Abstract
Background
Females are more vulnerable to developing cocaine addiction compared with males, a phenomenon that may be regulated by the steroid hormone 17β-estradiol. 17β-Estradiol enhances cocaine reward as measured by the conditioned place preference test. It is currently not known which estrogen receptor is involved or the neuroanatomical locations in which estrogen receptors act to enhance cocaine responses. The purpose of this study was to determine if the estrogen receptors ERα and ERβ regulate cocaine conditioned place preference in mice and whether they act in the nucleus accumbens, a brain region critically involved in the development of cocaine abuse.
Methods
Ovariectomized mice were treated with 17β-estradiol or agonists selective for ERα or ERβ and tested for cocaine conditioned place preference and for c-fos expression in the nucleus accumbens. Female mice with intact ovaries were also tested for cocaine conditioned place preference after RNA interference-mediated knockdown of ERα or ERβ in the nucleus accumbens.
Results
We found that mice treated with 17β-estradiol or an ERβ agonist exhibited increased cocaine conditioned place preference, while knockdown of ERβ, but not ERα, in the nucleus accumbens of females with intact ovaries abrogated cocaine conditioned place preference. Acute treatment with 17β-estradiol or an ERβ agonist induced expression of the immediate-early gene c-fos in the nucleus accumbens, whereas the ERα agonist did not.
Conclusions
These data indicate that ERβ in the nucleus accumbens regulates the development of cocaine conditioned place preference in female mice. 17β-Estradiol may activate neurons in the nucleus accumbens via ERβ. We speculate that this might increase the saliency of cocaine cues that predict drug reward.
Keywords: addiction, cocaine, estradiol, female, reward
Significance Statement
The biological mechanisms responsible for sex differences in the etiology of drug abuse are not well understood, yet knowledge of these mechanisms may be important for effectively treating males and females with cocaine use disorder. Females are particularly vulnerable to developing cocaine addiction, and the steroid hormone estradiol is thought to be one of the biological factors responsible for this increased vulnerability. The present study provides evidence that one of the estrogen receptors, ERβ, enhances the rewarding properties of cocaine in female mice. One of the neuroanatomical locations in which it acts is the nucleus accumbens, a brain region critically involved in cocaine reward. These data provide the basis for understanding sex differences in cocaine addiction and the development of new therapeutic approaches to treating females with cocaine addiction.
Introduction
Males and females respond differently to drugs of abuse. This has important implications for the treatment of substance use disorder (Becker and Koob, 2016). Clinical and preclinical studies indicate that females are more vulnerable to developing cocaine addiction. Women initiate cocaine use at an earlier age, more rapidly transition from occasional use to addiction, and experience higher levels of craving during abstinence compared with men (Griffin et al., 1989; Kosten et al., 1993; Robbins et al., 1999; Chen and Kandel, 2002; Potenza et al., 2012). Female rats acquire cocaine self-administration and conditioned place preference (CPP), a behavioral measure of cocaine reward, more readily and at lower doses than male rats (Lynch and Carroll, 1999; Carroll et al., 2002; Russo et al., 2003; Hu et al., 2004; Zakharova et al., 2009) and show greater cocaine-primed reinstatement of CPP after extinction of the behavior (Bobzean et al., 2010). The molecular mechanisms contributing to these sex differences are not well understood but are important to determine to more effectively treat females suffering from cocaine addiction.
17β-Estradiol (E2), the main circulating form of estrogen produced by the ovaries, enhances behavioral responses to cocaine. E2 treatment of ovariectomized (OVX) rats increases the rate of cocaine self-administration (Lynch et al., 2001; Hu et al., 2004) and augments cocaine CPP compared with controls (Segarra et al., 2010; Segarra et al., 2014). The effect of E2 on cocaine self-administration and CPP in rats may be due to the ability of E2 to act on the mesolimbic dopamine system to regulate reward and motivation. For example, a recent study demonstrated that E2 increases cocaine-stimulated dopamine release in the nucleus accumbens (Acb) (Tobiansky et al., 2016), a critical region of the mesolimbic system involved in addiction. E2 also augments amphetamine- and potassium-induced dopamine release in the Acb (Becker, 1990; Thompson and Moss, 1994) and alters signaling pathways and gene expression in the striatum (Le Saux et al., 2006; Grove-Strawser et al., 2010; Peterson et al., 2015, 2016).
E2 is a ligand for two well-studied estrogen receptors (ERs), ERα and ERβ, and the more recently described G-protein coupled receptor GPR30. These receptors are expressed in the brain and regulate important effects of E2 on sexual and aggressive behavior, learning and memory, and anxiety (Handa et al., 2012; Frick et al., 2015; Alexander et al., 2017). Although E2 clearly plays a role in behavioral responses to cocaine, it is currently not known which receptor mediates these effects and where in the brain ERs act to regulate cocaine reward.
In this study, we sought to answer these questions using the cocaine CPP test to measure drug reward. The CPP test is a conditioning task wherein animals learn to associate cues with the positive effects of a drug and reflects cue-induced drug seeking behavior. Here, we used receptor-specific agonists to activate ERα or ERβ in OVX mice. In a complementary approach, we used lentiviral vectors to attenuate the expression of ERα or ERβ in the Acb of gonadally intact female mice.
To begin to understand how E2 might affect signaling in the Acb, we measured c-fos in the Acb after treatment with E2 or estrogen receptor agonists and cocaine. c-fos is a widely used marker of neuronal activation in the central nervous system (Kovacs, 2008), and its expression is increased in the Acb following acute cocaine administration (Young et al., 1991). Together with our behavioral data, our results suggest that activation of ERβ regulates the rewarding properties of cocaine in females. We speculate that ERβ may engage signaling pathways in the Acb that increase the saliency of cocaine-associated cues.
Materials And Methods
Animals
Mice used for this study were female C57BL/6J (The Jackson Laboratory). Mice were purchased at 8 weeks of age and tested starting at the ages of 10 to 12 weeks. Mice were group-housed (3–5 mice per cage) and maintained on a 14-hour-light/10-hour-dark cycle with lights on at 6 am. Mice had ad libitum access to food and water for the entire duration of the study. All procedures with animals were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the UIC Institutional Animal Care and Use Committee.
Drug Administration
Water-soluble E2 (encapsulated in [2-Hydroxypropyl]-β-cyclodextrin) and (2-Hydroxypropyl)-β-cyclodextrin vehicle (VEH) were purchased from Sigma-Aldrich. E2 was dissolved in saline to a final concentration of 0.02 mg/mL and administered i.p. at a dose of 0.2 mg/kg according to Gresack et al (Gresack and Frick, 2006a, 2006b). VEH-treated mice received the same amount of β-cyclodextrin as E2-treated mice. The ERβ agonist diarylpropionitrile (DPN) and the ERα agonist 4,4’,4’’-(4-Propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT) were purchased from Tocris. Solutions were prepared in sesame oil with 10% ethanol VEH to a final concentration of 0.5 mg/ml. Volumes of 50 μL were injected s.c. for a dose of ~1 mg/kg. Cocaine hydrochloride (Mallinckrodt Pharmaceuticals) was dissolved in saline and administered i.p. at a dose of 5 mg/kg.
