Abstract
Context
It is presumed that the incidence of adrenal adenomas is symmetric between the left and right adrenal gland; however, anecdotal observations suggest a potential lateralizing asymmetry.
Objective
To investigate the symmetry in detection of adrenal adenomas and relevance to patient care.
Design
Cross-sectional and longitudinal studies.
Population and Setting
One thousand three hundred seventy-six patients with abdominal computed tomography or magnetic resonance imaging demonstrating benign-appearing adrenal adenomas.
Main Outcome
Location and size of adrenal adenomas.
Results
Left-sided adenomas were discovered in 65% of patients, right-sided in 21%, and bilateral adenomas in 14%. Among unilateral adenomas, 75% were left-sided. Left-sided adenomas were more prevalent than right-sided adenomas in each size category except the largest: <10 mm, 87%; 10 to 19 mm, 74%; 20 to 29 mm, 72%; ≥30 mm, 56% (P < 0.0001 for each category, except P = 0.19 when ≥30 mm). Among those with bilateral adenomas, the left-sided adenoma was significantly larger than the right one in 61% of patients (P < 0.001). There were no significant differences in the baseline prevalence or incidence of cardiometabolic diseases between patients with left-sided vs right-sided adenomas during 5.10 (4.2) years of follow-up.
Conclusions
Adrenal adenomas are substantially more likely to be identified on the left adrenal than the right. This observation may be due to detection bias attributed to the location of the right adrenal, which may preclude identification of right-sided adenomas until they are substantially larger. These findings suggest the potential for an underrecognition of right-sided adenomas that may also impair the accurate detection of bilateral adrenal diseases.
Keywords: adrenal adenoma, adrenal mass, adrenal tumor, asymmetry, laterality
Adrenal adenomas are more likely to be detected on the left than the right adrenal, highlighting a potential impaired accuracy in detecting right-sided adenomas and bilateral adrenal diseases.
The frequent use of abdominal cross-sectional imaging has resulted in a high incidence of incidentally discovered adrenal tumors [1–3]. Multiple large studies suggest that the prevalence of incidentally discovered adrenal tumors can range to be as high as 4% to 10% [4, 5]. Although the vast majority of adrenal tumors represent benign adenomas, even benign adrenal adenomas may secrete excessive adrenal hormones that may contribute to cardiometabolic disease [6, 7].
It is generally presumed that the detection of adrenal tumors is symmetric, with half the abnormalities detected on the left, and half detected on the right adrenal gland; however, prior studies focused on other objectives included data suggesting a potential lateralizing asymmetry that favored detection of left-sided adrenal tumors [8–10].
These previous observations suggest that the left adrenal gland may be more prone to developing adrenal neoplasia, or, alternatively, that left-sided adrenal tumors may be more readily apparent to radiologists and thus result in a detection bias. The anatomy and location of the left adrenal gland, with ample periadrenal adipose tissue, allow for easier visualization compared with the right adrenal gland, which is often compressed between the liver and right kidney. Therefore, we hypothesized that left-sided adrenal adenomas may be more frequently detected compared with right-sided adrenal adenomas. We speculated that this observation could have important clinical implications: a left-sided preference for detecting adrenal adenomas could result in the underdetection of right-sided adrenal adenomas and unreliable recognition of bilateral adrenal adenomas. Because the prognostication and treatment of adrenal disorders (i.e., primary aldosteronism, adrenal Cushing syndrome) may depend on reliable identification of unilateral vs bilateral disease, any lateralizing bias in recognizing adrenal adenomas could impact the delivery of quality clinical care to patients. In this study, we report the results of a large and dedicated systematic investigation focused on the laterality of adrenal adenoma detection.
1. Methods
A. Study Population
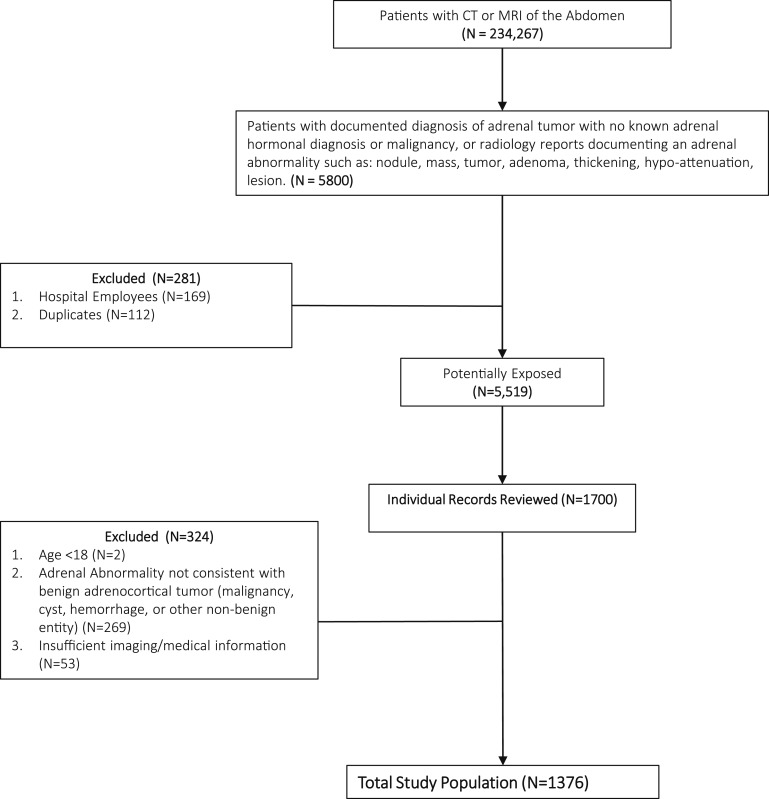
The study population was selected from an institutional research registry of all patients from the Brigham and Women’s Hospital, the Massachusetts General Hospital, and their affiliated partner hospitals (Fig. 1) [6]. From this registry, we developed a de-identified research dataset for the present study of patients who had undergone abdominal computed tomography (CT) or magnetic resonance imaging (MRI) from 1989 to 2016 and had accessible reports (n = 234,267). From this population, we selected for patients with either International Classification of Diseases 9 or 10 diagnosis codes for a benign adrenocortical tumor or text-search results from radiology reports that documented the presence of a benign adrenal “tumor,” “nodule,” “mass,” “adenoma,” “thickening,” “lesion,” “nodularity,” or “hypoattenuation.” We excluded patients with known clinical documentation of adrenal hormonal diagnoses (such as primary aldosteronism, Cushing syndrome, congenital adrenal hyperplasia, or pheochromocytoma) and adrenal malignancy to minimize confounding (because one of our objectives was to evaluate the association between laterality of adrenal adenomas and incident cardiometabolic outcomes) and bias (because these conditions usually trigger more frequent imaging and increased opportunities to detect adrenal abnormalities). From 5519 potentially eligible patients, we sequentially conducted detailed chart reviews and analyses on the first 1700 patients arbitrarily generated from our registry. Following this manual medical record review, we excluded 324 patients who were either <18 years old or who had an adrenal mass with radiographic characteristics that could be inconsistent with a benign adrenal adenoma (e.g., potential adrenal cysts, hemorrhage, malignancy, metastatic lesions), or who had insufficient imaging data, for a final sample size of 1376 patients with benign-appearing adrenocortical adenomas. We considered an individual as having a benign-appearing adenoma when the interpreting radiologist described the nodule as having lipid-rich attenuation (<10 Hounsfield units) on unenhanced CT imaging, high-contrast washout on CT imaging (when available), a marked drop in signal intensity on MRI imaging, and/or descriptors that are suggestive of a benign entity, such as “benign” or “adenoma” or “myelolipoma” [11, 12]. This study was conducted with the approval from the Institutional Research and Ethics Review Board of our Hospital.
Figure 1.
Patient selection process. The study population was selected from patients who underwent abdominal CT or MRI at our institution. We arbitrarily reviewed 1700 patients with adrenal tumors and excluded 324 patients who were <18 years old, had an adrenal abnormality not consistent with benign adrenocortical tumor, or had insufficient information for a final sample size of 1376.
B. Assessment of Baseline and Follow-up Characteristics
We reviewed each patient’s electronic medical record to gather data on the location and size of the adrenal tumor from the earliest available imaging report. The date of this earliest image was established to be the baseline time point. Very small adrenal abnormalities or tumors were sometimes reported as a “thickening” or “nodularity” that were too small to be measured. For these instances, a default size of 1 mm was assigned to the adenoma. For the remaining adenomas, we recorded the exact size and location measured by the radiologist who interpreted the CT or MRI.
We determined the most recent comprehensive clinical visit, which was established to be the final follow-up time point. Examples of comprehensive clinical visits included annual physical examination, primary care, and general internal medicine visits, medical subspecialty consultations, and preoperative anesthesia consultations. The average follow-up period was defined as the average time spanning from the earliest radiology report documenting an adrenal tumor (baseline) to the most recent comprehensive clinic visit (final follow-up). We collected pertinent demographic information [age, sex, race, and body mass index (BMI)], smoking status, creatinine level, medical comorbidities (hypertension, prediabetes, type 2 diabetes, hyperlipidemia, coronary artery disease, peripheral arterial disease, heart failure, myocardial infarction, ischemic stroke, atrial fibrillation, chronic kidney disease) at both baseline and final follow-up. We also collected data on medication use for hypertension (angiotensin converting enzyme inhibitors, angiotensin inhibitors, selective calcium channel blockers, beta-blockers, thiazides), diabetes (oral hypoglycemic, insulin), hyperlipidemia (statins, fibrates, niacin), and coronary artery disease (aspirin, nitrates) at both baseline and final follow-up.
We defined hypertension, chronic kidney disease, coronary artery disease, peripheral arterial disease, atrial fibrillation, myocardial ischemia, and ischemic stroke as any documented diagnosis. Prediabetes was defined as a documented diagnosis and/or two or more documented hemoglobin A1c value of 5.7% to 6.4% among patients not taking any hypoglycemic agent other than metformin. Type 2 diabetes was defined as a documented diagnosis and/or two or more documented hemoglobin A1c values ≥6.5%. Composite diabetes was defined as having either prediabetes or type 2 diabetes. Hyperlipidemia was defined as a documented diagnosis and/or a low-density lipoprotein cholesterol level of ≥150 mg/dL (3.89 mmol/L). Composite cardiovascular disease was defined as a documented diagnosis of hypertension, myocardial infarction, ischemic stroke, or peripheral vascular disease. Incident cardiovascular outcomes were defined as the development of any cardiovascular comorbidities during the follow-up period.
