Abstract
Objective:
The present study was performed to investigate the effect of hydroalcoholic extract of red cabbage and its fractions on sleeping behavior in mice.
Materials and Methods:
The extract and its fractions were injected to mice and sleep duration as well as sleep latency were recorded. Furthermore, toxicity of the extract was determined both in vivo and in vitro.
Results:
The extract increased sleep duration at doses of 50–200mg/kg (P < 0.001). This observed hypnotic effect was comparable to that of diazepam (3mg/kg) (P < 0.001 in comparison with control group). Ethyl acetate, n-butanol, and aqueous fractions could increase sleep duration (P < 0.001). The sleep latency was decreased by the extract (P < 0.001) and only ethyl acetate fraction (P < 0.001). LD50 value for red cabbage extract was 2.4g/kg. There was no toxic effect on viability of cultured neuronal cells (PC12). Rotarod test results showed that there were no significant differences between the extract groups and the control group.
Conclusion:
The results suggest that red cabbage potentiates pentobarbital hypnosis without any toxic effect. The main component(s) responsible for this effect is most likely to be intermediate polar agent(s) such as flavonoids, which are found in ethyl acetate fraction of this plant.
KEYWORDS: Diazepam, PC12, pentobarbital, red cabbage, sleep
INTRODUCTION
Insomnia is defined as repeated difficulty in falling asleep, difficulty maintaining sleep, and/or experiencing low-quality sleep, which results in some form of daytime disturbance.[1] Low sleeping leads to mental problem and fatigue. According to recent reports, 45% people suffer from sleeping disorder; this problem is higher in women than men.[2] There are some drugs such as benzodiazepines and antihistaminic agents that are used for inducing sleep.[2] γ -Aminobutyric acid receptor A (GABAA) is an important target for sleep-inducing drugs.[3] However, they are not suitable for long-time administration because of their tolerance-related issues and dependence.[4] There is a need to find new hypnotic drugs with lower adverse effects. Natural products have always been good sources for developing new treatments for the management of several diseases.[5] Some of the herbs that are used in insomnia include Humulus lupulus, Ziziphus jujuba (sour date), Valeriana officinalis, Passiflora incarnata (passion flower), Eschscholzia californica (California poppy), Piper methysticum, and Lactuca sativa.[6,7,8,9] The red cabbage (Brassica oleracea var. capitata f. rubra) belongs to Brassicaceae family (order: Brassicales).[10] Red cabbage is a cheap source of anthocyanin pigment.[11] Red cabbage contains flavonoids, and recent studies have shown that flavonoids have good therapeutic effects.[10] The pharmacological effects of red cabbage include reducing oxidative stress, decreasing blood glucose,[12,13,14] possessing anticancer properties,[15,16,17] and reducing blood cholesterol.[18] In some traditional medicinal books, it is reported that red cabbage has sedative–hypnotic effect.[19] There is no pharmacological report about the hypnotic effect of red cabbage. Therefore, this study has been designed to evaluate the sleep-prolonging effect of red cabbage extract and its different fractions. Also, flumazenil was used to guess possible sleep mechanism.
MATERIALS AND METHODS
Drugs and chemicals
Penicillin–streptomycin, pentobarbital sodium, and flumazenil were purchased from Sigma (St. Louis, MO, USA). Diazepam was purchased from Chemidarou Company (Tehran, Iran). Tween 80 was provided from Merck (Darmstadt, Germany). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco Life Technologies (Grand Island, NY, USA). Flumazenil, pentobarbital, and diazepam were dissolved in saline to make 30, 2, and 3mg/mL solutions, respectively.