Ovariectomy
Mice underwent bilateral ovary removal under anesthesia as described in the supplementary Methods. Mice were allowed to recover for 2 weeks prior to behavioral testing.
Lentiviral Vectors
A short hairpin RNA (shRNA) sequence was designed to target Esr1 (shEsr1) and cloned into the lentiviral vector pLL3.7 as previously described (Lasek et al., 2007). This sequence was verified by Musatov et al to knockdown Esr1 in the brain (Musatov et al., 2006). The lentiviral vector (GIPZ), expressing an shRNA targeting Esr2 (shEsr2), was purchased from GE Healthcare Dharmacon, Inc. (clone ID V3LMM_504017). Verification of mRNA knockdown efficacy was performed in Neuro-2a cells according to Lasek et al (Lasek et al., 2010). We did not quantify knockdown of Esr1 and Esr2 in the Acb, because we found that transcript levels as measured by qPCR are at the lower limit of detection (mean Cq for Esr1, 31.0; mean Cq for Esr2, 34.2) due to the small number of cells expressing the receptors in this brain region (Mitra et al., 2003; Milner et al., 2010). In addition, commercially available antibodies to ERβ are not specific (Snyder et al., 2010). The control vector expresses a nontargeting shRNA (shScr) and was used previously (Lasek et al., 2007, 2010). Lentiviral particles were prepared as described (Lasek et al., 2007). Vectors expressed green fluorescent protein to aid in identifying infected cells.
Stereotaxic Surgeries
Mice were bilaterally injected with lentiviral vectors with a titer of ~3 x 107 pg/mL p24 gag antigen (by ELISA) into the Acb (AP +1.7; ML ±0.9; DV -4.6) using stereotaxic surgery, under 100 mg/kg ketamine and 10 mg/kg xylazine anesthesia as previously described (Lasek et al., 2010). After surgery, mice were allowed to recover for 3 weeks before beginning behavioral testing. To verify infusion into the Acb, immunohistochemistry was performed on brain sections using an antibody specific for green fluorescent protein as previously described (Lasek et al., 2010).
Conditioned Place Preference (CPP)
CPP was performed in a 2-chambered apparatus essentially as described in Hilderbrand and Lasek (2014). Briefly, mice were tested for preference for 30 minutes on the first day (Test 1) prior to conditioning and assigned to the nonpreferred side for cocaine injections. We chose to assign the mice to the nonpreferred side for cocaine injections, because we found after reanalysis of our data (Hilderbrand and Lasek, 2014) that female mice only develop cocaine CPP when they are conditioned with cocaine on the nonpreferred side (supplementary Figure 1). Fifteen-minute cocaine (5 mg/kg) and saline conditioning sessions were performed over the next 6 days, with one conditioning session per day, for a total of 3 cocaine and 3 saline sessions. A 30-minute preference test was performed on day 8 (Test 2). Preference scores were calculated as the difference between the time spent on the cocaine-paired side during Test 2 minus Test 1. For E2 treatments, OVX mice were given a single E2 (n = 21) or VEH (n = 23) injection 20 minutes prior to each conditioning session. For PPT (n = 11), DPN (n = 12), or VEH (n = 16) treatment, OVX mice received injections 1 hour before each conditioning session. The timing and doses were based on the pharmacokinetics of E2, PPT, and DPN such that conditioning was performed when plasma concentrations of the compounds are at their peak (Gresack and Frick, 2006b; Sepehr et al., 2012). For the lentiviral injections into the Acb, 10 mice per group were tested for cocaine CPP.
C-fos Immunohistochemistry (IHC)
OVX mice received a single injection of E2, DPN, PPT, or VEH followed 1 hour later by a single injection of cocaine (5 mg/kg) or saline. One hour after receiving cocaine or saline, each mouse was anesthetized with pentobarbital and transcardially perfused with phosphate buffered saline followed by 4% paraformaldehyde. Brains were processed for immunohistochemistry as described in supplementary Methods. Primary antibody was a rabbit anti-c-fos (Santa Cruz Biotechnology). Four sections containing the Acb core from each mouse were selected and used for cell counting. All images were acquired using a Zeiss AxioScope A1 microscope (Carl Zeiss). c-fos positive cells were counted in an area of 242 μm x 242 μm from images acquired at 20x magnification using ImageJ software (National Institutes of Health). All cell counts were done with investigators blinded to treatment groups. Each group comprised 5 to 8 mice, with 4 sections per mouse for the Acb, 2 sections per mouse for the cingulate cortex (Cg), and 1 section per mouse for the infralimbic cortex (IL).
Statistical Analysis
Data are presented as the mean ± SEM and were analyzed using Prism 6 (GraphPad Software). For the CPP and c-fos data, we used 2-way repeated measures (RM) or 2-way ANOVA followed by posthoc Holm-Sidak’s multiple comparisons tests as appropriate. A Student’s t test or 1-way ANOVA followed by posthoc Holm-Sidak’s multiple comparisons test was used to compare preference scores.
Results
E2 Enhances Cocaine CPP
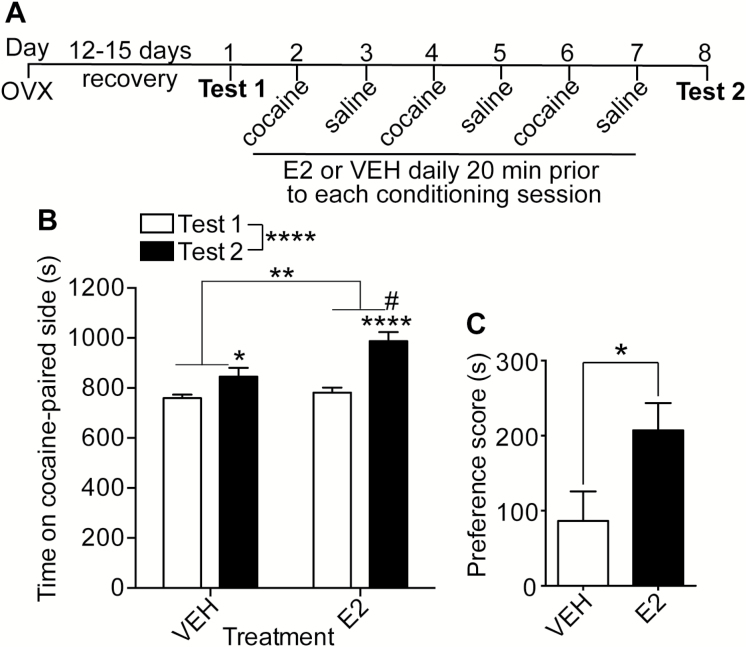
E2 increases the acquisition of cocaine CPP in rats (Segarra et al., 2010, 2014). However, the effect of E2 on cocaine CPP in mice has not been determined. We examined the effect of E2 treatment on the acquisition of cocaine CPP by treating OVX mice with E2 or VEH before each of the conditioning sessions. Figure 1A shows the procedural details. Overall, VEH- and E2-treated mice developed cocaine CPP (Figure 1B; 2-way RM ANOVA of time spent on the cocaine-paired side before and after conditioning, time: F1, 42 = 29.7, P < .0001, treatment: F1, 42 = 7.6, P = .0085, and treatment x time interaction: F1, 42 = 5.0, P = .031). Posthoc tests showed that both VEH- and E2-treated mice developed cocaine CPP (pre- vs postconditioning in VEH-treated mice, P = .025; pre- vs postconditioning in E2-treated mice, P < .0001). Posthoc tests also indicated that E2-treated mice spent significantly more time on the cocaine-paired side after conditioning compared with VEH-treated mice (P = .0013), whereas no difference was observed between VEH- and E2-treated mice before conditioning. In addition, the mean preference score was 58% lower in VEH-treated mice compared with E2-treated mice (Figure 1C; t42 = 2.24, P = .028). These data demonstrate that E2 enhances cocaine reward in OVX mice.