C. Statistical Analysis
Primary analyses were conducted to assess the prevalence, sidedness, and size of unilateral vs bilateral adrenal adenomas. Categorical variables for demographic and clinical characteristics were presented as counts and percentages whereas continuous variables were presented with means and standard deviations. Differences in means and frequencies were analyzed using t tests and χ2 tests, respectively. In secondary analyses, we used logistic regression to assess the risk for incident composite cardiovascular disease by laterality and sidedness of adrenal adenomas in the subset of patients who did not have baseline composite cardiovascular disease. Models were adjusted for pertinent confounders, including age, sex, race/ethnicity, BMI, smoking status, and the presence of baseline diabetes or hyperlipidemia. A two-tailed P value of <0.05 was considered statistically significant. All statistical analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC).
2. Results
A. Baseline Characteristics
Demographic and baseline characteristics of study population categorized by those with unilateral vs bilateral adrenal adenomas are presented in Table 1. The vast majority of patients (86.4%, n = 1189) had unilateral adrenal adenomas and were more likely to be younger, female, and nonsmokers when compared with those with bilateral adenomas. In addition to being older, patients with bilateral adrenal adenomas were more likely to have hypertension, peripheral arterial disease, and a composite of cardiovascular diseases when compared with those with unilateral adenomas (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics of Patients with Unilateral Versus Bilateral Adrenal Adenomas
| Characteristic | Unilateral | Bilateral | P Value |
|---|---|---|---|
| Patients, n (%) | 1189 (86.4) | 187 (13.6) | |
| Mean age, y | 61.11 (13.0) | 67.08 (11.6) | <0.0001 |
| Sex, % | |||
| Female | 67.9 | 60.4 | 0.054 |
| Male | 32.1 | 39.6 | |
| Race, % | |||
| White | 71.4 | 67.4 | 0.056 |
| Black | 7.7 | 7.0 | |
| Hispanic | 5.8 | 3.2 | |
| Other | 15.3 | 22.5 | |
| Mean BMI, kg/m2a | 30.0 (6.84) | 28.9 (6.73) | 0.061 |
| Creatinine, mg/dLb | 1.00 (0.76) | 0.97 (0.41) | 0.56 |
| Smoking status, % | |||
| Nonsmoker | 39.5 | 29.4 | <0.01 |
| Current or Past Smoker | 60.5 | 70.6 | |
| Comorbidity at baseline,c % | |||
| Hypertension | 58.5 | 69.5 | 0.004 |
| Prediabetes | 6.6 | 9.1 | 0.21 |
| Type 2 diabetes | 21.1 | 24.1 | 0.39 |
| Composite diabetes | 27.7 | 33.2 | 0.14 |
| Hyperlipidemia | 50.6 | 52.4 | 0.69 |
| Coronary artery disease | 15.5 | 15.0 | 0.91 |
| Peripheral arterial disease | 8.2 | 14.4 | <0.01 |
| Myocardial infarction | 6.5 | 10.2 | 0.087 |
| Stroke | 3.7 | 3.2 | 1.00 |
| Atrial fibrillation | 7.4 | 7.0 | 1.00 |
| Chronic kidney disease | 8.4 | 9.1 | 0.78 |
| Composite cardiovascular diseased | 72.3 | 84.3 | <0.001 |
| Medications at baseline,c % | |||
| Hypertension | 55.1 | 64.2 | 0.021 |
| Diabetes | 16.9 | 21.9 | 0.099 |
| Hyperlipidemia | 40.2 | 46.0 | 0.15 |
| Coronary heart disease | 33.8 | 42.8 | 0.020 |
| Average follow-up time,e y (standard deviation) | 5.2 (4.29) | 4.4 (3.61) | 0.031 |
Ninety-six patients did not have BMI measured at the time of the baseline imaging.
Seventy patients did not have creatinine measured at the time of the baseline imaging.
Baseline defined as date of earliest radiologic imaging demonstrating adrenal adenoma.
Composite cardiovascular disease defined as diagnosis of hypertension, myocardial infarction, cardiovascular disease, stroke, or peripheral arterial disease.
Follow-up time defined as the period of time from earliest radiologic image demonstrating adrenal adenoma to the most recent comprehensive clinic visit.
B. The Lateralizing Asymmetry of Unilateral Adrenal Adenomas
Among those with unilateral adrenal adenomas (n = 1189), left-sided adenomas (75%; n = 894) were detected three times more often than right-sided adenomas (25%; n = 295) (Table 2). This finding was similar throughout the duration of the study period. Patients with left-sided adrenal adenomas were more likely to be female (70.1% vs 61%, P < 0.01) and have lower BMI (29.6 vs 31.0, P < 0.01) when compared with those with right-sided adenomas. There was a marginally higher prevalence of history of prior stroke (4.4% vs 1.7%, P = 0.033) and peripheral arterial disease (9.1% vs 5.4%, P = 0.05) among those with left-sided adenomas; however, there were no other significant differences in demographics or comorbidities (Table 2).
Table 2.