PLANT COLLECTION AND EXTRACTION
Plant material
The red cabbage was purchased from a local market in Dargaz and the voucher specimen (No. 21373) was deposited in Dargaz Payame Noor University herbarium. The aerial parts of red cabbage were dried in shadow, powdered, and subjected to extraction with 70% ethanol with a Soxhlet apparatus for 48h. Then, the hydroalcoholic extract (HAE) was dried in a water bath, and the yield (19% w/w) was dissolved in saline containing 1% (v/v) of Tween 80. For preparation of fractions, a part of HAE was suspended in distilled water and transferred to a separator funnel. With solvent–solvent extraction, it was fractionated using ethyl acetate or n-butanol. The ethyl acetate fraction (EAF) and n-butanol fraction (NBF) were separated to obtain water fraction (WF).[9] The resulting fractions were dried on a water bath, and working solutions were made up in saline and saline containing 1% Tween 80 for WF and EAF or NBF, respectively.[20] The yields obtained from the extract fractionation were 73.5% WF, 12.5% EAF, and 14% NBF.
Animals
Male albino mice weighting 20–30g were maintained at a controlled temperature (22°C ± 1°C) with a 12-h light/dark cycle and free access to water and food. The study was carried out in accordance with ethical guidelines of Mashhad University of Medical Sciences (IR.MUMS.REC.1392.206). The animals (n = 88) were randomly divided into different groups (the number of animal in each group was 8); group 1 received normal saline and served as a negative control for HAE. Animals of group 2 received 3mg/kg diazepam as positive control. Mice in groups 3–7 received extracts at doses 12.5, 25, 50, 100, and 200mg/kg, respectively. Animals in groups 8–10 received 50mg/kg WF, 50mg/kg EAF, and 50mg/kg NBF, respectively. Moreover, animals were treated with 2mg/kg flumazenil as a diazepam antagonist before receiving diazepam (group 11), HAE (group 12), or EAF (group 13).
Evaluation of pentobarbital-induced sleep
The hypnotic assessment method is based on prolongation of sleep induced by pentobarbital.[20] Briefly, a single dose of HAE (12.5–200mg/kg), fractions of HAE, diazepam (3mg/kg), or vehicles was injected (i.p.) to the mice. After 30min, pentobarbital (30mg/kg, i.p.) was administrated to induce sleep.[21] Flumazenil (2mg/kg) was administrated (i.p.) 30min before diazepam or HAE.[21] The animals were considered asleep if stayed immobile and lost their righting reflex when positioned on its back. The time interval between administration of pentobarbital and onset of sleep was considered as sleep latency.
Rotarod behavioral method
The rotarod test was used to measure motor resistance and coordination. The experimental procedure for learning and adaptation was conducted for 3 consecutive days. On the next day, mice were placed on a rotating rod that accelerated smoothly from 4 to 40rpm over a period of 5min. The length of time they could maintain their balance on the turntable against the movement’s strength was recorded. Then, the extract (50mg/kg) or vehicle was injected, and after 30min, the animals were placed on rotarod again.[22] Each group included seven mice.
LD50 determination
Eight groups, each containing five mice, were used for determination of LD50 of HAE. Groups 1–7 were injected i.p. with 50, 100, 200, 400, 800, 1600, and 3200mg/kg, respectively, of HAE and group 8 received normal saline as vehicle. Mortality rate was observed and recorded for 24-h period. The highest dose that did not kill any mice and the lowest dose that led to death of one animal were recorded. The mean of these two doses was considered as the median lethal dose.[23]
Neurotoxicity assessment
The possible cytotoxicity of red cabbage was tested on rat pheochromocytoma-derived cells (PC12). The cells were cultured in 96-well plates for 24h in DMEM supplemented with 10% FBS, penicillin (100 units/mL), and streptomycin (100 µg/mL). Then, the culture medium was changed to fresh one containing vehicle dimethyl-sulfoxide (DMSO 1%) or HAE (50, 100, 150, 200, 400, and 800 µg/mL). The cells were incubated for 24h at 37°C in an atmosphere of 5% CO2. Cell proliferation was evaluated using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay as previously described.[9]
Statistics
All values are expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed using one-way analysis of variance followed by Tamhane’s T2 post hoc test. Differences were considered significant at P < 0.05.
RESULTS
Effects of red cabbage on duration of sleep
As expected, the reference drug diazepam was able to increase duration of sleep. The HAE at doses of 50, 100, and 200mg/kg could significantly increase sleep duration. As expected, pretreatment of mice with flumazenil decreased the sleep-prolonging effect of diazepam. Similarly, the effect of HAE on sleep duration was significantly inhibited by flumazenil [Figure 1].
