Abstract
Tumour necrosis factor α (TNF-α) inhibitors are frequently used for the treatment of immune-mediated diseases. Conversely, cytokine therapy has the potential to paradoxically induce autoimmunity. A number of case reports have emerged concerning sarcoid-like granulomatosis secondary to TNF-α therapy, an adverse effect that typically affects the pulmonary and cutaneous systems. Granulomatous interstitial nephritis (GIN) is a relatively unknown, relatively under-reported consequence of adalimumab therapy that can have important clinical implications. To our knowledge, this is the first case report of GIN secondary to anti-TNF-α therapy necessitating a prolonged period of dialysis and the first report demonstrating the successful use of secukinumab as an alternative immunomodulatory agent.
Keywords: acute kidney injury, anti-TNF alpha, dialysis, granulomatis interstitial nephritis, sarcoid-like granulomatosis
Background
Granulomatous interstitial nephritis (GIN) is a rare histological diagnosis, present in between 0.5 and 0.9% of native kidney biopsies [1]. Sarcoidosis, infections (particularly tuberculosis) and drugs remain the most common causes [1]. We report a case series of GIN following adalimumab therapy. In the setting of anti-TNF therapy, granulomatous nephritis represents a rare complication that can lead to rapidly progressive renal failure.
Case series
Patient 1
A 42-year-old man was referred following investigation for asymptomatic renal decline and moderate hypertension; his blood pressure (BP) was 150/90 mmHg. His creatinine had increased to 168 µmol/L from 82 µmol/L recorded 10 months prior. Medications included only amlodipine and carvedilol. He had been treated with adalimumab for his ankylosing spondylitis (AS) in the preceding 20 months, with significant benefit. On examination, his BP was 144/68 mmHg with a bland urinalysis.
His initial investigations, including inflammatory markers, chest X-ray and renal ultrasound, were unremarkable. A kidney biopsy was performed (Figure 1) that demonstrated GIN with non-caseating granulomas. He subsequently underwent an extensive evaluation for tuberculosis (TB), including a Quantiferon Gold assay, urine samples for Mycobacterium tuberculosis (MTB) culture, MTB complex DNA quantification, Ziehl–Neelsen staining and TB polymerase chain reaction of the biopsy. These were negative. Human immunodeficiency virus (HIV), brucellosis immunoglobulin G (IgG) and IgM serology and urine culture were negative. His serum angiotensin-converting enzyme (ACE) was mildly elevated at 86 Units of Angiotension Converting Enzyme (UECA) (normal 20–70). Computed tomography (CT) of the thorax, abdomen and pelvic regions illustrated no pathological lymphadenopathy.
Fig. 1.
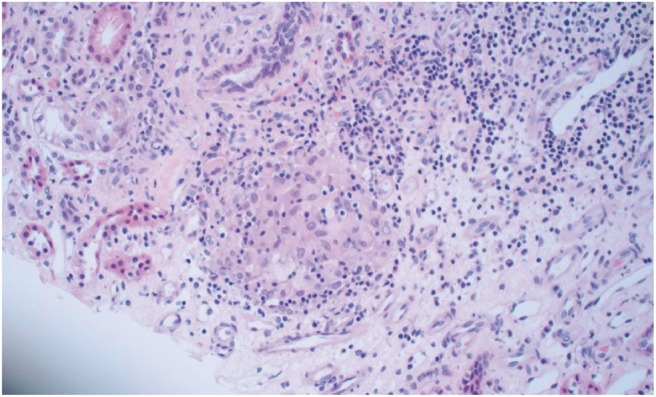
Renal biopsy from Patient 1: a core of renal parenchyma shows an interstitial inflammatory cell infiltrate including non-caseating granulomata admixed with other inflammatory cells including lymphocytes, plasma cells and occasional eosinophils.
Adalimumab was withdrawn. His kidney function stabilized over a period of 6 months to a creatinine of 120 µmol/L. His serum ACE decreased from 86 to 13 UECA. After 6 months off therapy he developed a severe systemic flare of symptoms of AS. He was started on secukinamab, a novel interleukin (IL)-17 inhibitor, to which he has had an excellent therapeutic response.