Baseline Demographic and Clinical Characteristics of Patients with Left Versus Right Unilateral Adrenal Adenomas
| Characteristic | Left Unilateral | Right Unilateral | P Value |
|---|---|---|---|
| Patients, n (%) | 894 (75.2) | 295 (24.8) | |
| Mean age, y | 61.5 (12.87) | 60.1 (13.2) | 0.11 |
| Sex, % | |||
| Female | 70.1 | 61.0 | 0.004 |
| Male | 29.9 | 39.0 | |
| Race, % | |||
| White | 72.2 | 68.8 | 0.24 |
| Black | 8.1 | 6.4 | |
| Hispanic | 5.2 | 7.5 | |
| Other | 14.6 | 17.3 | |
| Mean BMI, kg/m2a | 29.6 (6.70) | 31.0 (7.16) | <0.01 |
| Creatinine, mg/dLb | 1.01 (0.83) | 0.96 (0.50) | 0.37 |
| Smoking status, % | |||
| Nonsmoker | 39.0 | 41.0 | 0.58 |
| Current or past smoker | 61.0 | 59.0 | |
| Comorbidity at baseline,c % | |||
| Hypertension | 58.3 | 59.0 | 0.84 |
| Prediabetes | 6.3 | 7.5 | 0.50 |
| Type 2 diabetes | 20.8 | 22.0 | 0.68 |
| Composite diabetes | 27.1 | 29.5 | 0.45 |
| Hyperlipidemia | 51.0 | 49.2 | 0.59 |
| Coronary artery disease | 15.4 | 15.6 | 0.93 |
| Peripheral arterial disease | 9.1 | 5.4 | 0.0498 |
| Myocardial infarction | 6.5 | 6.4 | 1.00 |
| Stroke | 4.4 | 1.7 | 0.033 |
| Atrial fibrillation | 7.6 | 6.8 | 0.70 |
| Chronic kidney disease | 8.5 | 8.1 | 0.90 |
| Composite cardiovascular diseased | 72.3 | 72.3 | 1.00 |
| Medications at baseline,c % | |||
| Hypertension | 55.5 | 53.9 | 0.64 |
| Diabetes | 15.9 | 20.0 | 0.11 |
| Hyperlipidemia | 40.7 | 38.6 | 0.54 |
| Coronary heart disease | 34.2 | 32.5 | 0.62 |
| Average follow-up time,e y (standard deviation) | 5.1 (4.26) | 5.5 (4.38) | 0.18 |
Eighty-three patients did not have BMI measured at the time of the baseline imaging.
Sixty-two patients did not have creatinine measured at the time of the baseline imaging.
Baseline defined as date of earliest radiologic imaging demonstrating adrenal adenoma.
Composite cardiovascular disease defined as diagnosis of hypertension, myocardial infarction, cardiovascular disease, stroke, or peripheral arterial disease.
Follow-up time defined as the period of time from earliest radiologic image demonstrating adrenal adenoma to the most recent comprehensive clinic visit.
The excess detection of left-sided adenomas was evident across the entire range of adenoma sizes, except when adenomas were ≥30 mm (Table 3). Left-sided adrenal adenomas represented 87% of adenomas that were <10 mm in size, 74% of adenomas that were 10 to 19 mm in size, and 72% of adenomas that were 20 to 29 mm in size. However, once adenomas were ≥30 mm, the lateralizing asymmetry was attenuated such that the left-sided detection preference was no longer statistically significant (Table 3). In parallel with the left-sided detection asymmetry was a size asymmetry. The mean size of right-sided adenomas was significantly larger than the mean size of left-sided adenomas [19.2 (11.8) vs 14.8 (10.3) mm, P < 0.0001], a finding that was evident among small-sized (<10 mm) and larger sized (≥10 mm) adenomas (Table 4). However, this difference in detection size was predominantly driven by the smallest adenomas (<10 mm) where right-sided adenomas were clearly larger than left-sided adenomas; with the detection of larger adenomas, the mean size of left-sided and right-sided adenomas was similar (Table 4).
Table 3.
Distribution of Unilateral Adrenal Adenomas by Size Categories
| Size Category | Percentage Distribution Left | Percentage Distribution Right | P Value |
|---|---|---|---|
| Any size, % (n) | 75 (894) | 25 (295) | <0.0001 |
| <10 mm, % (n) | 87 (246) | 13 (37) | <0.0001 |
| ≥10 mm, % (n) | 72 (648) | 28 (258) | <0.0001 |
| 10–19 mm, % (n) | 74 (405) | 26 (139) | <0.0001 |
| 20–29 mm, % (n) | 72 (178) | 28 (68) | <0.0001 |
| ≥30 mm, % (n) | 56 (65) | 44 (51) | 0.19 |
Table 4.
Mean Size of Unilateral Adrenal Adenomas by Size Categories
| Size Category | Average Size Left | Average Size Right | P Value |
|---|---|---|---|
| Any size | 14.8 (10.3) | 19.2 (11.8) | <0.0001 |
| <10 mm | 4.3 (3.5) | 5.6 (3.3) | 0.023 |
| ≥10 mm | 18.8 (9.1) | 21.2 (11.3) | <0.001 |
| 10–19mm | 13.5 (2.9) | 13.6 (3.0) | 0.92 |
| 20–29 mm | 23.4 (2.7) | 23.7 (2.8) | 0.44 |
| ≥30 mm | 38.7 (11.2) | 38.5 (12.1) | 0.92 |
Because the amount of periadrenal adipose tissue is an important determinant for the visualization of the adrenal glands and adenomas, we investigated the frequency of left-sided vs right-sided unilateral adrenal adenomas detected by quartiles of BMI, where BMI represented a crude proxy for abdominal adiposity (Table 5). Left-sided adrenal adenomas were detected more frequently in every quartile of BMI; however, there was a nonsignificant trend suggesting that the proportion of right-sided adenomas detected was greater with higher BMI (Table 5).
Table 5.
