AE, Herndon MB, Anthony DL, et al. Are regional variations in end-of-life care intensity explained by patient preferences?: A Study of the US Medicare Population. Med Care. 2007;45(5):386–393. doi: 10.1097/01.mlr.0000255248.79308.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schenker Y, Tiver GA, Hong SY, White DB. Association between physicians’ beliefs and the option of comfort care for critically ill patients. Intensive Care Med. 2012;38(10):1607–1615. doi: 10.1007/s00134-012-2671-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uy J, White DB, Mohan D, Arnold RM, Barnato AE. Physicians’ Decision-Making Roles for an Acutely Unstable Critically and Terminally Ill Patient. Crit Care Med. 2013;41(6):1511–1517. doi: 10.1097/CCM.0b013e318287f0dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turnbull AE, Krall JR, Ruhl AP, et al. A Scenario-Based, Randomized Trial of Patient Values and Functional Prognosis on Intensivist Intent to Discuss Withdrawing Life Support. Crit Care Med. 2014 Feb; doi: 10.1097/CCM.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hart JL, Harhay MO, Gabler NB, Ratcliffe SJ, Quill CM, Halpern SD. Variability among us intensive care units in managing the care of patients admitted with preexisting limits on life-sustaining therapies. JAMA Intern Med. 2015;175(6):1019–1026. doi: 10.1001/jamainternmed.2015.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalkey N, Helmer O. An Experimental Application of the DELPHI Method to the Use of Experts. Manag Sci. 1963;9(3):458–467. doi: 10.1287/mnsc.9.3.458. [DOI] [Google Scholar]
- 18.Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PloS One. 2011;6(6):e20476. doi: 10.1371/journal.pone.0020476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuur JD, Carney DP, Lyn ET, et al. A top-five list for emergency medicine: a pilot project to improve the value of emergency care. JAMA Intern Med. 2014;174(4):509–515. doi: 10.1001/jamainternmed.2013.12688. [DOI] [PubMed] [Google Scholar]
- 20.Reay H, Arulkumaran N, Brett SJ. Priorities for Future Intensive Care Research in the UK: Results of a James Lind Alliance Priority Setting Partnership. J Intensive Care Soc. 2014;15(4):288–296. doi: 10.1177/175114371401500405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott ES, Murphy LS, Warshawsky NE. Nursing Administration Research Priorities: Findings From a Delphi Study. J Nurs Adm. 2016 Apr; doi: 10.1097/NNA.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson ND, Davis AM, Slutsky AS, Stewart TE. Development of a clinical definition for acute respiratory distress syndrome using the Delphi technique. J Crit Care. 2005;20(2):147–154. doi: 10.1016/j.jcrc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Campbell SM, Cantrill JA, Roberts D. Prescribing indicators for UK general practice: Delphi consultation study. BMJ. 2000;321(7258):425–428. doi: 10.1136/bmj.321.7258.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tugwell P, Boers M, Brooks P, Simon L, Strand V, Idzerda L. OMERACT: an international initiative to improve outcome measurement in rheumatology. Trials. 2007;8:38. doi: 10.1186/1745-6215-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinha IP, Smyth RL, Williamson PR. Using the Delphi technique to determine which outcomes to measure in clinical trials: recommendations for the future based on a systematic review of existing studies. PLoS Med. 2011;8(1):e1000393. doi: 10.1371/journal.pmed.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32(4):1008–1015. [PubMed] [Google Scholar]
