Abstract
Context:
Doxorubicin (DOX) and gemcitabine (GEM) are anticancer drugs that were combined in a nanoemulsion (NE) to reduce their adverse side effects.
Aim:
To detect the antitumor activity of the combination formulas of GEM and DOX, loaded either in water (GEM+DOX-Sol) or in NEs (GEM–DOX combination/loaded NE [GEM+DOX/LNE]), in female Swiss albino mice inoculated with Ehrlich ascites carcinoma (EAC).
Settings and Design:
The anticancer assessment of the NE formulas was implemented in 200 mice, which were divided into 10 groups.
Materials and Methods:
It includes the detection of the change in body weight, analysis of the hematological and serum biochemical profiles, and study of the histopathologic alterations of the heart tissues.
Statistical Analysis:
One-factor analysis of variance was used.
Results:
Mice treated with GEM + DOX/LNE, which have an z-average of 155.38±2.33nm and zeta potential of −38.5±1.3 mV, recorded a considerable improvement in the mean survival time (MST), which was 60 days, as compared to the EAC control group, which has an MST of 28 days. It also restored the hematological and serum biochemical parameters toward normal values.
Conclusions:
The combination of GEM and DOX in NE has significantly diminished the cardiotoxicity of DOX and hematotoxicity of GEM while improving their antitumor properties.
KEYWORDS: Cardiotoxicity, chemotherapeutic agents, mean survival time, z-average diameter, zeta potential
INTRODUCTION
Cancer is still considered one of the most challenging diseases in spite of all the advancement in the pharmaceutical industries in formulating enormous numbers of chemotherapeutic agents.[1] In fact, most of the antitumor drugs have serious side effects due to their nonspecificity and inability to target the cancer cells without affecting the healthy cells. Therefore, it has been proposed to combine more than one anticancer drug in a nanocarrier to reduce the administered drug concentration and ameliorate the targeted drug delivery.[2]
Gemcitabine (GEM), a nucleoside analog, and doxorubicin (DOX), an antibiotic, are chemotherapeutic agents that have been formulated previously and exhibited safe and effective effect in the treatment of hepatocellular carcinoma, metastatic breast cancer, and renal cell carcinoma with sarcomatoid features.[3,4,5] Nanoemulsion (NE) is a colloidal system that is composed of two immiscible liquids, such as oil and water, stabilized by an interfacial film of surfactant molecules.[6] NEs have been used extensively for solubilizing both hydrophobic and hydrophilic drugs and proved to improve the drug pharmacokinetic and pharmacodynamic properties.
The major objective of the present study was to in vivo assess the anticancer activity of the combination therapy of GEM and DOX encapsulated in NE.
SUBJECTS AND METHODS
Chemicals
The chemotherapeutic agents (GEM and DOX) were procured from Pharmacopeia, CA, USA. All the NE constituents were obtained from Leo Chem, Bengaluru, India.
Animals and tumor cell line
Female Swiss albino mice (n = 200, weight = 25–30g) were housed in accordance with King Abdulaziz University’s policy on the care and use of laboratory animals. The ethical approval was obtained from the research ethics committee in the Faculty of Medicine at King Abdulaziz University. Ehrlich ascites carcinoma (EAC) cells were procured from the American Type Tissue Culture Collection (Manassas, VA, USA).
Preparation of NE formulations
The NE formulation was prepared as described by Alkhatib and Albishi.[7] In brief, the surfactant mixtures of polyoxyethyleneglycerol trihydroxy stearate 40, soya phosphatidyl choline, and sodium oleate were blended at a ratio of 3.5:3.0:3.5, respectively, followed by the addition of cholesterol (CHO), 1-octanol, and warm Tris–HCl buffer (pH 7.22). The resulted NE formula was stored at room temperature.
The NE formulas were used as blank nanoemulsion (Bl-NE); GEM loaded in NE (GEM/LNE), prepared by solubilizing 60.0mg GEM/kg of mouse in 0.2mL NE; DOX loaded in NE (DOX/LNE), prepared by solubilizing 2.0mg DOX/kg of mouse in 0.2mL NE; and a 1:1 ratio of GEM–DOX combination loaded in NE (GEM+DOX/LNE), produced by solubilizing 30.0mg GEM/kg of mouse and 1.0mg DOX/kg of mouse in 0.2mL NE.[8,9] Another groups of formulas, produced by dissolving the drugs in 0.2mL distilled water instead of 0.2mL NE, were designated as GEM-Solution (GEM-Sol), DOX-Solution (DOX-Sol), and combination-Solution (GEM+DOX-Sol).