Figure 1.
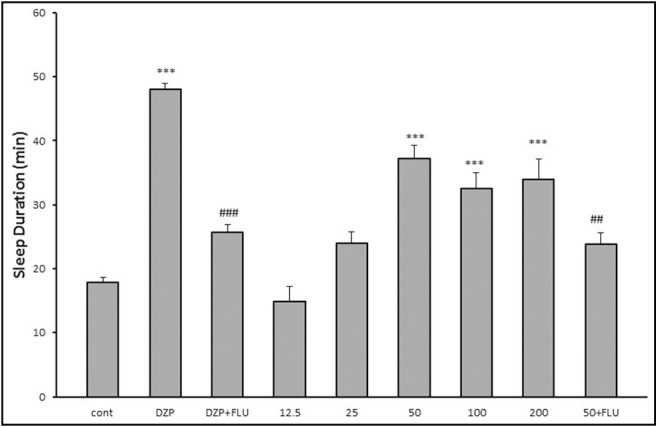
Effects of red cabbage HAE on sleeping time in pentobarbital-induced hypnotic test. Data are mean ± SEM of eight animals in each group. FNx18P < 0.001 significantly different from the control group. ##P < 0.01, ###P < 0.001 significantly different from the same group plus flumazenil (2mg/kg). DZP: diazepam; Flu: flumazenil
Effects of red cabbage on sleep latency
Diazepam (min, P < 0.001) and HAE at doses 50, 100, and 200mg/kg significantly (P < 0.001) reduced the latency in comparison to the saline [Figure 2]. Results showed that flumazenil (2mg/kg, i.p.) reversed the effects of diazepam and 50mg/kg of HAE. Therefore, in flumazenil-treated mice, there was an increased latency to sleep in pentobarbital-induced hypnotic test.
Figure 2.
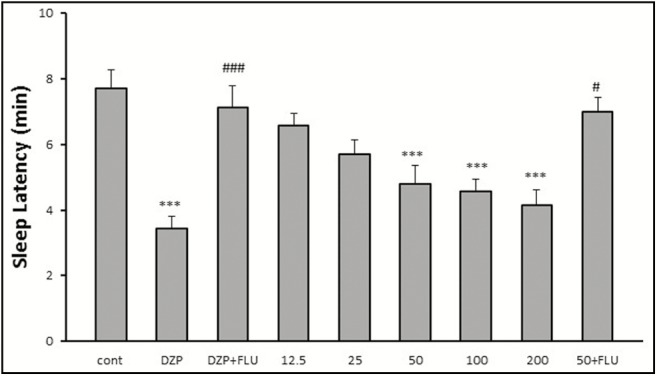
Effects of red cabbage hydro-alcoholic extract on sleeping latency in pentobarbital-induced hypnotic test. Data are mean ± SEM of eight animals in each group. FNx18P < 0.001 significantly different from the control group. #P < 0.05, ###P < 0.001 significantly different from the same group plus flumazenil (2mg/kg). DZP: diazepam; FLU: flumazenil
Effects of fractions on duration of sleep
As shown in Figure 3, all of fractions increased sleep duration, but the effect of EAF was more than that of NBF and WF (EAF: P < 0.001; WF: P < 0.001; NBF: P < 0.001). In addition, flumazenil reversed the effects of EAF.
Figure 3.