Distribution of Unilateral Adrenal Adenomas by Quartiles of BMI
| BMI Quartiles | Left, % (n) | Right, % (n) | P Value |
|---|---|---|---|
| 1. <24.88 | 76.5 (267) | 23.5 (82) | 0.28 |
| 2. 24.88–29.0 | 78.1 (217) | 21.9 (61) | |
| 3. 29.0–33.8 | 74.6 (203) | 25.4 (69) | |
| 4. >33.8 | 71.4 (207) | 28.6 (83) |
C. Lateralizing Asymmetries of Bilateral Adrenal Adenomas
Among the 187 patients with bilateral adrenal adenomas, 30% (n = 56) of patients had equally sized left-sided and right-sided adenomas. Of the remaining 70% (n = 131) of patients with asymmetrically sized bilateral adenomas, most (61%) had a larger left-sided adenoma, whereas the minority (39%) had a larger right-sided adenoma (P < 0.001).
D. Incident Clinical Outcomes
Patients with unilateral adrenal adenomas were followed longitudinally with serial imaging for 5.2 (4.3) years whereas patients with bilateral adrenal adenomas were followed for 4.4 (3.6) years. Among patients with unilateral adenomas, left-sided adenomas were longitudinally followed for 5.1 (4.3) years whereas right-sided adenomas were followed for 5.5 (4.4) years. There were no significant differences in any incident composite cardiovascular outcomes between patients with unilateral vs bilateral adenomas, or unilateral left-sided vs right-sided adenomas.
3. Discussion
It is commonly presumed that the frequency and ability to detect adrenal adenomas is symmetric between the left and the right adrenal glands. In contrast, our findings suggested a marked threefold greater detection of left-sided unilateral adrenal adenomas compared with the right side. We observed that left-sided adrenal adenomas were detected more frequently even when smaller in size than right-sided adenomas; alternatively, right-sided adenomas were larger than left-sided adenomas when detected. Furthermore, when bilateral adrenal adenomas were detected, the left-sided adenoma tended to be larger in size than the right-sided adenoma. Collectively, these findings suggest a potential bias toward the detection of left-sided adrenal adenomas, but perhaps more importantly, a concern that right-sided adrenal adenomas need to be significantly larger than left-sided adenomas before they are detected. This may have two important implications for patient care: (1) the recognition of right-sided adrenal adenomas may be delayed until a later stage of progressive growth; and (2) the underrecognition of right-sided adrenal adenomas may result in a substantial inaccuracy in identifying true bilateral adrenal abnormalities, which may be most relevant for conditions such as primary aldosteronism and adrenal Cushing syndrome where treatment decisions rely on reliable lateralization.
To our knowledge, the present study is the first study dedicated to investigating the asymmetry in detecting adrenal adenomas, and it is the largest study to date with data on this topic. Prior studies by Kim et al. [8], Debono et al. [9], and Sangwaiya et al. [10] all included data that also suggested a potential left-sided asymmetry, but had small study populations and focused on other research objectives. Note that a survey on adrenal incidentalomas by Mantero et al. [13] observed more right-sided adrenal tumors than those on the left side; however, the imaging modality used was predominantly ultrasound, which is not regarded as sensitive as CT or MRI, and is more often performed to evaluate the right-sided abdominal organs. Indeed, when Mantero et al. [13] restricted their analysis to the 247 adrenal adenomas detected by CT imaging alone, the asymmetry was no longer apparent. Therefore, our findings substantially extend and build upon these prior observations. Our study was not designed to investigate the underlying cause for the asymmetry we observed; however, we suspect that the preferential identification of left-sided adrenal adenomas may be attributable to radiologic detection bias due to the anatomic differences between the left and right adrenal glands. The left adrenal gland is surrounded by a layer of hypoattenuating retroperitoneal fat and is located above the left kidney without compression from surrounding structures, thus allowing for better visualization on abdominal cross-sectional imaging. In contrast, the right adrenal gland has less surrounding retroperitoneal fat, abuts the liver, and lies between the liver and kidney, which may obscure recognition of small abnormalities. Indeed, the right adrenal gland is often compressed in a manner such that the lateral limbs are indistinguishable from one another. The proposition of an imaging detection bias favoring the left adrenal gland is further supported by the observation that left-sided adrenal adenomas were detected despite being significantly smaller than those on the right, particularly for adrenal adenomas in smaller size ranges. As the size of the adenomas increased, the ability to detect both left-sided and right-sided adenomas appeared to equilibrate. This prompted our attempt to investigate whether BMI, as a crude proxy of visceral adiposity and periadrenal adipose tissue, influenced the detection asymmetry. We observed only a mild and nonsignificant trend suggesting that patients with higher BMI (or greater adiposity) may have less asymmetry in the detection of adrenal adenomas; however, BMI is not an accurate measure of abdominal adiposity, and further studies using quantification of visceral and periadrenal adipose tissue would be required to better test this hypothesis.