Characterization of NE formulations
The physical properties of the NE-dispersed droplets were identified with the Zetasizer (3,000 HS; Malvern Instruments, Malvern, UK). The desired NE formula was prepared by diluting a 100 µL sample in a 400-µL Tris buffer (pH 7.2).
In vivo antitumor activity
The antitumor efficacy of the tested drug formulas was assessed as mentioned previously.[10] The mice were divided into 10 groups (n = 20) according to the administered drugs:
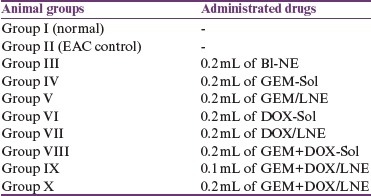
On zero-day, mice were weighed followed by intraperitoneal injection with 2.5×106 EAC cells/mouse except group I (normal), which was kept without inoculation with EAC. Group II served as EAC control that did not get administered with drugs. After 3 days, animals in groups III–IX were treated with 4 doses whereas group X was treated with 3 doses of the desired drug given every 3 days.[8]
After 12h of fasting on the 18th day, the blood of the weighed mice (n = 10) from each group was collected for the analysis of the hematological and serum parameters followed by killing them and resecting their hearts to weigh them and analyze their tissues as mentioned earlier.[11] The other 10 mice of each group were left to determine the mean survival time (MST) as described elsewhere.[12]
Statistical analysis
The differences between the groups were assessed using MegaStat Excel (version 10.3; Butler University, Indianapolis, IN). The significant difference was considered when the P-value <0.05.
RESULTS
Characterization of the drug-loaded NEs
The nanoparticles of the drug formulas were physically characterized using the Zetasizer. As illustrated in Table 1, the z-average diameters of all the drug-loaded NEs were significantly greater than Bl-NE. The nanodroplets’ sizes of GEM+DOX/LNE were greater than DOX/LNE but were less than those of GEM/LNE. In fact, GEM/LNE has the least zeta negative potential, whereas GEM+DOX/LNE has the maximum negative potential. Interestingly, both Bl-NE and DOX/LNE have comparable zeta potentials that significantly differ from GEM/LNE and GEM+DOX/LNE. It is noteworthy to mention that the drug formulas have net negative charges on their surfaces as revealed by the values of the zeta potentials. In addition, the coefficients of variations of all the formulas were less than 0.18, indicating homogenous distribution of the nanodroplets’ sizes.
Table 1.
z-average, zeta potential, and the coefficient of variation of the drugs-LNE determined by Zetasizer. Data were expressed as X̄ ± SD

In vivo antitumor efficacy of drug formulations
Body weight change
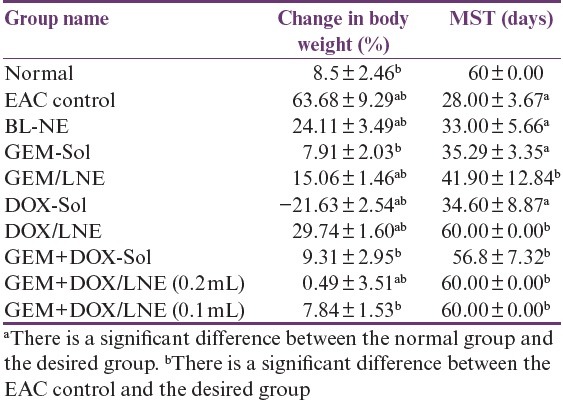
The effect of the drug formulations on % changes in body weight is illustrated in Table 2. All the groups have positive % body weight changes except DOX-Sol group, which has a negative % body weight change. The EAC control group has the most increase in the % body weight that significantly differs from the other groups. Interestingly, the % body weight changes of both Bl-NE and DOX/LNE were comparable. In addition, there were no significant differences in the % body weight changes between the combination formulas, GEM+DOX-Sol and GEM+DOX/LNE (0.1mL).
Table 2.
Effect of drug formulations on the body weight(g), change in EAC-bearing mice. Data were represented as X̄ ± SD
It is worth mentioning that the volumes of EAC fluid (mL) came out from the peritonea of the slaughtered mice after 18 days of inoculations were 27.1±1.1, 4.67±4.51, and 0.17±0.29 in the groups of EAC control, Bl-NE, and GEM-Sol, respectively, whereas the other groups did not have any fluid in their peritonea.