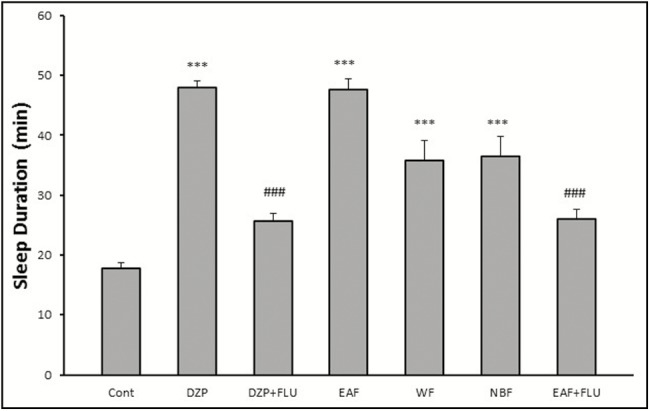
Effects of HAE fractions of red cabbage on sleeping time in pentobarbital-induced hypnotic test. Data are mean ± SEM of eight animals in each group. FNx18P < 0.001 significantly different from the control group. ###P < 0.001 significantly different from the same group plus flumazenil (2mg/kg)
Effects of fractions on sleep latency
As shown in Figure 4, only EAF decreased sleep latency significantly. In addition, flumazenil reversed the effects of EAF.
Figure 4.
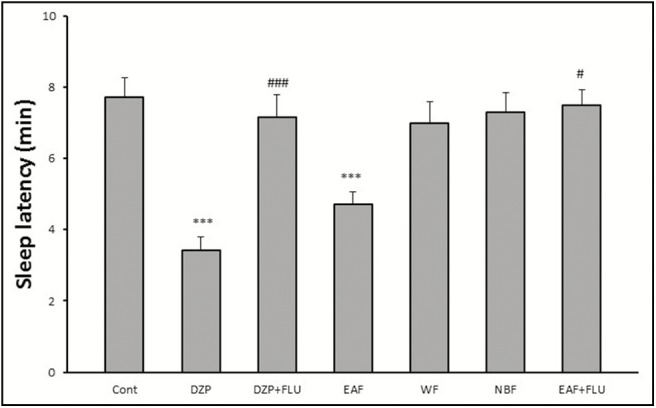
Effects of HAE fractions of red cabbage on sleeping latency in pentobarbital-induced hypnotic test. FNx18P < 0.001 significantly different from the control group. #P < 0.05, ###P < 0.001 significantly different from the same group plus flumazenil (2mg/kg)
Toxicity assessments
The highest dose, which did not kill any mice, and the lowest dose, which led to death of mouse, were 1.6 and 3.2g/kg, respectively. The mean of these two doses (2.4g/kg) was considered as LD50.
Result of MTT showed that none of the HAE concentrations (200, 400, 800, and 1600 µg/mL) decreased the proliferation of PC12 cells. Similarly, the HAE fractions exhibited no cytotoxicity [Figure 5].
Figure 5.
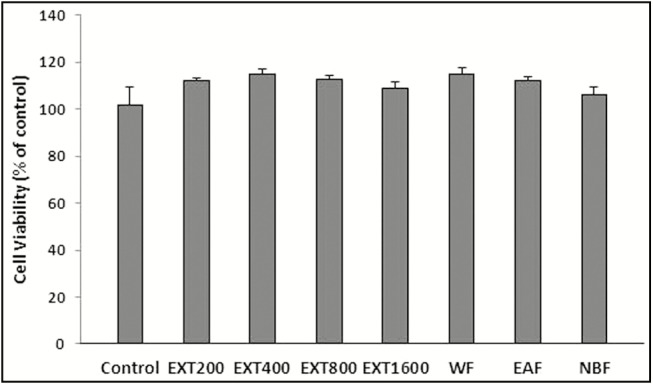
Effect of HAE and its fractions of red cabbage on PC12 cell viability. Cell viability was quantitated by MTT assay. Results are mean ± SEM
Effect of red cabbage on motor coordination
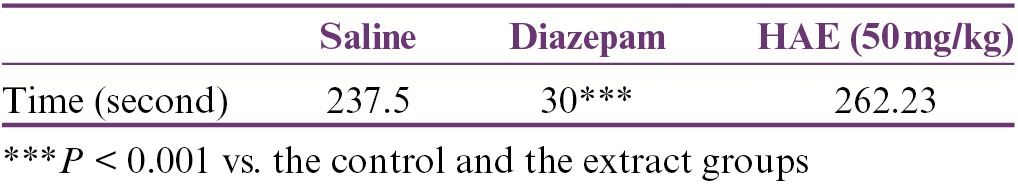
Considering the results from the rotarod test, there were no significant differences between the groups when the animals were examined 30min after injection of the extract. The results showed that injection of diazepam (3mg/kg) significantly shortened the length of time (P < 0.001) that the mice maintained their balance on rotarod apparatus compared to the control and extract groups [Table 1].