Patient 2
A 58-year-old man was referred with a 4-week history of fatigue, dyspnoea and a dry cough. Routine blood tests revealed an increased creatinine of 250 µmol/L from 97 µmol/L. He had been on adalimumab 40 mg monthly for 5 years for rheumatoid arthritis (RA).
On physical examination he had bibasal coarse crepitations, with a BP of 140/86 mmHg and a bland urine dipstick. His creatinine increased to 555 µmol/L with a urea of 38 mmol/L, resulting in clinical uraemia. A pyraexia (38.2°C) developed, with rapidly worsening respiratory symptoms. CT of the thorax showed diffuse ground glass change and bilateral mediastinal lymphadenopathy. He was commenced on intermittent haemodialysis. Antimicrobial treatment was initiated with intravenous amoxicillin/clavulanic acid, oral clarithromycin, clindamycin and primaquine.
Blood tests showed a peak corrected calcium of 3.05 mmol/L. Serum ACE levels were elevated at 100 UECA. A vasculitic screen, connective tissue screen, complement levels, serum protein electrophoresis and immunoglobulin levels were normal. Blood cultures, hepatitis, HIV screen, Chlamydia, Brucella, Mycoplasma and leptospirosis serology were negative. Extensive testing to exclude TB as the unifying diagnosis was negative. Bronchoscopy and bronchoalveolar lavage samples were negative for Pneumocystis pneumonia, cytomegalovirus, TB and cryptococcal antigen. There was no relevant travel or occupational exposure to indicate further testing for histoplasmosis. Antimicrobial therapy was discontinued.
Renal biopsy showed granulomatous nephritis without evidence of necrosis. An endobronchial ultrasound and fine-needle aspiration cytology demonstrated granulomatous lymphadenitis.
Adalimumab was withdrawn and empiric prednisolone 60 mg daily was commenced. He remained dialysis dependent for 3 months. Four months after discontinuing adalimumab, dialysis was successfully stopped and steroid therapy was tapered slowly. His kidney function stabilized to a creatinine of 160 μmol/L with resolution of his respiratory symptoms clinically and radiographically. He is currently on methotrexate with good control of his RA.
Discussion
Sarcoid-like granulomatosis secondary to anti-TNF-α therapy is an emerging clinical entity. Typically patients present with pulmonary and cutaneous reactions [2], with isolated cases reporting orbital involvement [3]. However, data on anti-TNF-α-induced granulomatous renal disease have been limited [4–6].
Our incident patient illustrated a clear temporal association between the initiation of TNF-α therapy and the development of GIN. The exposure delay of 10 months prior to the onset of disease and the recovery time of 6 months is in keeping with previous studies. In a case series on primarily pulmonary and cutaneous sarcoid-like granulomatosis, the median delay between TNF-α drug onset and diagnosis was estimated at 18 months for etanercept and 11 months for adalimumab [2]. Our second case presented atypically, with a delay in onset of 5 years. Delays of this magnitude have not been reported with adalimumab use but have been seen in isolated cases of infliximab-related sarcoidosis [7]. Similar to our series, serum ACE was positive in six patients and extensive TB testing proved negative [2]. In both patients, cessation of therapy led to an objective improvement in renal indices, suggesting adalimumab as the precipitant of renal dysfunction.
The mechanism of autoimmune induction by TNF-α agents is poorly understood. Both TNF-α therapies and interferon (IFN)-γ have been reported to induce anti-nuclear antibody production, overt systemic lupus erythematous or sarcoid [2, 4]. It has been suggested that anti-TNF-α therapies may restore a Type 1 T helper cells (Th1) response leading to induction of IFN-γ, subsequently precipitating granulomatosis [2].
Notably, GIN appears to carry a favourable renal prognosis, regardless of the cause. In the largest case series of GIN so far, only 1 of 18 patients required dialysis, although most patients did not recover fully. Secondary fibrosis from healed granulomatous inflammation could represent the mechanism of permanent kidney damage. In this instance, concomitant use of steroids in addition to withdrawal of the offending medication may reduce the risk of renal impairment [8].