Alternatively, another hypothesis for the lateralizing asymmetry we observed could involve a fundamental predisposition for left-sided adrenal neoplasia. There have been prior studies that have suggested asymmetry in the detection of malignancies of the breast [14–17]; however, to our knowledge, there is limited evidence to support a predisposition for asymmetric adrenal neoplasia. One of the earliest studies to note a lateralizing asymmetry of adrenal adenomas was an autopsy series published by Russi et al. [18] in 1945 that noted that among 68 unilateral adrenal adenomas, 34 (50%) were found on the left adrenal, 21 (31%) were found on the right adrenal, and 13 (19%) were found on bilateral adrenal glands. One potential explanation for this asymmetry may include potential morphologic differences between the left and right adrenal glands. In one autopsy study in 2001, Lam et al. [19] examined the adrenal glands of 333 patients and found a significantly greater dimension and mean weight of the left adrenal gland compared with the right. Similarly, multiple fetal adrenal development studies have found an asymmetry in the development of the adrenal glands such as greater mass, volume, thickness, and surface area of the left adrenal gland compared with the right adrenal gland during gestation [20–22]. A study by Aliab’ev and Paderov [23] on the adrenal glands of 161 men of different ages also found an asymmetry marked by greater mass of the left adrenal and its cortex over the right and that the increasing adrenal mass with age was predominantly due to the growth of cortex in the left adrenal. Adult adrenal volumetric studies have reported conflicting results on whether there exists a significant difference between the left and right mean adrenal volume [24–27]. In an analysis of 154 patients, Carsin-Vu et al. [24] found that the mean volume of left adrenal gland (4.5 ± 1.6cm3) was significantly greater than the mean volume of the right adrenal gland (3.8 ± 1.3cm3). Similarly, in a study of 105 patients, Schneller et al. [25] found a significantly greater left adrenal mean volume (4.84 ± 1.67cm3 vs 3.62 ± 1.23 cm3) and left adrenal total width (18.96 ± 3.37 mm vs 15.80 ± 3.05 mm). However, studies with smaller sample sizes of 40 patients by Nougaret et al. [26] and 81 patients by Wang et al. [27] failed to demonstrate any significant difference between the left and right adrenal gland volume. If the left adrenal gland is indeed larger in dimension and greater in volume, then it may be possible that greater tissue mass in the left adrenal may potentially lead to greater opportunities for neoplastic transformation. It may also be possible that the greater left adrenal volume and size could lead to better visualization of any abnormalities. In addition to differences in morphology, other differences such as innervation, vascular supply, and venous drainage may also play a role in the higher frequency of adenomas observed in the left adrenal gland. Tóth et al. [28] conducted a study using viral transneuronal tracing techniques that demonstrated an asymmetry with greater supraspinal innervation of the left adrenal gland compared with the right adrenal gland in rodents. Furthermore, although it is recognized that adrenal venous drainage is asymmetric, studies have also demonstrated asymmetry in vascular supply with an additional group of posterior arteries supplying the posterior surface of left adrenal gland in some individuals [29, 30]. These differences in vascular supply and drainage as well as innervation may further contribute to differences in size and susceptibility to neoplasia.
The findings of this study have several important clinical implications. Our data suggest that in the current era of frequent adrenal incidental findings: (1) there is a substantial detection bias toward identifying left-sided adrenal adenomas; (2) the detection of right-sided adrenal adenomas may be delayed until they are significantly larger, thus resulting in a window of time where recommended biochemical screening and surveillance are not conducted and exposure to autonomous adrenal hormone secretion may go unrecognized; and (3) the ability to detect bilateral adrenal diseases may be unreliable, particularly when only a left-sided adrenal abnormality is seen in conditions such as primary aldosteronism and adrenal Cushing syndrome. For example, in a prior study by Young et al. [31], when treatment of primary aldosteronism relied on CT findings alone, 25% of patients would have had unnecessary or inappropriate unilateral adrenalectomy due to incorrect identification of the responsible adrenal gland or failure to detect bilateral disease. Similarly, Kempers et al. [32] conducted a systematic review of 950 patients that demonstrated that CT/MRI failed to identify bilateral disease in 14.6% of patients and identified the wrong adrenal gland in 3.9% of patients.
An alternative viewpoint is that this detection bias may result in “overevaluation” of patients with left-sided adrenal adenomas. In other words, the superior ability to recognize left-sided adenomas may result in more biochemical evaluations and resultant medical or surgical interventions for those with left-sided adenomas when compared with right-sided adenomas. Indeed, the higher incidence of left-sided adrenalectomies has been reported many times before [33–38]. Rieder et al. [33] examined consecutive patients receiving laparoscopic adrenalectomy between 1998 and 2007 in Southern California Kaiser Permanente Hospital and reported 109 left-sided adrenalectomies and 54 right-sided adrenalectomies (twice as many left-sided interventions). Similarly, a study from Nancy University Hospital in France documented 64 left-sided and 36 right-sided adrenalectomies from November 2001 to November 2007 in 100 consecutive patients (78% more left-sided interventions) [34].
Our findings must be interpreted within the context of our study design. The main limitations of this study are related to the interpretation of radiographic imaging. Very small adrenal abnormalities (<5 mm) sometimes could not be quantified with a reliable size measurement, and we therefore assigned them default size of 1 mm; however, we present our data in both continuous and categorical formats to demonstrate that our observations related to size were consistent. Furthermore, measurement technique of adrenal adenomas can vary depending on how radiologists manually place measurement bars in their imaging software, and our study relied on radiology interpretations made by many heterogeneous evaluators; however, our findings reflect “real-world” observations and therefore provide a practical representation of what may be encountered in medical practice. As with all observational studies such as ours, there is a risk of bias in the selection of patients; the patients in the present study did not undergo systematic prospective imaging, rather they were retrospectively selected based on predefined inclusion criteria from a large registry. Our study focused on nonfunctional and benign-appearing adrenal adenomas to minimize confounding and bias attributed to hormonally active adenomas and adrenal malignancies because they are known to impart higher risk for cardiometabolic disease and are associated with increased frequency of imaging; thus, our results may not be generalizable to individuals with functional or malignant adrenal tumors. Furthermore, we did not have reliable outcomes assessments of all potential surgical interventions, growth in adenoma sizes, or development of incident hormone excess, because our study design and electronic medical record system was not designed to uniformly capture all future events. Thus, even though we suggest that a potential implication of our findings could be the unreliable biochemical screening and detection of bilateral adrenal diseases, our present study was not designed to specifically evaluate this.