Table 1.
Effects of HAE of red cabbage on motor performance in rats. Data are expressed as mean ± SEM of seven animals in each group
DISCUSSION
According to the ancient Ayurveda literature, red cabbage has anticonvulsive, sedative, and hypnotic effects;[24] also, it is used in aromatherapy for relieving stress and insomnia.[24] In this study, we evaluated the hypnotic effect of HAE of red cabbage and its fractions in mice. To study the comprehensive effect of red cabbage, the following issues were investigated in mice: sleep latency, sleep duration, loss of motor coordination, and neurotoxicity, and its fraction effects on sleep latency and duration. In this study, the hypnotic evaluation method was based on potentiation of sleep induced by pentobarbital. This method is used for investigating sedative–hypnotic agents.[9,21,25] In the previous reports and as expected, diazepam significantly enhanced the sleeping time induced by pentobarbital, indicating that our experimental method was well optimized.[26] The effect of HAE of red cabbage on sleep duration was not dose dependent in the range of given doses, and the maximum effect was observed with a dose of 50mg/kg. The higher doses (100 and 200mg/kg) led to lesser sleep in comparison with 50mg/kg. It may be related to the fact that the solution that is concentrated at higher doses leads to pain and causes lower sleep in comparison to the lower dose. We observed that when high dose was injected to mice, it led to righting syndrome. The hypnotic effect was comparable to that of diazepam, but HAE did not affect the animals’ performance on the rotarod test; it seems that its effects on sleeping time and sleep latency are not due to affecting motor movement while diazepam decreased motor movement. To obtain a better insight into the nature of compounds responsible for the sleep-prolonging effect of this plant, three fractions were prepared from HAE of red cabbage. The WF contained polar compounds such as tannins, glycosides, and some alkaloids. The EAF included intermediate polarity such as flavonoids. The NBF contained low polar agents including alkanes, sterols, and terpenoids.[27,28,29] Our data showed that among these three fractions, EAF prolonged the duration of sleep and could decrease the sleep latency more than other fractions. Therefore, it can be concluded that the active constituents responsible for sleep-prolonging effects of red cabbage are intermediate polar agents in EAF. Several neurotransmitters have been shown to be involved in the regulation of sleep behavior. GABA is released from neurons that are located in the anterior hypothalamus and inhibits wake-promoting areas of the hypothalamus and brainstem.[30,31] Barbiturates such as pentobarbital tend to bind to GABA receptor ionophore complex to facilitate GABA action. Diazepam, a benzodiazepine agonist, is used in the management of sleep disorders such as insomnia; this compound has a binding site on GABAA ionophore complex.[3,32] It decreases activity, moderates excitement, and calms the recipient. Substances such as diazepam reduce the onset and increase duration of barbiturate-induced sleep and reduce exploratory activity, possessing potentials as sedative.[33] Our findings showed pretreatment with flumazenil inhibits sleep-prolonging effect of diazepam. Also, we found that in the presence of flumazenil, red cabbage is unable to increase the duration of sleep or decrease sleep latency time. This observation may be related to the effect of extract on GABA receptors and possible involvement of GABAergic transmission. Previously, it has been reported that flavonoids as natural active compounds tend to bind to benzodiazepine GABAA receptors, and they act pharmacologically as partial agonists. Some semisynthetic flavonoid derivatives are much more potent than diazepam in vivo.[34,35,36] Baicalin, a flavonoid extracted from Scutellaria lateriflora (Lamiaceae), exerts anxiolytic activity that is antagonized by a GABAA-specific antagonist.[37] It has also been suggested that chrysin, another natural flavonoid from Passiflora caerulea (Passifloraceae), exerts anxiolytic effects without any sedative and muscle relaxation activities. Recent studies have shown the presence of flavonoids in red cabbage. Therefore, it is rational to assume that the sleep-prolonging action of red cabbage is mediated, at least in part, by potentiating GABAergic system by flavonoids. Although we did not investigate the exact compound responsible for the sleep-prolonging effect of extract, further studies should be performed to test and to isolate active compounds from red cabbage. The rotarod test is a widely used method to evaluate the motor coordination or muscle relaxant effect in rodents. It is well established that some benzodiazepines such as diazepam cause muscle weakness,[38] decrease of ambulatory activity, and sedation, consequently impairing the performance of rodents in the rotarod.[39,40] As expected, we also found that diazepam caused muscle relaxation in the animals, causing a decrement of the falling time in rotarod but extract did not have muscle relaxation effect. Also, evaluation of the toxicity of red cabbage showed that LD50 value for HAE is 2.4g/kg. This value was markedly higher than that of hypnotic doses of extract (50–200mg/kg). Similarly, HAE and its fractions did not diminish the viability of neuronal cells even at high concentrations. Therefore, it seems that the sleep-prolonging effect of red cabbage is not accompanied by a neurotoxic action.