Mean survival time
As shown in Table 2, MSTs of DOX/LNE, GEM+DOX/LNE (0.1mL), and GEM+DOX/LNE (0.2mL), which were comparable to the normal group, were the highest among the other treated groups and had significantly increased as compared to the EAC control group. The least MST was observed in the DOX-Sol group. The MST of GEM+DOX-Sol group was significantly more than GEM-Sol and DOX-Sol groups. It should be mentioned that the MST of the EAC group did not significantly differ from the MST of Bl-NE, DOX-Sol, and GEM-Sol groups.
Toxic effect of the drug formulations on the complete blood count
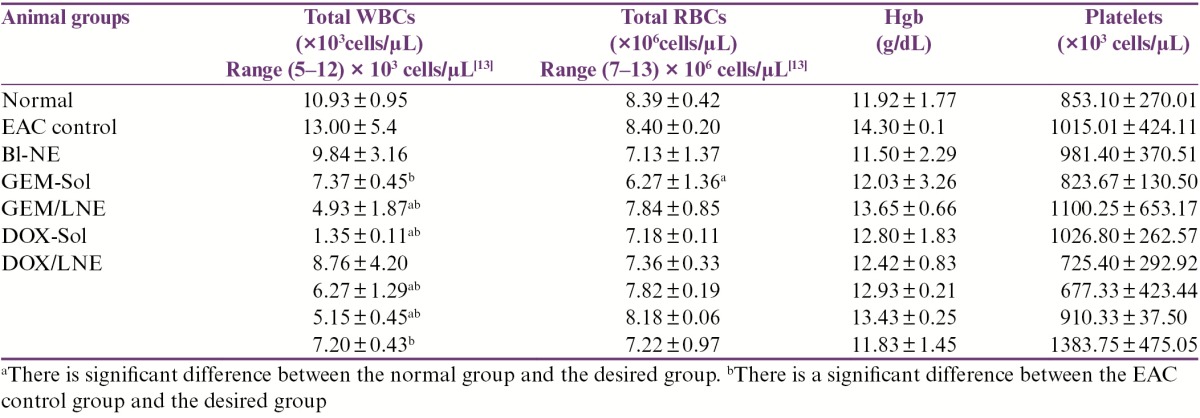
Complete blood counts (CBCs) of the tested animals are shown in Table 3. Compared to the normal group, white blood cell (WBC) count has been significantly decreased in DOX-Sol, GEM/LNE, GEM+DOX-Sol, and GEM+DOX/LNE (0.1mL) groups, whereas other treated groups did not show any significant differences. In fact, the WBC counts of DOX-Sol and EAC control groups were out of the standard normal range of WBC count in the mice, which is (5–12) × 103 cells/µL. On the other hand, it was only GEM-Sol group that was less than the standard range of the red blood cell (RBC) count in the mice, which is (7–13) × 106 cells/µL, and has shown a significant decrease in RBC count when compared to the normal group. All treated groups showed no significant change in the hemoglobin (Hgb) content and platelets levels when compared to the normal group.
Table 3.
Effect of drug formulations on the hematological parameters in EAC-bearing mice. Data were expressed as X̄ ± SD
Toxic effect of the drug formulations on the heart
Relative heart weights
Figure 1 displays the effect of the drug formulations on the heart weight relative to the body weight. It was only DOX-Sol group that has enlarged the heart as the relative heart weight differs significantly from all of the tested groups.
Figure 1.
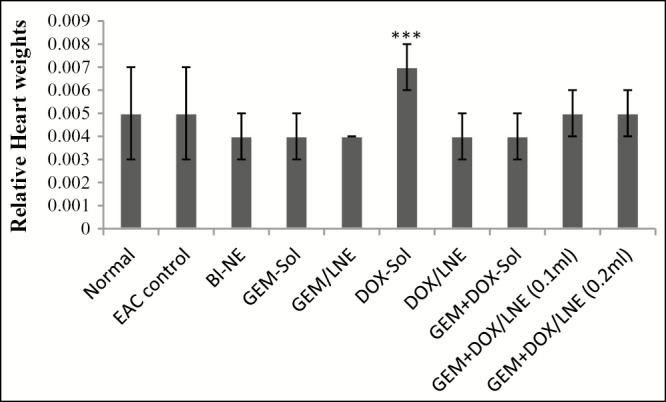
Relative heart weights of the tested animal groups
Cardiac enzymes
The effect of different treatments on heart enzymes is elaborated in Table 4. An elevated level of creatine phosphokinase (CK) from the standard range of 15–110 U/L was observed in DOX-Sol group. In fact, their levels of CK were significantly greater than all the other studied groups that were within the range. It is noteworthy to mention that GEM+DOX/LNE (0.1mL) has similar levels of CK compared to the normal group. Likewise, the levels of creatine phosphokinase-MB (CK-MB) were elevated from the standard range (up to 16 U/L) in DOX-Sol group. Actually, the levels of CK-MB have significantly decreased in EAC control and all the treated groups when compared to the normal group except GEM+DOX-Sol, DOX-Sol, and DOX/LNE groups have elevated levels. In fact, the levels of CK-MB in both DOX/LNE and GEM+DOX-Sol groups were within the standard range, whereas DOX-Sol has elevated level.