Figure 1.
17β-estradiol (E2) treatment increases cocaine conditioned place preference (CPP) in ovariectomized mice. (A) Timeline and design of the CPP experiment (5 mg/kg cocaine). (B) Graph of the time spent on the cocaine-paired side (in seconds) before conditioning (Test 1) and after conditioning (Test 2) in vehicle (VEH; n = 23)- and E2 (n = 21)-treated mice. Significant main effects by 2-way RM ANOVA: **P < .01 and ****P < .0001. #Indicates a significant difference (P < .01) between VEH and E2 on Test 2; significant difference (*P < .05 and ****P < .0001) between Test 1 and Test 2 within each treatment group by posthoc Holm-Sidak’s multiple comparisons test. (C) Preference scores (Test 2 –Test 1, in seconds) of VEH- and E2-treated mice (*P < .05 by Student’s t test). Data are presented as the mean ± SEM.
Activation of ERβ Is Sufficient to Enhance Cocaine CPP
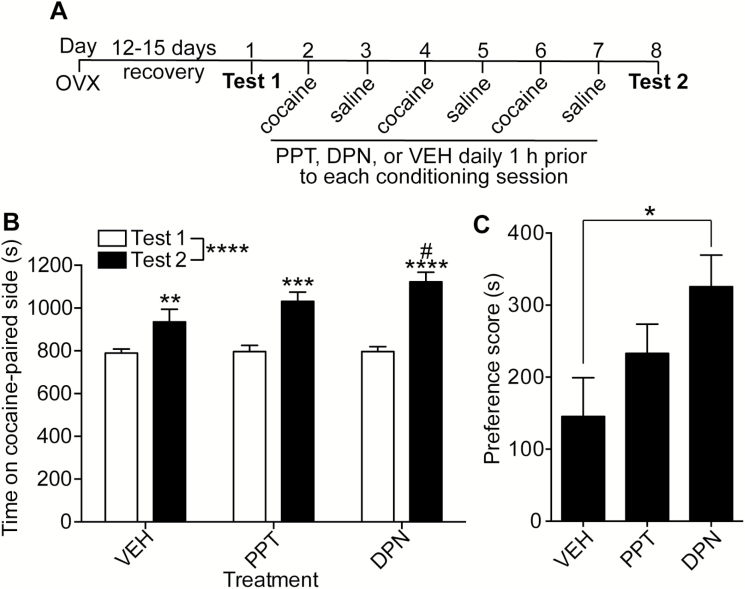
To determine which estrogen receptors underlie the ability of E2 to enhance cocaine CPP, OVX mice were treated with selective ERα or ERβ agonists. PPT is an ERα agonist with a 410-fold higher affinity for ERα versus ERβ. DPN is an ERβ agonist with a 70-fold higher affinity for ERβ versus ERα (Stauffer et al., 2000; Meyers et al., 2001). The experimental procedure for the cocaine CPP experiment is outlined in Figure 2A. Mice treated with DPN developed greater cocaine CPP compared with VEH-treated mice (Figure 2B; 2-way RM ANOVA, time: F1, 36 = 66.9, P <.0001, treatment x time interaction: F2, 36 = 3.57, P = .034). Posthoc comparisons indicated that each group of mice developed cocaine CPP (pre- vs postconditioning: VEH, P = .023; PPT, P = .0002; DPN, P < .0001). However, mice treated with DPN spent significantly more time on the cocaine-paired side after conditioning than VEH-treated mice (P = .0039), while PPT-treated mice did not differ from VEH. The mean preference score of DPN-treated mice was 124% higher than VEH-treated mice (Figure 2C, P = .034). These results suggest that activation of ERβ is sufficient for the E2-mediated enhancement of cocaine CPP.
Figure 2.
Activation of ERβ is sufficient to enhance cocaine conditioned place preference (CPP) in ovariectomized (OVX) mice. (A) Timeline and design of the CPP experiment (5 mg/kg cocaine). (B) Graph of the time spent on the cocaine-paired side (in seconds) before conditioning (Test 1) and after conditioning (Test 2) of vehicle (VEH; n = 16), the ERα agonist (PPT; n = 11), and the ERβ agonist (DPN; n = 12)-treated mice. Significant main effect by 2-way RM ANOVA: ****P < .0001. #Indicates a significant difference between VEH - and DPN-treated mice on Test 2 (P < .01); Significant difference (**P < .01, ***P < .001, and ****P < .0001) between Test 1 and Test 2 within each treatment group by posthoc Holm-Sidak’s multiple comparisons test. (C) Preference scores (Test 2-Test 1, in seconds) of VEH-, PPT-, and DPN-treated mice. A 1-way ANOVA of preference scores demonstrated a significant difference between treatment groups (P < .05). Posthoc analysis revealed a significant increase in preference score in DPN- vs VEH-treated mice (*P < .05). Data are presented as the mean ± SEM.