In conclusion, our findings suggest a strong preferential detection for left-sided vs right-sided adrenal adenomas. Left-sided adenomas are detected at significantly smaller sizes than right-sided adenomas, and patients with bilateral adrenal abnormalities more frequently present with larger left-sided adrenal adenomas. Taken together, these findings may implicate a radiologic detection bias that has important clinical implications. First, this asymmetric detection could lead to underrecognition of right-sided adrenal adenomas that may delay hormonal screening as well as result in overevaluation and treatment of left-sided adrenal adenomas. Second, these findings may provide one explanation for why detection of bilateral disease is unreliable on cross-sectional imaging. Clinicians should be aware of this lateralizing detection asymmetry when evaluating abdominal imaging and managing patients with adrenal adenomas.
Acknowledgments
Financial Support: M.H. was supported by the Endocrine Society Summer Research Fellowship, as well as by Scholars in Medicine at Harvard Medical School. A.V. was supported by National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health Grant R01 DK107407 and by Grant 2015085 from the Doris Duke Charitable Foundation.
Disclosure Statement: The authors have nothing to disclose.
Glossary
Abbreviations:
- BMI
body mass index
- CT
computed tomography
- MRI
magnetic resonance imaging.
References and Notes
- 1. Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, Tabarin A, Terzolo M, Tsagarakis S, Dekkers OM. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175(2):G1–G34. [DOI] [PubMed] [Google Scholar]
- 2. Nieman LK. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab. 2010;95(9):4106–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Young WF., Jr Clinical practice. The incidentally discovered adrenal mass. N Engl J Med. 2007;356(6):601–610. [DOI] [PubMed] [Google Scholar]
- 4. Bovio S, Cataldi A, Reimondo G, Sperone P, Novello S, Berruti A, Borasio P, Fava C, Dogliotti L, Scagliotti GV, Angeli A, Terzolo M. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. 2006;29(4):298–302. [DOI] [PubMed] [Google Scholar]
- 5. Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B. Incidentally discovered adrenal masses. Endocr Rev. 1995;16(4):460–484. [DOI] [PubMed] [Google Scholar]
- 6. Lopez D, Luque-Fernandez MA, Steele A, Adler GK, Turchin A, Vaidya A. “Nonfunctional” adrenal tumors and the risk for incident diabetes and cardiovascular outcomes: a cohort study. Ann Intern Med. 2016;165(8):533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Di Dalmazi G, Vicennati V, Garelli S, Casadio E, Rinaldi E, Giampalma E, Mosconi C, Golfieri R, Paccapelo A, Pagotto U, Pasquali R. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol. 2014;2(5):396–405. [DOI] [PubMed] [Google Scholar]
- 8. Kim J, Bae KH, Choi YK, Jeong JY, Park KG, Kim JG, Lee IK. Clinical characteristics for 348 patients with adrenal incidentaloma. Endocrinol Metab (Seoul). 2013;28(1):20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Debono M, Bradburn M, Bull M, Harrison B, Ross RJ, Newell-Price J. Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J Clin Endocrinol Metab. 2014;99(12):4462–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sangwaiya MJ, Boland GW, Cronin CG, Blake MA, Halpern EF, Hahn PF. Incidental adrenal lesions: accuracy of characterization with contrast-enhanced washout multidetector CT—10-minute delayed imaging protocol revisited in a large patient cohort. Radiology. 2010;256(2):504–510. [DOI] [PubMed] [Google Scholar]
- 11. Buitenwerf E, Korteweg T, Visser A, Haag CM, Feelders RA, Timmers HJ, Canu L, Haak HR, Bisschop PH, Eekhoff EMW, van der Kleij-Corssmit EP, Krak NC, Rasenberg E, van den Bergh J, Stoker J, Greuter MJ, Dullaart RPF, Links TP, Kerstens MN. Unenhanced CT imaging is highly sensitive to exclude pheochromocytoma: a multicenter study [published online ahead of print 21 February 2018]. Eur J Endocrinol. doi: 10.1530/EJE-18-0006. [DOI] [PubMed]
- 12. Marty M, Gaye D, Perez P, Auder C, Nunes ML, Ferriere A, Haissaguerre M, Tabarin A. Diagnostic accuracy of computed tomography to identify adenomas and benign tumors amongst adrenal incidentalomas in an endocrinological population [published online ahead of print 21 February 2018]. Eur J Endocrinol. doi: 10.1530/EJE-17-1056. [DOI] [PubMed]
- 13. Mantero F, Terzolo M, Arnaldi G, Osella G, Masini AM, Alì A, Giovagnetti M, Opocher G, Angeli A; Study Group on Adrenal Tumors of the Italian Society of Endocrinology . A survey on adrenal incidentaloma in Italy. J Clin Endocrinol Metab. 2000;85(2):637–644. [DOI] [PubMed] [Google Scholar]
- 14. Roychoudhuri R, Putcha V, Møller H. Cancer and laterality: a study of the five major paired organs (UK). Cancer Causes Control. 2006;17(5):655–662. [DOI] [PubMed] [Google Scholar]