Table 4.
Effect of drug formulations on the heart function in EAC-bearing mice in terms of serum analysis. Data were represented as X̄ ± SD
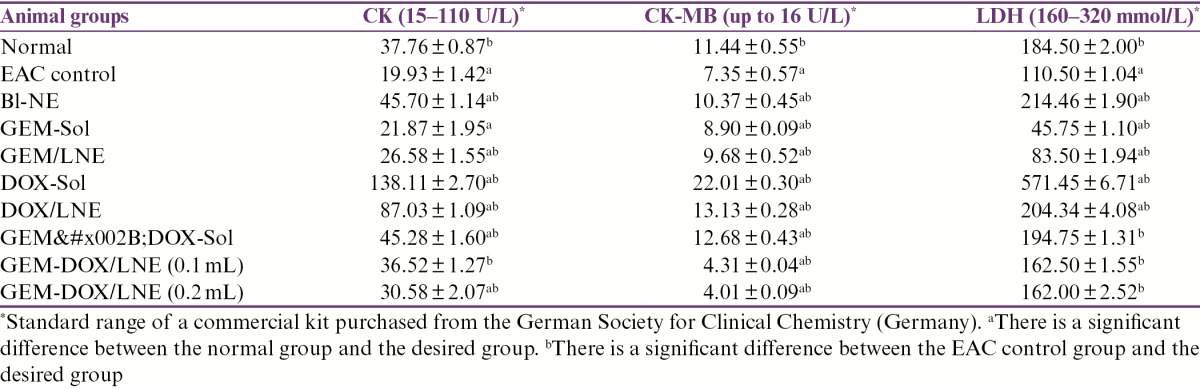
There were no significant differences in the levels of lactate dehydrogenase (LDH) in the combination formulas, GEM+DOX-Sol, GEM+DOX/LNE (0.2mL), and GEM+DOX/LNE (0.1mL), compared to the normal group as they were within the standard range (160–320 mmol/L). The levels of LDH in both DOX-Sol and DOX/LNE were significantly greater than the normal group and close to the maximum limit of the standard range, whereas EAC control, GEM/LNE, and GEM-Sol groups showed a significant decrease in the LDH levels when compared to the normal group and were lower than the standard range.
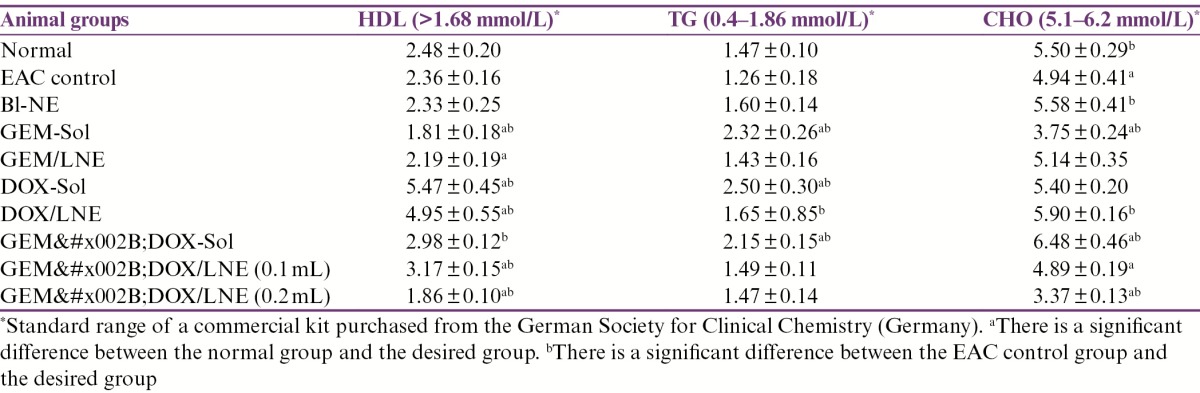
Lipid profile
The lipid profile of all the groups is exhibited in Table 5. The levels of high-density lipoprotein (HDL) in all of the studied groups were within the standard range (>1.68 mmol/L). In contrast, the levels of triglyceride (TG) that were significantly greater than the normal group and elevated from the standard range (0.4–1.86 mmol/L) were detected in the groups treated with the solution formulas. Meanwhile, the levels of TG for EAC control and formulas/LNE were comparable to the normal group. On the other hand, EAC control, GEM-Sol, GEM+DOX/LNE (0.1mL), and GEM+DOX/LNE (0.2mL) treatments have caused a significant reduction in CHO levels compared to the normal group. It was only GEM+DOX-Sol group that raised the CHO level in the mice relative to the normal group to be out of the standard range (5.1–6.2 mmol/L).