ERβ Is Necessary for Cocaine CPP in Female Mice
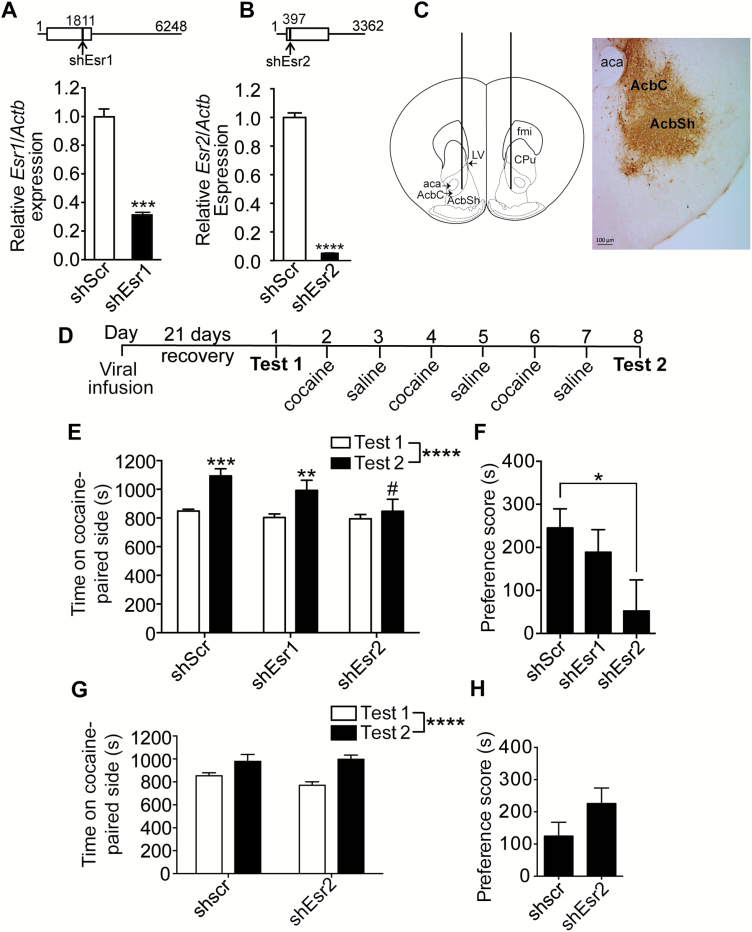
To determine if ERα or ERβ is necessary for the development of cocaine CPP under more natural conditions, we manipulated ERs in female mice with intact ovaries. The second goal of this experiment was to determine a neuroanatomical location in which ERs might act to regulate cocaine reward. To do this, we generated lentiviral vectors that express shRNAs that specifically target the genes encoding ERα (Esr1) or ERβ (Esr2) for RNA interference-mediated attenuation of gene expression (shEsr1 and shEsr2, respectively). To validate the shRNAs, we transfected Neuro-2a cells with lentiviral plasmids and tested mRNA levels by qPCR. Transfection of cells with shEsr1 plasmid reduced Esr1 expression by 69% compared with transfection of the shScr control plasmid (Figure 3A; P = .0003). Similarly, cells transfected with shEsr2 plasmid showed a 95% reduction in Esr2 expression compared with controls (Figure 3B; P < .0001). This demonstrates that each shRNA is effective at reducing expression of Esr1 or Esr2.
Figure 3.
ERβ is necessary for cocaine conditioned place preference (CPP) in female mice. (A, B) Top panels show the location of the short hairpin RNA (shRNA) targeting ERα (shEsr1, A) and ERβ (shEsr2, B). The horizontal line represents the reference sequence transcript, with the nucleotide position indicated by a number. The open box shows the position of the protein coding sequence in the transcript, and the black vertical line shows the location of the 19 nucleotide targeting sequence in the shRNA. The bottom graphs show the relative mRNA expression of Esr1 (A) and Esr2 (B) by quantitative real-time PCR in Neuro-2a cells transfected with lentiviral plasmids expressing each of the shRNAs. Asterisks indicate a significant difference in Esr1 (A) and Esr2 (B) expression in cells transfected with shEsr1 or shEsr2, respectively, compared with the control shRNA (shScr, ***P < .001 or ****P < .0001 by Student’s t test, n = 3). (C) The left panel is a drawing of a coronal section of the mouse brain at 1.7 mm anterior to bregma, with the location of the cannulas used to infuse the lentivirus bilaterally into the nucleus accumbens (Acb) indicated by vertical lines. Adapted from the Mouse Brain Atlas (Franklin and Paxinos, 3rd ed.). The right panel is a representative image of a coronal section of lentivirus infection in the Acb as measured by expression of green fluorescent protein, indicated by dark brown staining. (D) Timeline and design of the CPP experiment. (E) Graph of the time spent on the cocaine-paired side (in seconds) before conditioning (Test 1) and after conditioning (Test 2) with 5 mg/kg cocaine of mice expressing shEsr1, shEsr2, or shScr in the Acb. Data are presented as the means ±SEM. Significant main effect by two-way RM ANOVA (****P < .0001, n = 10). #Indicates significant difference between shScr and shEsr2 on Test 2 (P < .01); Significant difference between Test 1 and Test 2 within each treatment group (**P < .01 and ***P < .001) by posthoc multiple comparisons test. (F) Preference scores (Test 2-Test 1, in seconds) of mice expressing shScr, shEsr1, and shEsr2 in the Acb. A 1-way ANOVA showed a trend toward an effect of shRNA (P = .067, n = 10) with posthoc multiple comparisons test indicating a significant difference between mice expressing shEsr2 compared with shScr (P < .05). (G) Time on cocaine-paired side before conditioning (Test 1) and after conditioning (Test 2) in male mice expressing shScr or shEsr2 in the Acb (n = 8). There was a significant effect of time (****P < .0001), but no significant effect of shRNA. (H) Mean preference scores of male mice expressing shScr or shEsr2 in the Acb after cocaine CPP. aca, anterior commissure; AcbC, nucleus accumbens core; AcbSh, nucleus accumbens shell; fmi, forceps minor of the corpus callosum; LV, lateral ventricle; CPu, caudate putamen. Scale bar, 100 μm.
Lentiviruses derived from these plasmids were injected into the Acb and mice were tested for cocaine CPP. A representative image of infection in the Acb is shown in Figure 3C. We measured estrous cycles in a separate group of mice expressing shEsr1 and shEsr2 in the Acb and found that estrous cycles were not altered compared with control mice, indicating that knockdown of these receptors in the Acb does not affect the reproductive cycle (supplementary Table 1). The cocaine CPP procedure is outlined in Figure 3D. Mice expressing shEsr2 in the Acb exhibited a marked attenuation of cocaine CPP compared with mice expressing shScr and shEsr1 (Figure 3E; 2-way RM ANOVA, time: F1, 27 = 23.9, P < .0001, shRNA: F2, 27 = 3.03, P = .065, shRNA x time interaction: F2, 27 = 2.99, P = .067). Posthoc multiple comparisons test demonstrated that mice expressing shScr and shEsr1 developed cocaine CPP (P = .0006 and P = .0056, respectively), whereas mice expressing shEsr2 did not. Moreover, there was a significant difference after conditioning between mice expressing shScr and shEsr2 (P = .0042), but no difference between these groups before conditioning. Finally, the mean preference score of mice expressing shEsr2 was reduced by 79% compared with mice expressing shScr in the Acb (Figure 3F; P = .049). We also injected lentivirus expressing shEsr2 or shScr into the Acb of male mice and tested them for cocaine CPP. There was no difference in cocaine CPP between male mice expressing shScr compared with shEsr2 in the Acb (Figure 3G-H; 2-way RM ANOVA, time: F1, 14 = 29.23, P < .0001, shRNA: F1, 14 = 0.46, P = .508, shRNA x time interaction: F1, 14 = 2.44, P = .141). These results suggest that ERβ in the Acb is required for the development of cocaine CPP in female mice.