- 15. Amer MH. Genetic factors and breast cancer laterality. Cancer Manag Res. 2014;6:191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ekbom A, Adami HO, Trichopoulos D, Lambe M, Hsieh CC, Pontén J. Epidemiologic correlates of breast cancer laterality (Sweden). Cancer Causes Control. 1994;5(6):510–516. [DOI] [PubMed] [Google Scholar]
- 17. Senie RT, Rosen PP, Lesser ML, Snyder RE, Schottenfeld D, Duthie K. Epidemiology of breast carcinoma II: factors related to the predominance of left-sided disease. Cancer. 1980;46(7):1705–1713. [DOI] [PubMed] [Google Scholar]
- 18. Russi S, Blumenthal HT, Gray SH. Small adenomas of the adrenal cortex in hypertension and diabetes. Arch Intern Med (Chic). 1945;76(5):284–291. [DOI] [PubMed] [Google Scholar]
- 19. Lam KY, Chan AC, Lo CY. Morphological analysis of adrenal glands: a prospective analysis. Endocr Pathol. 2001;12(1):33–38. [DOI] [PubMed] [Google Scholar]
- 20. Nowak D, Góralczyk K, Zurada A, Gielecki J. Morphometrical analysis of the human suprarenal gland between the 4th and 7th months of gestation. Ann Anat. 2007;189(6):575–582. [DOI] [PubMed] [Google Scholar]
- 21. Özgüner G, Sulak O, Koyuncu E. A morphometric study of suprarenal gland development in the fetal period. Surg Radiol Anat. 2012;34(7):581–587. [DOI] [PubMed] [Google Scholar]
- 22. Zhang Z, Meng H, Hou Z, Ma J, Feng L, Lin X, Tang Y, Zhang X, Liu Q, Liu S. Fetal adrenal gland in the second half of gestation: morphometrical assessment with 3.0T post-mortem MRI. PLoS One. 2013;8(10):e75511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aliab’ev FB, Paderov IuM. [Age-related structural asymmetry of human adrenal glands]. Morfologiia. 2004;125(2):61–64. [PubMed] [Google Scholar]
- 24. Carsin-Vu A, Oubaya N, Mulé S, Janvier A, Delemer B, Soyer P, Hoeffel C. MDCT linear and volumetric analysis of adrenal glands: normative data and multiparametric assessment. Eur Radiol. 2016;26(8):2494–2501. [DOI] [PubMed] [Google Scholar]
- 25. Schneller J, Reiser M, Beuschlein F, Osswald A, Pallauf A, Riester A, Tietze JK, Reincke M, Degenhart C. Linear and volumetric evaluation of the adrenal gland—MDCT-based measurements of the adrenals. Acad Radiol. 2014;21(11):1465–1474. [DOI] [PubMed] [Google Scholar]
- 26. Nougaret S, Jung B, Aufort S, Chanques G, Jaber S, Gallix B. Adrenal gland volume measurement in septic shock and control patients: a pilot study. Eur Radiol. 2010;20(10):2348–2357. [DOI] [PubMed] [Google Scholar]
- 27. Wang X, Jin ZY, Xue HD, Liu W, Sun H, Chen Y, Xu K. Evaluation of normal adrenal gland volume by 64-slice CT. Chin Med Sci J. 2013;27(4):220–224. [DOI] [PubMed] [Google Scholar]
- 28. Tóth IE, Wiesel O, Tóth DE, Boldogkoi Z, Halász B, Gerendai I. Transneuronal retrograde viral labeling in the brain stem and hypothalamus is more intense from the left than from the right adrenal gland. Microsc Res Tech. 2008;71(7):503–509. [DOI] [PubMed] [Google Scholar]
- 29. Bianchi H, Ferrari A. The arterial circulation of the left suprarenal gland. Surg Radiol Anat. 1991;13(2):113–116. [DOI] [PubMed] [Google Scholar]
- 30. Pityński K, Skawina A, Polakiewicz J, Walocha J. Extraorganic vascular system of adrenal glands in human fetuses. Ann Anat. 1998;180(4):361–368. [DOI] [PubMed] [Google Scholar]
- 31. Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004;136(6):1227–1235. [DOI] [PubMed] [Google Scholar]
- 32. Kempers MJ, Lenders JW, van Outheusden L, van der Wilt GJ, Schultze Kool LJ, Hermus AR, Deinum J. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med. 2009;151(5):329–337. [DOI] [PubMed] [Google Scholar]
- 33. Rieder JM, Nisbet AA, Wuerstle MC, Tran VQ, Kwon EO, Chien GW. Differences in left and right laparoscopic adrenalectomy. JSLS. 2010;14(3):369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brunaud L, Ayav A, Zarnegar R, Rouers A, Klein M, Boissel P, Bresler L. Prospective evaluation of 100 robotic-assisted unilateral adrenalectomies. Surgery. 2008;144(6):995–1001, discussion 1001. [DOI] [PubMed] [Google Scholar]
- 35. Asari R, Koperek O, Niederle B. Endoscopic adrenalectomy in large adrenal tumors. Surgery. 2012;152(1):41–49. [DOI] [PubMed] [Google Scholar]
- 36. Karabulut K, Agcaoglu O, Aliyev S, Siperstein A, Berber E. Comparison of intraoperative time use and perioperative outcomes for robotic versus laparoscopic adrenalectomy. Surgery. 2012;151(4):537–542. [DOI] [PubMed] [Google Scholar]
- 37. Nordenström E, Westerdahl J, Hallgrimsson P, Bergenfelz A. A prospective study of 100 roboticallyassisted laparoscopic adrenalectomies. J Robot Surg. 2011;5(2):127–131. [DOI] [PubMed] [Google Scholar]
- 38. Sroka G, Slijper N, Shteinberg D, Mady H, Galili O, Matter I. Laparoscopic adrenalectomy for malignant lesions: surgical principles to improve oncologic outcomes. Surg Endosc. 2013;27(7):2321–2326. [DOI] [PubMed] [Google Scholar]