Table 5.
Effect of drug formulations on the levels of the lipid profiles in EAC-bearing mice. Data were expressed as X̄ ± SD
Cardiac tissue analysis
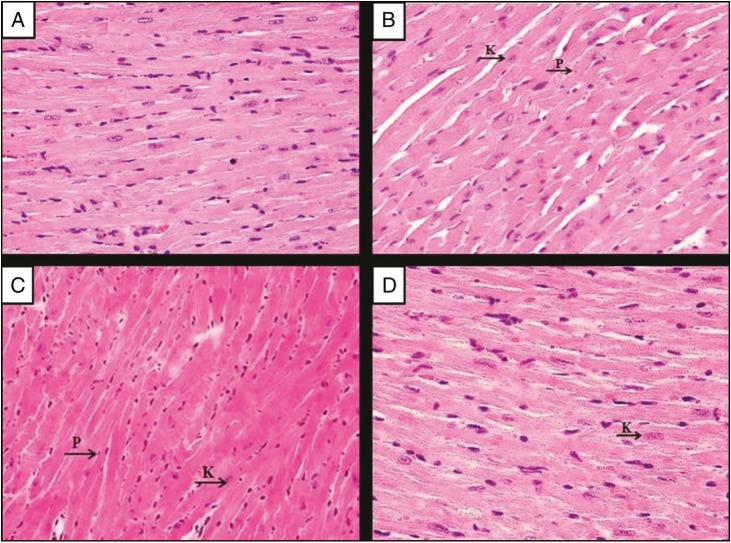
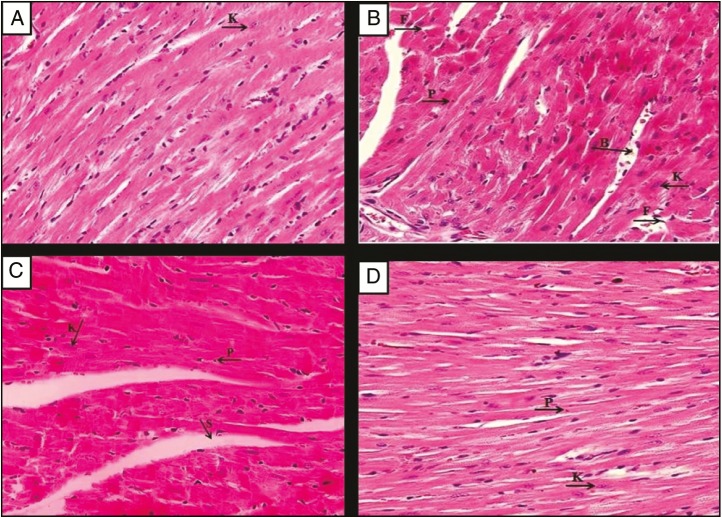
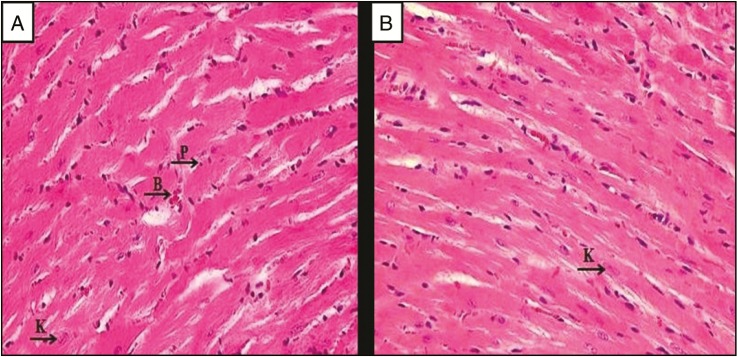
As displayed in Figure 2A, the heart tissues of the normal group showed normal cardiac myocytes with their centrally placed nuclei were seen. Figure 2B displays section of heart tissue of untreated EAC-bearing mice (EAC control group), which exhibits nearly normal cardiac myocytes architectures with mild nuclear changes in the form of pyknosis, which is defined as the degeneration of the cell in which the nucleus shrinks in size and the chromatin irreversibly condenses to a solid, and karyolysis, which is the dissolution of the cell nucleus by necrosis, and wide dilated blood vessels that could be due to the enzymatic degradation by endonucleases. In contrast, Bl-NE group has less dilated myocytes spaces with dark nuclei, and some myocytes have endured pyknosis and karyolysis as shown in Figure 2C. Mice treated with GEM-Sol [Figure 2D] and GEM/LNE [Figure 3A] showed normal cardiac myocytes with their centrally placed nuclei and less dilated blood vessels. However, Figure 3B presented damages in the heart tissues of DOX-Sol group, including some nuclei enduring karyolysis and pyknosis. In contrast, DOX/LNE group had a minor disruption of heart muscle fibers as revealed in Figure 3C. In terms of the combination formulas, light microscopy examination of the heart tissue of GEM+DOX-Sol group, viewed in Figure 3D, revealed the morphological changes as widened spaces between myocytes, and some myocytes have endured pyknosis and karyolysis. Meanwhile, GEM+DOX/LNE (0.1mL) and GEM+DOX/LNE (0.2mL) groups, as illustrated in Figure 4A and B, respectively, showed less morphological modification than DOX-Sol and GEM+DOX-Sol groups as moderate widening spaces between the myocytes, and fewer myocytes have undergone pyknosis and karyolysis.
Figure 2.
Photomicrographs of the myocardium of (A) the normal group, (B) the EAC group, (C) EAC-bearing mice treated with Bl-NE group, and (D) the EAC-bearing mice treated with GEM-Sol group. Hematoxylin and eosin stain (H&E) × 400. P: pyknosis; K: karyolysis
Figure 3.
Photomicrographs of the myocardium of the EAC-bearing mice treated with (A) GEM/LNE, (B) DOX-Sol, (C) DOX/LNE, and (D) GEM+DOX-Sol. H&E × 400. P: pyknosis; K: karyolysis; B: blood vessels; S: spaces between myocytes
Figure 4.
Photomicrographs of the myocardium of the EAC-bearing mice treated with (A) GEM+DOX/LNE (0.1mL) and (B) GEM+DOX/LNE (0.2mL). H&E × 400