- 27.Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67(4):401–409. doi: 10.1016/j.jclinepi.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Powell C. The Delphi technique: myths and realities. J Adv Nurs. 2003;41(4):376–382. doi: 10.1046/j.1365-2648.2003.02537.x. [DOI] [PubMed] [Google Scholar]
- 29.Okoli C, Pawlowski SD. The Delphi method as a research tool: an example, design considerations and applications. Inf Manage. 2004;42(1):15–29. doi: 10.1016/j.im.2003.11.002. [DOI] [Google Scholar]
- 30.Haberle TH, Shinkunas LA, Erekson ZD, Kaldjian LC. Goals of care among hospitalized patients: a validation study. Am J Hosp Palliat Care. 2011;28(5):335–341. doi: 10.1177/1049909110388505. [DOI] [PubMed] [Google Scholar]
- 31.Brandt DS, Shinkunas LA, Gehlbach TG, Kaldjian LC. Understanding Goals of Care Statements and Preferences among Patients and Their Surrogates in the Medical ICU. J Hosp Palliat Nurs JHPN Off J Hosp Palliat Nurses Assoc. 2012;14(2):126–132. doi: 10.1097/NJH.0b013e3182389dd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gehlbach TG, Shinkunas LA, Forman-Hoffman VL, Thomas KW, Schmidt GA, Kaldjian LC. Code status orders and goals of care in the medical ICU. Chest. 2011;139(4):802–809. doi: 10.1378/chest.10-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. [Accessed April 8, 2016];Johns Hopkins Clinical Research Network (JHCRN) | ICTR. http://ictr.johnshopkins.edu/clinical/clinical-resources/human-subjects-research-core/jhcrn/
- 34.Flaatten H, Bonde J, Ruokonen E, Wins O. Classification for coding procedures in the intensive care unit. Acta Anaesthesiol Scand. 2002;46(8):994–998. doi: 10.1034/j.1399-6576.2002.460811.x. [DOI] [PubMed] [Google Scholar]
- 35.Weissman DE, Meier DE. Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17–23. doi: 10.1089/jpm.2010.0347. [DOI] [PubMed] [Google Scholar]
- 36.Nelson JE, Curtis JR, Mulkerin C, et al. Choosing and Using Screening Criteria for Palliative Care Consultation in the ICU: A Report From the Improving Palliative Care in the ICU (IPAL-ICU) Advisory Board*. Crit Care Med. 2013;41(10):2318–2327. doi: 10.1097/CCM.0b013e31828cf12c. [DOI] [PubMed] [Google Scholar]
- 37.Mosenthal AC, Weissman DE, Curtis JR, et al. Integrating palliative care in the surgical and trauma intensive care unit: A report from the Improving Palliative Care in the Intensive Care Unit (IPAL-ICU) Project Advisory Board and the Center to Advance Palliative Care. Crit Care Med. 2011 Nov; doi: 10.1097/CCM.0b013e31823bc8e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American Geriatrics Society Ethics Committee and Clinical Practice and Models of Care Committee. American Geriatrics Society Feeding Tubes in Advanced Dementia Position Statement. J Am Geriatr Soc. 2014;62(8):1590–1593. doi: 10.1111/jgs.12924. [DOI] [PubMed] [Google Scholar]
- 39.Bukata R. I Hereby Declare the End of the Reflexive NG Tube. [Accessed April 30, 2016];Emerg Physicians Mon. 2016 Apr; http://epmonthly.com/article/i-hereby-declare-the-end-of-the-reflexive-ng-tube/
- 40. [Accessed April 25, 2016];Force-Feeding: Cruel at Guantánamo, but O.K. for Our Parents - The New York Times. http://www.nytimes.com/2015/11/24/opinion/force-feeding-cruel-at-guantanamo-but-ok-for-our-parents.html?smid=pl-share.
- 41.M.D JNZ. [Accessed April 25, 2016];Food and the Dying Patient. Well. http://well.blogs.nytimes.com/2014/08/21/food-and-the-dying-patient/. Published 1408593787.
- 42.Span P. When Demented Patients Receive Feeding Tubes. [Accessed April 25, 2016];The New Old Age Blog. http://newoldage.blogs.nytimes.com/2011/05/09/feeding-tube-decisions-often-made-in-haste/. Published 1304958164.