E2 Induces c-fos Expression in the Acb
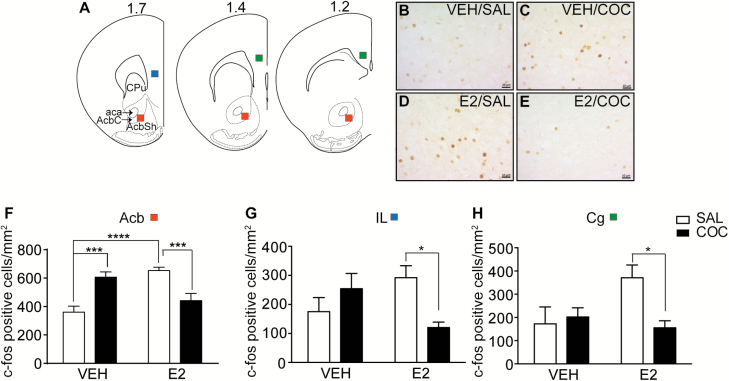
C-fos is an immediate-early gene that acts a reporter for neuronal activation. It is rapidly induced in the Acb by acute cocaine treatment (Hope et al., 1992). We examined c-fos expression by counting cells positive for c-fos immunoreactivity in the Acb of OVX mice following VEH or E2 and saline or cocaine treatments to determine if acute treatment with E2 and/or low-dose cocaine could alter neuronal activation in the Acb of females. Data analysis showed a significant interaction between cocaine and E2 (Figure 4; 2-way ANOVA, cocaine x E2 interaction: F1, 84 = 34.8, P < .0001). Post-hoc multiple comparisons tests demonstrated that there was a difference in the number of c-fos positive cells between saline- and cocaine-treated mice in the VEH-treated group (P = .0001). As expected, cocaine increased the number of c-fos positive cells in the Acb by 68% compared with saline. In addition, there was a significant difference in the number of c-fos positive cells between VEH- and E2-treated mice in the saline-treated group (P < .0001). E2 increased the number of c-fos positive cells in the Acb by 81%. Surprisingly, we found that the combination of E2 and cocaine led to a normalization of the number of c-fos positive cells to control levels. These data demonstrate that E2 and cocaine independently increase neuronal activation in the Acb as measured by c-fos, whereas the combination of cocaine and E2 interact to return c-fos expression to baseline levels.
Figure 4.
17β-estradiol (E2) induces c-fos expression in the nucleus accumbens (Acb). (A) Illustration of coronal sections containing the Acb with red boxes showing the counting areas. Green and blue boxes show the counting areas for the cingulate (Cg) and infralimbic (IL) areas of the prefrontal cortex, respectively. Adapted from the Mouse Brain Atlas (Franklin and Paxinos, 3rd ed.). (B–E) Representative images of c-fos immunoreactivity in the Acb core of ovariectomized mice injected with vehicle (VEH) or E2 and saline (SAL) or cocaine (COC). (F–H) Graphs of the numbers of c-fos positive cells counted per mm2 in the Acb (F), IL (G), and Cg (H) areas after treatments. Data are presented as means ± SEM. Asterisks indicate significant interactions by 2-way ANOVA (*P < .05, ***P < .001, **** P < .0001; n = 5 for VEH/SAL, VEH/COC, and E2/COC; n = 6 for E2/SAL). Scale bar, 10 μm.
To determine if the effects of E2 and cocaine on c-fos expression were specific to the Acb, we also measured c-fos immunoreactivity in the IL and cingulate (Cg) regions of the prefrontal cortex (Figure 4). In both these regions, there was a significant interaction between cocaine and E2 treatment (IL: F1, 22 = 9.83, P = .0048; Cg: F1, 20 = 5.56, P = .029), but no main effects of cocaine or E2. Posthoc multiple comparisons tests indicated that the interaction was due to a difference between saline and cocaine in the E2-treated group only (IL, P = .028; Cg, P = .049), with c-fos cell counts significantly lower in the cocaine-treated mice compared with saline-treated mice. In contrast to the Acb, c-fos expression did not increase in the IL and Cg in the VEH-treated mice after cocaine treatment, possibly because of the low dose of cocaine used.
Activation of ERβ Induces c-fos Expression in the Acb
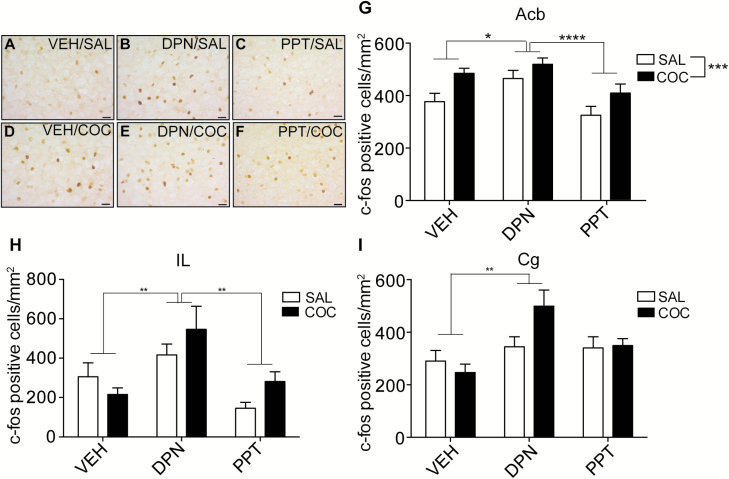
Next, we tested whether ERβ might be responsible for the ability of E2 to increase c-fos expression in the Acb. OVX mice were treated with VEH, DPN (the ERβ agonist), or PPT (the ERα agonist) and saline or cocaine. There were significant main effects of estrogen receptor agonist and cocaine but no significant interaction (Figure 5; 2-way ANOVA, cocaine: F1, 186 = 11.44, P = .0009; agonist: F2, 186 = 9.21, P = .0002). To parse out the differences between the 2 estrogen receptor agonists, main effects of VEH, PPT, and DPN were examined posthoc. There was a significant difference between PPT and DPN (P < .0001). VEH vs PPT and VEH vs DPN comparisons were also nearly significant (P = .053). These results demonstrate that cocaine and DPN independently increase c-fos in the Acb. Interestingly, the effect of PPT was the opposite to that of DPN, with PPT decreasing c-fos in the Acb. These data suggest that activation of ERβ is likely responsible for the increase in c-fos expression elicited by E2 in the absence of cocaine in the Acb.
Figure 5.