CONCLUSION
This study showed that red cabbage potentiates the pentobarbital-induced sleeping behaviors in mice. The sleep-prolonging effect was comparable to that induced by diazepam and accompanied with no neuron toxicity. The main components responsible for the effects are most likely of polar agents found in EAF. Isolation of the active compound(s) may yield novel sleep-prolonging agents.
Financial support and sponsorship
Nil.
Conflicts of interest
None.
REFERENCES
- 1.ZOLONG Study Group. Krystal AD, Erman M, Zammit GK, Soubrane C, Roth T. Long-term efficacy and safety of zolpidem extended-release 12.5mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: A 6-month, randomized, double-blind, placebo-controlled, parallel-group, multicenter study. Sleep. 2008;31:79–90. doi: 10.1093/sleep/31.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noor ZM, Smith AJ, Smith SS, Nissen LM. A study protocol: A community pharmacy-based intervention for improving the management of sleep disorders in the community settings. BMC Health Serv Res. 2014;14:74. doi: 10.1186/1472-6963-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang F, Xiong Y, Xu L, Ma S, Dou C. Sedative and hypnotic activities of the ethanol fraction from Fructus Schisandrae in mice and rats. J Ethnopharmacol. 2007;110:471–5. doi: 10.1016/j.jep.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Oh S, Oh K, Cho H, Eun J. Effects of the combined-preparation of Germinated Brown rice, cultured mountain Ginseng and Longanae Arillus on pentobarbital induced sleeping time. Kor J Oriental Physiol Pathol. 2010;24:598–601. [Google Scholar]
- 5.Meletis CD, Zabriskie N. Natural approaches for optimal sleep. J Altern Complement Med. 2008;14:181–88. [Google Scholar]
- 6.Sarris J, Byrne GJ. A systematic review of insomnia and complementary medicine. Sleep Med Rev. 2011;15:99–106. doi: 10.1016/j.smrv.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Sarris J, Panossian A, Schweitzer I, Stough C, Scholey A. Herbal medicine for depression, anxiety and insomnia: A review of psychopharmacology and clinical evidence. Eur Neuropsychopharmacol. 2011;21:841–60. doi: 10.1016/j.euroneuro.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Edinger JD, Means MK. Cognitive-behavioral therapy for primary insomnia. Clin Psychol Rev. 2005;25:539–58. doi: 10.1016/j.cpr.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Ghorbani A, Rakhshandeh H, Sadeghnia HR. Potentiating effects of Lactuca sativa on pentobarbital-induced sleep. Iran J Pharm Res. 2013;12:401–6. [PMC free article] [PubMed] [Google Scholar]
- 10.Asif M, Khodadadi E. Medicinal uses and chemistry of flavonoid contents of some common edible tropical plants. J Paramed Sci. 2013;3:119–38. [Google Scholar]
- 11.Serafini M, Peluso I, Raguzzini A. Flavonoids as anti-inflammatory agents. Proc Nutr Soc. 2010;69:273–8. doi: 10.1017/S002966511000162X. [DOI] [PubMed] [Google Scholar]
- 12.Roman-Ramos R, Flores-Saenz JL, Alarcon-Aguilar FJ. Anti-hyperglycemic effect of some edible plants. J Ethnopharmacol. 1995;48:25–32. doi: 10.1016/0378-8741(95)01279-m. [DOI] [PubMed] [Google Scholar]