DISCUSSION
NEs consist of safe materials that are arranged to produce nanodroplets suitable to solubilize the drugs in their cores and hence protect them from degradation when applied into the body. It is very important to identify the nanodroplets’ sizes and charges to determine their effect on the drug’s efficacy and toxicity.[14] In this study, it had been found that droplet sizes of all the drug-loaded NEs were considerably greater than the size of the Bl-NE, which indicates that the drugs were loaded in the cores of the nanodroplets. In addition, the droplet diameters of all the NE formulations are sufficient to be adsorbed and for uptake by the cells because they are within the range of 100–200nm.[15] The high negative amounts of the zeta potential of all the NE formulations imply that they are stable formulas due to the presence of large repulsion forces between the nanoparticles that prevent them from aggregation.[16]
The antitumor efficacy of the drug formulas was shown in terms of % change of body weight and MST. The variations in the body weight could be a sign of the drug toxicity that precede the animal death. The EAC control group has significantly increased in body weight than the other groups, which is attributed to the growth of the EAC in the peritoneal cavity.[12] By comparing the results of our experimental groups and previous studies, treatment with the DOX/LNE and DOX+GEM/LNE (0.1 and 0.2mL) prevented the body weight from the great loss induced by DOX and huge increase that happened in the EAC control group.[9,17,18] Furthermore, the MST of the tumor-bearing mice treated with GEM/LNE, DOX/LNE, GEM+DOX-Sol, GEM+DOX/LNE (0.1mL), and GEM+DOX/LNE (0.2mL) formulations exceeded the MSTs of Bl-NE, GEM-Sol, and DOX-Sol treatment, which implies an enhancement in the efficacy of the antitumor drugs.[19]
Myelosuppression caused by the increase of WBCs and anemia are the most common problems that are associated with the chemotherapeutic-agent treatment.[20] The finding of this study showed that the largest WBC count was recorded for the EAC control group, whereas the least count was observed in the DOX-Sol group, which can be due to the bone marrow depression caused by DOX.[21] In contrast, the RBC count in the mice treated with GEM-Sol formula was significantly less than the normal group and all studied groups due to the hematological toxicity of GEM.[22] In this study, GEM in combination with DOX-LNE showed nontoxic effect on hematological parameters, indicating that NE formulas had a selective affinity to the cancer cell.