Activation of ERβ induces c-fos expression in the nucleus accumbens (Acb), infralimbic (IL), and cingulate (Cg) cortex. (A–F) Representative images of c-fos immunoreactivity in the Acb core of ovariectomized mice treated with vehicle (VEH), the ERβ agonist (DPN), or the ERα agonist (PPT) and saline (SAL) or cocaine (COC). (G–I) Graphs of the numbers of c-fos positive cells counted per mm2 in the Acb (G), IL (H), and Cg (I) areas after treatments. Data are presented as means ± SEM. Asterisks indicate significant main effects of treatment by 2-way ANOVA, (*P = 0.05, **P < .01, ****P < .0001, n = 8 for all groups). Note that DPN treatment was significantly different than VEH and PPT treatment in the Acb and IL. Scale bar, 10 μm.
We also examined c-fos induction in the IL and Cg with these treatments. In both the IL and Cg, there were significant main effects of estrogen receptor agonist (Figure 5; IL: F2, 42 = 9.64, P = .0004; Cg: F2, 81 = 6.82, P = .0018) but no significant effect of cocaine treatment. Posthoc analysis of main effects of estrogen receptor agonist treatment demonstrated significant differences between VEH and DPN (IL, P = .0026; Cg, P = .0015), with DPN treatment increasing c-fos expression in these brain regions. In the IL, c-fos expression was also higher in the DPN-treated group than in the PPT-treated group (P = .0026). Interestingly, in the Cg, there was a trend toward an interaction between cocaine and estrogen receptor agonist treatment by 2-way ANOVA (F2, 81 = 3.04, P = .053), with posthoc testing indicating an increase in c-fos after cocaine treatment in the DPN-treated group (P = .033). Together, these results indicate that DPN increases c-fos expression in the Acb, IL, and Cg regions of the brain.
Discussion
To our knowledge, this is the first study to demonstrate that E2 promotes cocaine reward in female mice, that activation of ERβ is both necessary and sufficient to enhance cocaine reward, and that ERβ functions in the Acb. Interestingly, we found that under circumstances in which circulating E2 is absent (OVX mice), preference scores were significantly lower than in OVX mice treated with E2 and in mice with intact ovaries (~100 seconds for OVX and ~200 seconds for OVX plus E2 and intact females). Since we were able to restore preference scores to levels seen in non-OVX females by treating OVX mice with E2, our data demonstrate that E2 produced by the ovaries is important for cocaine reward in female mice. This is consistent with a report by Mirbaha et al., who found that E2 enhances morphine CPP in female mice (Mirbaha et al., 2009).
The main novel finding of this study is that E2 can act through ERβ to increase cocaine reward. Activation of ERβ by DPN increases the mean preference score to similar levels as found with E2 treatment. Similarly, Silverman and Koenig demonstrated that treatment of OVX rats with DPN increases amphetamine CPP to the same level as in rats treated with E2 (Silverman and Koenig, 2007). Our cocaine CPP results also show interesting parallels with a study by Larson and Carroll demonstrating that activation of ERβ is important for drug-primed reinstatement of extinguished cocaine self-administration in OVX female rats (Larson and Carroll, 2007). Reinstatement of cocaine self-administration is a model for drug-seeking behavior, indicating that activation of ERβ may contribute to cocaine relapse.
Our data also demonstrate that, in addition to activation of ERβ being sufficient to increase cocaine CPP, ERβ in the Acb may be necessary for the development of cocaine CPP in female mice. We found that knockdown of ERβ in the Acb of mice with intact ovaries essentially eliminated the development of cocaine CPP. The advantage of performing studies in mice with intact ovaries is that it more closely models the natural state, whereas OVX dramatically and rapidly eliminates circulating hormones, induces a menopause-like condition, and may lead to neuroadaptations. We also found that knockdown of ERβ in the Acb of male mice did not affect cocaine CPP, indicating a potentially different mechanism for cocaine reward in male and female mice. It is interesting to consider why knockdown of ERβ in the Acb led to sex differences in the response to cocaine. Although male mice apparently express ERβ in the Acb to the same extent as females (Milner et al., 2010), they may not be responding in the same way to ERβ activation. This might be due to organizational differences in the brain guided by E2 during developmental sexual differentiation of the nervous system or due to acute differences in ERβ activation. Differential localization of ERβ at a subcellular level or different isoforms generated by alternative splicing in males and females might account for this. Wissman et al. have also observed sexually dimorphic effects of cocaine in the Acb on dendritic spine density and synaptic responses (Wissman et al., 2011). To our knowledge, this is the first demonstration that ERβ is crucial for the development of cocaine CPP in naturally cycling females.
In contrast to our results with ERβ manipulation, we found that OVX mice treated with PPT did not demonstrate a significant difference in cocaine CPP compared with VEH- or DPN-treated mice. PPT-treated mice showed an intermediate level of cocaine CPP, with the mean preference score falling between the scores of VEH- and DPN-treated mice. We cannot rule out that ERα might be involved in the enhancement of cocaine CPP by E2, because we have only tested one dose of PPT. Testing higher doses of PPT might increase cocaine CPP, but higher doses would potentially lead to activation of ERβ, since PPT can activate both receptors at higher concentrations. However, our results do demonstrate that activation of ERβ by DPN is sufficient to enhance cocaine CPP. We also found that knockdown of ERα in the Acb of gonadally intact females had no significant effect on cocaine CPP. Similar to the PPT results, mice expressing shEsr1 in the Acb demonstrated an intermediate level of cocaine CPP that was not significantly different from mice expressing the control shRNA or shEsr2 in the Acb. One technical limitation is that knockdown of ERα in the Acb with this shRNA may not have been sufficient to observe a behavioral effect. Our in vitro knockdown data indicated that the shEsr1 construct was able to knockdown Esr1 by ~70%, whereas the shEsr2 construct was able to knockdown Esr2 by 95%. Thus, it is possible that both ERα and ERβ are involved in the enhancement of cocaine CPP by E2. However, our data with the ERβ agonist and shRNA do indicate a role for ERβ in cocaine reward in females.
In this study, we have shown that one site where ERβ acts to modulate cocaine reward is the Acb. The lentiviral vector that we employed infects cell bodies at the injection site and is not transported to other brain regions, so our results demonstrate a direct role for ERβ in the Acb in cocaine CPP. The specific cell types in the Acb responsible for this effect are not known and will be important to determine in future experiments. It is possible that ERβ also acts in brain regions other than the Acb to enhance cocaine CPP.