- 13.Grover JK, Yadav SP, Vats V. Effect of feeding Murraya koeingii and Brassica juncea diet on [correction] kidney functions and glucose levels in streptozotocin diabetic mice. J Ethnopharmacol. 2003;85:1–5. doi: 10.1016/s0378-8741(02)00355-0. [DOI] [PubMed] [Google Scholar]
- 14.Yokozawa T, Kim HY, Cho EJ, Yamabi N, Choi JS. Protective effects of mustard leaf (Brassica juncea) against diabetic oxidative stress. J Nutr Sci Vitaminol (Tokyo) 2003;49:87–93. doi: 10.3177/jnsv.49.87. [DOI] [PubMed] [Google Scholar]
- 15.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A. 1997;94:10367–72. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komatsu W, Miura Y, Yagasaki K. Induction of tumor necrosis factor production and antitumor effect by cabbage extract. Nutr Cancer. 2002;43:82–9. doi: 10.1207/S15327914NC431_10. [DOI] [PubMed] [Google Scholar]
- 17.Fowke JH, Chung FL, Jin F, Qi D, Cai Q, Conaway C, et al. Urinary isothiocyanate levels, brassica, and human breast cancer. Cancer Res. 2003;63:3980–6. [PubMed] [Google Scholar]
- 18.Komatsu W, Miura Y, Yagasaki K. Suppression of hypercholesterolemia in hepatoma-bearing rats by cabbage extract and its component, S-methyl-L-cysteine sulfoxide. Lipids. 1998;33:499–503. doi: 10.1007/s11745-998-0233-7. [DOI] [PubMed] [Google Scholar]
- 19.Mirheydar H. Herbal information: Usage of plants in prevention and treatment of diseases. Tehran, Iran: Islamic Culture Press Center; 1993. p. 40. [Google Scholar]
- 20.Rakhshandah H, Shakeri MT, Ghasemzadeh MR. Comparative hypnotic effect of Rosa damascena fractions and diazepam in mice. Iran J Pharm Res. 2012;6:193–7. [Google Scholar]
- 21.Rakhshandeh H, Sadeghnia HR, Ghorbani A. Sleep-prolonging effect of Coriandrum sativum hydro-alcoholic extract in mice. Nat Prod Res. 2012;26:2095–8. doi: 10.1080/14786419.2011.613388. [DOI] [PubMed] [Google Scholar]
- 22.Pritchet K, Mulder GB. The rotarod. J Am Assoc Lab Anim Sci. 2002;42:40–49. [PubMed] [Google Scholar]
- 23.Shetty Akhila J, Alwar M. Acute toxicity studies and determination of median lethal dose. Curr Sci. 2007;93:917–20. [Google Scholar]
- 24.Khorasani A. Tehran, Iran: Publishers and Education of Islamic Revolution; 2nd ed. Tehran, Iran: Publishers and Education of Islamic Revolution; 1992. Makhzanoladvieh; pp. 742–3. [Google Scholar]
- 25.Hosseini A, Ghorbani A, Sadeghnia HR, Rajabian A, Rakhshandeh H. Potentiating effects of Lactuca serriola on pentobarbital-induced sleep. Res Opin Anim Vet Sci. 2014;4:601–7. [Google Scholar]
- 26.Emamghoreishi M, Heidari-Hamedani G. Sedative-hypnotic activity of extracts and essential oil of coriander seeds. Iran J Basic Med Sci. 2006;31:22–27. [Google Scholar]
- 27.Seidel V. Initial and bulk extraction. In: Sarker SD, Latif Z, Gray AI, editors. Natural Products Isolation. Totowa, New Jersey: Humana Press; 2005. pp. 27–46. [Google Scholar]