DOX, a well-known chemotherapeutic agent, could cause cardiac injury due to the generation of the reactive oxygen species in the myocytes that cause damage in myocardium and thereby release the enzymes, such as CK, CK-MB, and LDH.[4,9] It is necessary to determine the cardiac toxicity of the NEs loaded with DOX to corroborate our hypothesis that NEs will have a lower cardiotoxicity when compared to DOX alone due to their tumor-targeted approach. In addition, this study endows with the significant indication of the beneficial effects of GEM on DOX/LNE-induced cardiomyopathy because GEM is generally not considered as one of the cardiotoxic agents.[23] In this study, the cardiotoxicity of the drug formulas in the tested mice was assessed in terms of measuring the relative heart weights, analyzing the serum, lipid profile, and the histological changes in the myocytes of the heart tissue. Compared to the other groups, DOX-Sol was very toxic to the cardiac myocytes of the mice because they have had an enlarged relative heart weight, damaged myocytes exhibited in the heart tissue, and elevated levels of CK, CK-MB, LDH, HDL, and TG. In fact, DOX interferes with the metabolism and biosynthesis of lipids, thereby increasing the TG level.[24] In contrast, when GEM+DOX/LNE (0.1mL) was administered into the tumor-bearing mice, it has reduced the levels of CK, CK-MB, LDH, and CHO while increasing the amount of HDL.
The outstanding remarkable findings were that GEM prevented the DOX-induced altered lipid parameters and myocardial marker enzymes and tissue because GEM is not considered cardiotoxic.[23] In this study, the levels of LDH in GEM-Sol and GEM/LNE groups were below the normal range, whereas the levels of CK and CK-MB in mice treated with GEM-Sol and GEM/LNE were within the normal range. Our findings suggest that GEM+DOX/LNE protects the myocardium through numerous aspects against DOX-induced cardiomyopathy.
CONCLUSIONS
Incorporating GEM into DOX/LNE formulation enhanced the antitumor activity of GEM and DOX while reducing their side effect. On the basis of this study, it is recommended to establish further human researches to give a complementary study of the effect of the combination NE formulas on the body tissues. In addition, further studies have to be performed on other types of chemotherapy drugs.
Financial support and sponsorship
The study was supported by King Abdulaziz City for Science and Technology.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Murugan A, Kumari Shanmugasundaram K. Antiproliferative effect of silver nanoparticles synthesized using vitex negundo on HEPG2 cell line. World J Pharm Res. 2014;3:2277–7105. [Google Scholar]
- 2.Hu CM, Aryal S, Zhang L. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther Deliv. 2010;1:323–34. doi: 10.4155/tde.10.13. [DOI] [PubMed] [Google Scholar]
- 3.Zhu F, Jiang Y, Luo F, Li P. Effectiveness of localized ultrasound-targeted microbubble destruction with doxorubicin liposomes in H22 mouse hepatocellular carcinoma model. J Drug Target. 2015;23:323–34. doi: 10.3109/1061186X.2014.996759. [DOI] [PubMed] [Google Scholar]
- 4.Lale SV, Kumar A, Naz F, Bharti AC, Koul V. Multifunctional ATRP based pH responsive polymeric nanoparticles for improved doxorubicin chemotherapy in breast cancer by proton sponge effect/endo-lysosomal escape. Polym Chem. 2015;6:2115–32. [Google Scholar]
- 5.Haas NB, Lin X, Manola J, Pins M, Liu G, McDermott D, et al. A phase II trial of doxorubicin and gemcitabine in renal cell carcinoma with sarcomatoid features: ECOG 8802. Med Oncol. 2012;29:761–7. doi: 10.1007/s12032-011-9829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alkhatib MH, Albishi HM, Mahassni SH. Impact of nanoparticles on cancer therapy. Trop J Pharm Res. 2012;11:1001–11. [Google Scholar]
- 7.Alkhatib M, Albishi H. In vitro evaluation of antitumor activity of doxorubicin loaded nanoemulsion in MCF-7 human breast cancer cells. J Nanopart Res. 2013;15:1489–504. [Google Scholar]