In this study we also found that acute administration of E2 and the ERβ agonist DPN induce c-fos expression in the Acb. These data suggest that E2, acting through ERβ, increases signaling and neural activity in the Acb. We did not observe an increase in c-fos after treatment with the ERα agonist PPT in the Acb, and in fact, PPT actually decreased c-fos in the Acb. Selective activation of ERα and ERβ can have opposite effects on c-fos induction (Weiser and Handa, 2009). It has been known for quite some time that acute injections of cocaine increase c-fos expression in the Acb and that the induction of c-fos depends on activation of the dopamine D1 receptor (Young et al., 1991; Hope et al., 1992; Bertran-Gonzalez et al., 2008). Increased c-fos in the Acb by E2 and DPN (in the absence of cocaine) could be due to a direct effect of ERβ signaling on c-fos transcription in dopamine D1-receptor expressing Acb neurons. Alternatively, this activation may be the result of increased dopamine release in the Acb through actions of ERβ in another brain region. Tobiansky et al. found that lesions of the medial preoptic area increase cocaine-induced c-fos expression and dopamine release in the Acb and that injection of E2 into the medial preoptic area increases dopamine release in the Acb (Tobiansky et al., 2013, 2016). The fact that we also observed increased c-fos in the IL and Cg areas of the prefrontal cortex in addition to the Acb after systemic DPN treatment argues that activation of ERβ in another brain region might lead to increased dopamine release in dopaminergic target regions, which include the prefrontal cortex and Acb, and increase c-fos levels. The increase in c-fos expression after treatment with E2 or DPN in the absence of cocaine may be due to an activation of the same neurons and signal transduction cascades that are activated by acute cocaine treatment. Activation of these neurons by E2 or DPN may prime them to respond to cocaine-stimulated dopamine release in the Acb, which could manifest later as an enhanced association between cocaine-associated cues. Since we administered DPN systemically, we do not know the mechanism that is responsible for the increase in c-fos. Future experiments will examine whether the increase in c-fos after DPN treatment is due to direct activation of ERβ-expressing neurons in specific brain regions by DPN, or the result of ERβ acting in another brain region to increase dopamine release in these brain regions.
We originally hypothesized that the combination of E2 plus acute cocaine would augment c-fos expression in the Acb more than either treatment alone. Curiously, we found that the combination of E2 plus cocaine led to a normalization of c-fos in the Acb. We do not have a plausible explanation for this, but it is similar to what has been observed with chronic cocaine treatment, in which c-fos levels are normalized after repeated cocaine injections (Hope et al., 1992). Treatment with DPN plus cocaine did not lead to enhanced induction of c-fos expression in the Acb compared with the increase seen with DPN or cocaine treatment alone. Different pharmacokinetics of E2 and DPN in the brain might have contributed to differences between their effects on c-fos expression when combined with cocaine. It is notable that Niyomchai et al. found that the combination of E2 plus cocaine treatment in female rats did not increase c-fos mRNA levels in the caudate putamen more than cocaine treatment alone (Niyomchai et al., 2006), similar to what we found with DPN plus cocaine. It is possible that a more detailed study incorporating multiple time points (and knowing brain levels of E2 and DPN at these time points) would answer these questions. Alternatively, there might be a role for ERα in decreasing c-fos expression after E2 and cocaine treatments, since we found that PPT, in contrast to DPN, decreased c-fos in the Acb. At this point, it is difficult to reconcile our c-fos findings with the results of the CPP tests, but the goal in performing the c-fos experiments was to determine if E2 and/or DPN might acutely activate neurons in the same brain region (Acb) in which we observed an effect of ERβ knockdown. To our knowledge, this is the first demonstration that activation of ERβ alone increases c-fos in the Acb and prefrontal cortex regions.
Estrogen receptors have historically been known as ligand-activated transcription factors that act in the nucleus to regulate gene expression by binding to estrogen response elements at the promoters of specific genes, but have more recently been discovered to act as rapid signal transducers at the plasma membrane (Almey et al., 2015). It is currently not known whether ERβ is acting through genomic or rapid signaling (or both) to increase cocaine CPP in females. DPN is known to alter the transcription of many genes in the hippocampus, including genes involved in neurotransmission that could conceivably regulate cocaine reward (Sarvari et al., 2016). It will be important to determine changes in gene expression and rapid signal transduction cascades in the Acb elicited by E2 and DPN in the future to begin to sort out the molecular mechanisms leading to ERβ-induced enhancement of cocaine CPP.
Although the CPP test is a surrogate measure for the rewarding properties of a drug, it is an associative conditioning task dependent on learning and memory processes. E2 has been shown to improve memory in hippocampal-dependent behavioral tests (Fernandez et al., 2008) and to promote extinction of cocaine CPP (Twining et al., 2013), a process that is dependent on memory processes. Activation of either ERα or ERβ is sufficient to increase hippocampal-dependent memory (Boulware et al., 2013). Using the CPP test, we are unable to disentangle whether the ability of E2 to enhance cocaine CPP is due to an enhanced memory for cocaine-associated-cues, or if it is due to a direct effect on the pleasurable response to cocaine. E2 has been shown to increase cocaine reward in the intracranial self-stimulation paradigm, which is a more direct test for measuring drug reward (Galankin et al., 2010). This suggests that E2 may enhance cocaine CPP through an effect on reward. The knowledge gained from the studies presented here gives a greater mechanistic understanding of sex-specific mechanisms involved in addiction, a currently understudied area, and provides a framework for developing novel treatments for cocaine abuse that may be effective in women.
Supplementary Material
Supplementary data are available at International Journal of Neuropsychopharmacology online.
Supplementary Material
Acknowledgments
We thank Subhash Pandey, Amynah Pradhan, Antonia Savarese, and Elisa Hilderbrand for comments on the manuscript, and Hu Chen and Mahathi Challapalli for assistance with c-fos counting.
This work was supported by the National Institutes of Health (grant no. R01 DA033429 to A.W.L.).
Statement of Interest
None.