- 43.Steinhauser KE, Christakis NA, Clipp EC, McNeilly M, McIntyre L, Tulsky JA. Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA. 2000;284(19):2476–2482. doi: 10.1001/jama.284.19.2476. [DOI] [PubMed] [Google Scholar]
- 44.Garland A, Connors AF. Physicians’ influence over decisions to forego life support. J Palliat Med. 2007;10(6):1298–1305. doi: 10.1089/jpm.2007.0061. [DOI] [PubMed] [Google Scholar]
- 45.Larochelle MR, Rodriguez KL, Arnold RM, Barnato AE. Hospital staff attributions of the causes of physician variation in end-of-life treatment intensity. Palliat Med. 2009;23(5):460–470. doi: 10.1177/0269216309103664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barnato AE, Arnold RM. The Effect of Emotion and Physician Communication Behaviors on Surrogates’ Life-Sustaining Treatment Decisions: A Randomized Simulation Experiment. Crit Care Med. 2013 May; doi: 10.1097/CCM.0b013e31828a233d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anesi GL, Halpern SD. Choice architecture in code status discussions with terminally ill patients and their families. Intensive Care Med. 2016 Mar;:1–3. doi: 10.1007/s00134-016-4294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halpern SD, Loewenstein G, Volpp KG, et al. Default Options In Advance Directives Influence How Patients Set Goals For End-Of-Life Care. Health Aff (Millwood) 2013;32(2):408–417. doi: 10.1377/hlthaff.2012.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Majesko A, Hong SY, Weissfeld L, White DB. Identifying family members who may struggle in the role of surrogate decision maker*. Crit Care Med. 2012;40(8):2281–2286. doi: 10.1097/CCM.0b013e3182533317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oberndorfer TA, Anoff DL, Wald HL. Surgical intervention in terminal illness—doing everything: A teachable moment. JAMA Intern Med. 2015 Nov;:1–2. doi: 10.1001/jamainternmed.2015.6335. [DOI] [PubMed] [Google Scholar]
- 51.Gries CJ, Engelberg RA, Kross EK, et al. Predictors of symptoms of posttraumatic stress and depression in family members after patient death in the ICU. Chest. 2010;137(2):280–287. doi: 10.1378/chest.09-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davidson JE, Jones C, Bienvenu OJ. Family response to critical illness: postintensive care syndrome-family. Crit Care Med. 2012;40(2):618–624. doi: 10.1097/CCM.0b013e318236ebf9. [DOI] [PubMed] [Google Scholar]
- 53.Andresen M, Guic E, Orellana A, Diaz MJ, Castro R. Posttraumatic stress disorder symptoms in close relatives of intensive care unit patients: Prevalence data resemble that of earthquake survivors in Chile. J Crit Care. 2015;30(5):1152.e7–1152.e11. doi: 10.1016/j.jcrc.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 54.Beusekom I van, Bakhshi-Raiez F, Keizer NF de, Dongelmans DA, Schaaf M van der. Reported burden on informal caregivers of ICU survivors: a literature review. Crit Care. 2016;20(1):16. doi: 10.1186/s13054-016-1185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bernacki RE, Block SD for the American College of Physicians High Value Care Task Force. Communication about serious illness care goals: A review and synthesis of best practices. JAMA Intern Med. 2014 Oct; doi: 10.1001/jamainternmed.2014.5271. [DOI] [PubMed] [Google Scholar]
- 56.Blumenthal-Barby JS, Burroughs H. Seeking Better Health Care Outcomes: The Ethics of Using the “Nudge”. Am J Bioeth. 2012;12(2):1–10. doi: 10.1080/15265161.2011.634481. [DOI] [PubMed] [Google Scholar]
- 57.Nieuwenhuijze MJ, Korstjens I, Jonge A de, Vries R de, Lagro-Janssen A. On speaking terms: a Delphi study on shared decision-making in maternity care. BMC Pregnancy Childbirth. 2014;14(1):223. doi: 10.1186/1471-2393-14-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oostendorp LJ, Durand M-A, Lloyd A, Elwyn G. Measuring organisational readiness for patient engagement (MORE): an international online Delphi consensus study. BMC Health Serv Res. 2015;15(1):61. doi: 10.1186/s12913-015-0717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harman NL, Bruce IA, Kirkham JJ, et al. The Importance of Integration of Stakeholder Views in Core Outcome Set Development: Otitis Media with Effusion in Children with Cleft Palate. PLoS ONE. 2015;10(6):e0129514. doi: 10.1371/journal.pone.0129514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teno JMGP. Change in end-of-life care for medicare beneficiaries: Site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470–477. doi: 10.1001/jama.2012.207624. [DOI] [PMC free article] [PubMed] [Google Scholar]