- 8.Cividalli A, Mauro F, Livdi E, Ceciarelli F, Altavista P, Cruciani G, et al. Schedule dependent toxicity and efficacy of combined gemcitabine/paclitaxel treatment in mouse adenocarcinoma. J Cancer Res Clin Oncol. 2000;126:461–7. [PubMed] [Google Scholar]
- 9.Jiang SP, He SN, Li YL, Feng DL, Lu XY, Du YZ, et al. Preparation and characteristics of lipid nanoemulsion formulations loaded with doxorubicin. Int J Nanomedicine. 2013;8:3141–50. doi: 10.2147/IJN.S47708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alkreathy HM, Damanhouri ZA, Ahmed N, Slevin M, Osman AM. Mechanisms of cardioprotective effect of aged garlic extract against doxorubicin-induced cardiotoxicity. Integr Cancer Ther. 2012;11:364–70. doi: 10.1177/1534735411426726. [DOI] [PubMed] [Google Scholar]
- 11.Herzig MC, Kolly C, Persohn E, Theil D, Schweizer T, Hafner T, et al. LRRK2 protein levels are determined by kinase function and are crucial for kidney and lung homeostasis in mice. Hum Mol Genet. 2011;20:4209–23. doi: 10.1093/hmg/ddr348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raju AB, Ravindranath A, Kalpana G. Antitumor activity of Diospyros peregrina on Ehrlich ascites carcinoma in mice. J Sci Res. 2011;3:413–19. [Google Scholar]
- 13.McGarry M, Protheroe C, Lee J. Mouse hematology. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2010. [Google Scholar]
- 14.Honary S, Zahir F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (part 1) Trop J Pharm Res. 2013A;12:255–64. [Google Scholar]
- 15.Ha HK, Kim JW, Lee MR, Jun W, Lee WJ. Cellular uptake and cytotoxicity of β-lactoglobulin nanoparticles: The effects of particle size and surface charge. Asian-Australas J Anim Sci. 2015;28:420–7. doi: 10.5713/ajas.14.0761. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Sabeti B, Noordin MI, Mohd S, Hashim R, Dahlan A, Javar HA. Development and characterization of liposomal doxorubicin hydrochloride with palm oil. Biomed Res Int 2014. 2014:765426. doi: 10.1155/2014/765426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hao H, Ma Q, Huang C, He F, Yao P. Preparation, characterization, and in vivo evaluation of doxorubicin loaded BSA nanoparticles with folic acid modified dextran surface. Int J Pharm. 2013;444:77–84. doi: 10.1016/j.ijpharm.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 18.Cuong NV, Jiang JL, Li YL, Chen JR, Jwo SC, Hsieh MF. Doxorubicin-loaded PEG-PCL-PEG micelle using xenograft model of nude mice: Effect of multiple administration of micelle on the suppression of human breast cancer. Cancers (Basel) 2010;3:61–78. doi: 10.3390/cancers3010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph MM, Aravind SR, George SK, Pillai RK, Mini S, Sreelekha TT. Co-encapsulation of doxorubicin with galactoxyloglucan nanoparticles for intracellular tumor-targeted delivery in murine ascites and solid tumors. Transl Oncol. 2014;7:525–36. doi: 10.1016/j.tranon.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sainadh N, PKM N, Kulkarni SC. Evaluation of anti-cancer activity of Kigelia africana on EAC induced solid tumors. J Pharmacol Toxicol Studies. 2013;1:20–5. [Google Scholar]
- 21.Al-Harbi MM, Al-Gharably NM, Al-Shabanah OA, Al-Bekairi AM, Osman AMM, Tawfik HN. Prevention of doxorubicin-induced myocardial and haematological toxicities in rats by the iron chelator desferrioxamine. Cancer Chemoth Pharm. 1992;31:200–204. doi: 10.1007/BF00685548. [DOI] [PubMed] [Google Scholar]
- 22.Pitrubhakta AB, Shinde AJ, Jadhav NR. Design, development and characterization of pegylated liposomes of gemcitabine hydrochloride. Der Pharma Lett. 2012;4:314–29. [Google Scholar]
- 23.Khan MF, Gottesman S, Boyella R, Juneman E. Gemcitabine-induced cardiomyopathy: A case report and review of the literature. J Med Case Rep. 2014;8:220. doi: 10.1186/1752-1947-8-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chennuru A, Saleem MT. Antioxidant, lipid lowering, and membrane stabilization effect of sesamol against doxorubicin-induced cardiomyopathy in experimental rats. Biomed Res Int 2013. 2013:934239. doi: 10.1155/2013/934239. [DOI] [PMC free article] [PubMed] [Google Scholar]