References
- Alexander A, Irving AJ, Harvey J(2017)Emerging roles for the novel estrogen-sensing receptor GPER1 in the CNS. Neuropharmacology 113:652–660. [DOI] [PubMed] [Google Scholar]
- Almey A, Milner TA, Brake WG(2015)Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm Behav 74:125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB.(1990)Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett 118:169–171. [DOI] [PubMed] [Google Scholar]
- Becker JB, Koob GF(2016)Sex differences in animal models: Focus on addiction. Pharmacol Rev 68:242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA(2008)Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci 28:5671–5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobzean SA, Dennis TS, Addison BD, Perrotti LI(2010)Influence of sex on reinstatement of cocaine-conditioned place preference. Brain Res Bull 83:331–336. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Heisler JD, Frick KM(2013)The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci 33:15184–15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK(2002)Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology 161:304–313. [DOI] [PubMed] [Google Scholar]
- Chen K, Kandel D(2002)Relationship between extent of cocaine use and dependence among adolescents and adults in the United States. Drug Alcohol Depend 68:65–85. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM(2008)Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci 28:8660–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Kim J, Tuscher JJ, Fortress AM(2015)Sex steroid hormones matter for learning and memory: estrogenic regulation of hippocampal function in male and female rodents. Learn Mem 22:472–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galankin T, Shekunova E, Zvartau E(2010)Estradiol lowers intracranial self-stimulation thresholds and enhances cocaine facilitation of intracranial self-stimulation in rats. Horm Behav 58:827–834. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM (2006a) Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav 84:112–119. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM (2006b) Effects of continuous and intermittent estrogen treatments on memory in aging female mice. Brain Res 1115:135–147. [DOI] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U(1989)A comparison of male and female cocaine abusers. Arch Gen Psychiatry 46:122–126. [DOI] [PubMed] [Google Scholar]
- Grove-Strawser D, Boulware MI, Mermelstein PG(2010)Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience 170:1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Mani SK, Uht RM(2012)Estrogen receptors and the regulation of neural stress responses. Neuroendocrinology 96:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilderbrand ER, Lasek AW(2014)Sex differences in cocaine conditioned place preference in C57BL/6J mice. Neuroreport 25:105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope B, Kosofsky B, Hyman SE, Nestler EJ(1992)Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc. Natl. Acad. Sci. U.S.A. 89:5764–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB(2004)Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology 29:81–85. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ(1993)Gender differences in cocaine use and treatment response. J Subst Abuse Treat 10:63–66. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ.(2008)Measurement of immediate-early gene activation- c-fos and beyond. J Neuroendocrinol 20:665–672. [DOI] [PubMed] [Google Scholar]
- Larson EB, Carroll ME(2007)Estrogen receptor beta, but not alpha, mediates estrogen’s effect on cocaine-induced reinstatement of extinguished cocaine-seeking behavior in ovariectomized female rats. Neuropsychopharmacology 32:1334–1345. [DOI] [PubMed] [Google Scholar]
- Lasek AW, Janak PH, He L, Whistler JL, Heberlein U(2007)Downregulation of mu opioid receptor by RNA interference in the ventral tegmental area reduces ethanol consumption in mice. Genes Brain Behav 6:728–735. [DOI] [PubMed] [Google Scholar]
- Lasek AW, Kapfhamer D, Kharazia V, Gesch J, Giorgetti F, Heberlein U(2010)Lmo4 in the nucleus accumbens regulates cocaine sensitivity. Genes Brain Behav 9:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saux M, Morissette M, Di Paolo T(2006)ERbeta mediates the estradiol increase of D2 receptors in rat striatum and nucleus accumbens. Neuropharmacology 50:451–457. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME(1999)Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology 144:77–82. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Mickelberg JL, Carroll ME(2001)Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav 68:641–646. [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA(2001)Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem 44:4230–4251. [DOI] [PubMed] [Google Scholar]
- Milner TA, Thompson LI, Wang G, Kievits JA, Martin E, Zhou P, McEwen BS, Pfaff DW, Waters EM(2010)Distribution of estrogen receptor beta containing cells in the brains of bacterial artificial chromosome transgenic mice. Brain Res 1351:74–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirbaha H, Tabaeizadeh M, Shaterian-Mohammadi H, Tahsili-Fahadan P, Dehpour AR(2009)Estrogen pretreatment modulates morphine-induced conditioned place preference in ovariectomized mice. Pharmacol Biochem Behav 92:399–403. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE(2003)Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology 144:2055–2067. [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S(2006)RNAi-mediated silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc. Natl. Acad. Sci. U.S.A. 103:10456–10460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyomchai T, Jenab S, Festa ED, Akhavan A, Quinones-Jenab V(2006)Effects of short- and long-term estrogen and progesterone replacement on behavioral responses and c-fos mRNA levels in female rats after acute cocaine administration. Brain Res 1126:193–199. [DOI] [PubMed] [Google Scholar]
- Peterson BM, Mermelstein PG, Meisel RL(2015)Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct Funct 220:2415–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BM, Martinez LA, Meisel RL, Mermelstein PG(2016)Estradiol impacts the endocannabinoid system in female rats to influence behavioral and structural responses to cocaine. Neuropharmacology 110:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R(2012)Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry 169:406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP(1999)Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend 53:223–230. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V(2003)Sex differences in the conditioned rewarding effects of cocaine. Brain Res 970:214–220. [DOI] [PubMed] [Google Scholar]
- Sarvari M, Kallo I, Hrabovszky E, Solymosi N, Rodolosse A, Liposits Z(2016)Long-term estrogen receptor beta agonist treatment modifies the hippocampal transcriptome in middle-aged ovariectomized rats. Front Cell Neurosci 10:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra AC, Agosto-Rivera JL, Febo M, Lugo-Escobar N, Menendez-Delmestre R, Puig-Ramos A, Torres-Diaz YM(2010)Estradiol: a key biological substrate mediating the response to cocaine in female rats. Horm Behav 58:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra AC, Torres-Diaz YM, Silva RD, Puig-Ramos A, Menendez-Delmestre R, Rivera-Bermudez JG, Amadeo W, Agosto-Rivera JL(2014)Estrogen receptors mediate estradiol’s effect on sensitization and CPP to cocaine in female rats: role of contextual cues. Horm Behav 65:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehr E, Lebl-Rinnova M, Mann MK, Pisani SL, Churchwell MI, Korol DL, Katzenellenbogen JA, Doerge DR(2012)Pharmacokinetics of the estrogen receptor subtype-selective ligands, PPT and DPN: quantification using UPLC-ES/MS/MS. J Pharm Biomed Anal 71:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Koenig JI(2007)Evidence for the involvement of ERbeta and RGS9-2 in 17-beta estradiol enhancement of amphetamine-induced place preference behavior. Horm Behav 52:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder MA, Smejkalova T, Forlano PM, Woolley CS(2010)Multiple ERbeta antisera label in ERbeta knockout and null mouse tissues. J Neurosci Methods 188:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA(2000)Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem 43:4934–4947. [DOI] [PubMed] [Google Scholar]
- Thompson TL, Moss RL(1994)Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J Neurochem 62:1750–1756. [DOI] [PubMed] [Google Scholar]
- Tobiansky DJ, Roma PG, Hattori T, Will RG, Nutsch VL, Dominguez JM(2013)The medial preoptic area modulates cocaine-induced activity in female rats. Behav Neurosci 127:293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiansky DJ, Will RG, Lominac KD, Turner JM, Hattori T, Krishnan K, Martz JR, Nutsch VL, Dominguez JM(2016)Estradiol in the preoptic area regulates the dopaminergic response to cocaine in the nucleus accumbens. Neuropsychopharmacology 41:1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining RC, Tuscher JJ, Doncheck EM, Frick KM, Mueller D(2013)17beta-estradiol is necessary for extinction of cocaine seeking in female rats. Learn Mem 20:300–306. [DOI] [PubMed] [Google Scholar]
- Weiser MJ, Handa RJ(2009)Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience 159:883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissman AM, McCollum AF, Huang GZ, Nikrodhanond AA, Woolley CS(2011)Sex differences and effects of cocaine on excitatory synapses in the nucleus accumbens. Neuropharmacology 61:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ST, Porrino LJ, Iadarola MJ(1991)Cocaine induces striatal c-fos-immunoreactive proteins via dopaminergic D1 receptors. Proc. Natl. Acad. Sci. U.S.A. 88:1291–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Wade D, Izenwasser S(2009)Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol. Biochem. Behav. 92:131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.