- 28.Tian S, Shi Y, Zhou X, Ge L, Upur H. Total polyphenolic (flavonoids) content and antioxidant capacity of different Ziziphora clinopodioides Lam. extracts. Pharmacogn Mag. 2011;7:65–8. doi: 10.4103/0973-1296.75904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edewor-Kuponiyi TI. Plant-derived compounds with potential sedative and anxiolytic activities. Int J Basic Appl Sci. 2013;2:63–78. [Google Scholar]
- 30.Datta S. Cellular and chemical neuroscience of mammalian sleep. Sleep Med. 2010;11:431–40. doi: 10.1016/j.sleep.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murillo-Rodríguez E, Arias-Carrión O, Sanguino-Rodríguez K, González-Arias M, Haro R. Mechanisms of sleep–wake cycle modulation. CNS Neurol Disord Drug Targets. 2009;8:245–53. doi: 10.2174/187152709788921654. [DOI] [PubMed] [Google Scholar]
- 32.Herrera-Ruiz M, Gutiérrez C, Enrique Jiménez-Ferrer J, Tortoriello J, Mirón G, León I. Central nervous system depressant activity of an ethyl acetate extract from Ipomoea stans roots. J Ethnopharmacol. 2007;112:243–7. doi: 10.1016/j.jep.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Roy-Byren PP. The GABA-benzodiazepine receptor complex: Structure, function, and role in anxiety. J Clin Psychiatry. 2004;66:14–20. [PubMed] [Google Scholar]
- 34.Medina JH, Viola H, Wolfman C, Marder M, Wasowski C, Calvo D, et al. Overview—flavonoids: A new family of benzodiazepine receptor ligands. Neurochem Res. 1997;22:419–25. doi: 10.1023/a:1027303609517. [DOI] [PubMed] [Google Scholar]
- 35.Paladini AC, Marder M, Viola H, Wolfman C, Wasowski C, Medina JH. Flavonoids and the central nervous system: From forgotten factors to potent anxiolytic compounds. J Pharm Pharmacol. 1999;51:519–26. doi: 10.1211/0022357991772790. [DOI] [PubMed] [Google Scholar]
- 36.Wolfman C, Viola H, Marder M, Wasowski C, Ardenghi P, Izquierdo I, et al. Anxioselective properties of 6,3’-dinitroflavone, a high-affinity benzodiazepine receptor ligand. Eur J Pharmacol. 1996;318:23–30. doi: 10.1016/s0014-2999(96)00784-4. [DOI] [PubMed] [Google Scholar]
- 37.Liao JF, Hung WY, Chen CF. Anxiolytic-like effects of baicalein and baicalin in the Vogel conflict test in mice. Eur J Pharmacol. 2003;464:141–6. doi: 10.1016/s0014-2999(03)01422-5. [DOI] [PubMed] [Google Scholar]
- 38.López-Rubalcava C, Hen R, Cruz SL. Anxiolytic-like actions of toluene in the burying behavior and plus-maze tests: Differences in sensitivity between 5-HT(IB) knockout and wild-type mice. Behav Brain Res. 2000;115:85–94. doi: 10.1016/s0166-4328(00)00241-2. [DOI] [PubMed] [Google Scholar]
- 39.Farkas S, Berzsenyi P, Kárpáti E, Kocsis P, Tarnawa I. Simple pharmacological test battery to assess efficacy and side effect profile of centrally acting muscle relaxant drugs. J Pharmacol Toxicol Methods. 2005;52:264–73. doi: 10.1016/j.vascn.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Estrada-Reyes R, Martínez-Vázquez M, Gallegos-Solís A, Heinze G, Moreno J. Depressant effects of Clinopodium mexicanum Benth. Govaerts (Lamiaceae) on the central nervous system. J Ethnopharmacol. 2010;130:1–8. doi: 10.1016/j.jep.2010.03.012. [DOI] [PubMed] [Google Scholar]