Treatment options for the underlying inflammatory disease, such as AS or RA, poses a clinical dilemma. Sarcoid-like granulomatosis has been reported with both TNF soluble receptors and monoclonal TNF antibodies [2, 4, 5]. Conversely, infliximab, adalimumab and etanercept have all been successfully used as alternative rheumatological therapies in patients who have recovered from GIN [7]. Similarly, there have been two cases of primary sarcoid disease causing GIN treated effectively with adalimumab [9]. Given the conflicting nature of the literature, we opted to use secukinumab, an anti-IL-17A monoclonal antibody that has been used in the treatment of both anti-TNF-α-intolerant and anti-TNF-α-naïve patients in AS [10].
To our knowledge, this is the fourth reported case of GIN secondary to TNF-α therapy. It is the first report of GIN successfully treated using secukinumab as an alternative immunomodulatory treatment. Our incident case was characterized by the absence of systemic symptoms, normoalbuminuria and isolated renal involvement, suggesting the importance of a low threshold for kidney biopsy in patients on anti-TNF therapy who present with an otherwise unexplained increase in creatinine. Although withdrawal of the medication typically leads to recovery, patient 2 remained dialysis dependent for 4 months after diagnosis. Given the asymptomatic presentation of our index case, we furthermore strongly suggest regular monitoring of renal indices while on anti-TNF therapy. GIN secondary to adalimumab is evidently an underrecognized complication that can lead to rapid progressive renal decline.
Conflict of interest statement
None declared.
References
- 1. Shivani S, Naima CM, Atta MG.. Granulomatous interstitial nephritis. Clin Kidney J 2015; 8: 516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Daïen CI, Monnier A, Claudepierre P. et al. Sarcoid-like granulomatosis in patients treated with tumor necrosis factor blockers: 10 cases. Rheumatology (Oxford) 2009; 48: 883–886. [DOI] [PubMed] [Google Scholar]
- 3. Wladis EJ, Tarasen AJ, Roth ZJ. et al. Orbital sarcoid-like granulomatosis after inhibition of tumor necrosis factor-α. Ophthal Plast Reconstr Surg 2016; 32: e30–2 [DOI] [PubMed] [Google Scholar]
- 4. Korsten P, Sweiss NJ, Nagorsnik U. et al. Drug-induced granulomatous interstitial nephritis in a patient with ankylosing spondylitis during therapy with adalimumab. Am J Kidney Dis 2010; 56: e17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morgane V, Jean-Charles C, Nicolas M. et al. Renal sarcoid-like granulomatosis during anti-TNF therapy. Kidney Int 2014; 86: 215. [DOI] [PubMed] [Google Scholar]
- 6. Tong D, Manolios N, Howe G. et al. New onset sarcoid-like granulomatosis developing during anti-TNF therapy: an under-recognised complication. Intern Med J 2012; 42: 89–94 [DOI] [PubMed] [Google Scholar]
- 7. Toussirot E, Pertuiset E., Kantelip B.. et al. Sarcoidosis occuring during anti-TNF treatment for inflammatory rheumatic diseases: report of two cases. Clin Exp Rheumatol 2008; 26: 471–475 [PubMed] [Google Scholar]
- 8. Joss N, Morris S, Young B.. et al. Granulomatous interstitial nephritis. CJASN 2007, 2: 222–230 [DOI] [PubMed] [Google Scholar]
- 9. van der Stoep D, Braunstahl GJ, Jende VZ. et al. Sarcoidosis during anti-TNF factor a therapy: no relapse after rechallenge. J Rheumatol 2009; 36: 2847–2848 [DOI] [PubMed] [Google Scholar]
- 10. Guta R, Beudat L, Moore J. et al. Treatment of sarcoid granulomatous interstitial nephritis with adalimumab. NDT Plus 2009; 2: 139–142 [DOI] [PMC free article] [PubMed] [Google Scholar]


