Abstract
Background:
Marine organisms produce a variety of compounds with pharmacological activities including anticancer effects. This study attempt to find cytotoxicity of hexane (HEX), dichloromethane (DCM), and butanol (BUTOH) partitions of Sargassum angustifolium.
Materials and Methods:
S. angustifolium was collected from Bushehr, a Southwest coastline of Persian Gulf. The plant was extracted by maceration with methanol-ethyl acetate. The extract was evaporated under vacuum and partitioned by Kupchan method to yield HEX, DCM, and BUTOH partitions. The cytotoxic activity of the extract (150, 450, and 900 μg/ml) was investigated against MCF-7 (breast cancer), HeLa (cervical cancer), and human umbilical vein endothelial cells cell lines by mitochondrial tetrazolium test assay after 72 h.
Results:
The cell survivals of HeLa and MCF-7 cell were decreased by increasing the concentration of extracts from 150 μg/ml to 900 μg/ml. The median growth inhibitory concentration value of HEX partition was 71 and 77 μg/ml against HeLa and MCF-7, dichloromethane partition was 36 and 88 μg/ml against HeLa and MCF-7, respectively. BUTOH partition was 25 μg/ml against MCF-7.
Conclusion:
This study reveals that different partitions of S. angustifolium have cytotoxic activity against cancer cell lines.
Keywords: Cancer, cytotoxic, Persian Gulf, Sargassum
Introduction
Seaweed is a marine macro alga comprised many genuses and species. These marine organisms have been traditionally used as food sources and therapeutic agents, especially in some parts of Asia such as Japan, Korea, and China.[1] Seaweed dietary values are due to the existence of many compounds, such as vitamins, fatty acids, minerals, trace elements, dietary fibers included soluble, insoluble and total dietary fibers, and amino acids.[1]
According to photosynthetic pigments, seaweed is classified into three major groups; green, brown, and red algae.[2] They produce several kinds of secondary metabolites such as phlorotannins, fucoidans, alginate, laminaran, and terpenoids that have shown a wide range of biological effects. Different species of Sargassum from sargassaceae family have shown antioxidant,[3] antibacterial,[4] antifungal,[5] anticancer,[6] antitumor,[7] anti-Herpes,[8] and antiallergic effects.[9] hepatoprotective,[10] antihistamine,[11,12] anti-inflammatory,[11] and anticholinergic.[13]
Cancer is one of the most important diseases in the world, and cancer chemotherapy is the gold standard of treatment. Most of the anticancer drugs currently used in chemotherapy are cytotoxic to normal cells too.[14]
Some metabolites isolated from the algae, influence proliferation, apoptosis, and cell cycle arrest with different mechanisms. Some mechanisms which are suggested included increasing natural killer cells,[15] activation of nonspecific immune system,[16] inhibition of cell growth,[17] angiogenesis,[18] and induction of apoptosis.[19]
Iran has coastal lines about 1260 km along the Persian Gulf and the Oman Sea. More than 250 species of different algae have been identified in this area. Despite the existence of a great extent of marine algae in this region, there are only a few studies on the phytochemical analysis and biological activities of these seaweeds.[20] This study investigates the cytotoxicity of the seaweed Sargassum angustifolium and its different partitions.
Materials and Methods
Authentication of plant material
S. angustifolium was collected in autumn 2015 from Bushehr, a Southwest coastline of Persian Gulf. Voucher specimens were made and deposited in the herbarium of the School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences (Code: 2662) and were identified by the Agricultural and Natural Resources Research Center of Bushehr.
Preparation of the extracts
The algae were air-dried in the shade at room temperature and ground to a powder with a Philip's mill and then extracted by maceration with methanol-ethyl acetate (1:1) at room temperature. The extract was evaporated under vacuum and partitioned by Kupchan method to yield hexane (HEX), dichloromethane (DCM), butanol (BUTOH), and water partitions.[21]
The partitions were subjected to cytotoxic test.
Cytotoxic test
After providing these partitions at the second step, for cytotoxic screening, we used three cell lines: MCF-7, HeLa, and human umbilical vein endothelial cell (HUVEC). At first, three cell lines were growing in the Dulbecco's modified Eagle's medium cell culture medium which supplemented with 10% fetal bovine serum. Penicillin and streptomycin were added to the media. All cell lines were cultured at 37°C in air/carbon dioxide (95:5) atmosphere.
At the second, the concentration of 150, 450, and 900 μg/ml[22] from all partitions were tested for each cell line. Samples were dissolved in dimethyl sulfoxide (DMSO) and further diluted with cell culture medium. The final DMSO concentration used was 1% of total volume of medium in all treatments including the control group.
For mitochondrial tetrazolium test (MTT) assay, 1 × 105 cells/well were plated into 96-well plates and incubated for 24 h before addition of extracts. The plates were allowed to proliferate and reach their exponential phase of growth. The incubation time for each cell line was assigned according to the normal growth curve of that cell line and was determined twice as long as the doubling time of each cell line. And then, 20 μl from all partitions were added to the media.
After 72 h of incubation for these type of cells, 30 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reagent (5 mg/ml) in phosphate buffered serum was added to each well. The plates were incubated at 37°C for 4 h. At the end of the incubation period, the medium was removed and 100 μl cell culture grade DMSO was added to each well. The formazan salts were quantified by reading the absorbance at 540 nm on a microplate reader.
Cell viability in MTT assays was calculated as a percentage of untreated cells (control value). The cytotoxicity value was presented as the median growth inhibitory concentration (IC50) of the reagents compared to control.
Statistical analysis
All experiments were done in triplicate. Results are expressed as the mean ± standard error of the mean. Statistical analysis was done using SIGMASTAT™ (Jandel Software, version 12, CA, USA), which was used to perform statistical tests. Analysis of variance followed by the Student–Newman–Keuls test was used to see the differences among the groups. Significance was assumed at 5% level.
Results and Discussion
The cytotoxicity results obtained from the extracts against MCF-7, HeLa, and HUVEC cells were shown in Table 1. Values were modified from those of NCI and Geran et al.[23] as follows: IC50 ≤20 μg/ml = highly active, IC50 21–200 μg/ml = moderately active, IC50 201–500 μg/ml = weakly active, and IC50 >501 μg/ml = inactive.
Table 1.
The median growth inhibitory concentration values (μg/ml) of dichloromethane, butanol, and hexane partition fraction of extract of Sargassum angustifolium
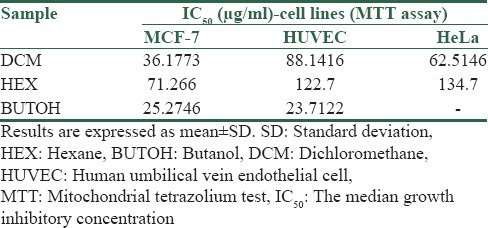
Figure 1 shows the MTT test results for different S. angustifolium extract partition with different concentrations on the HUVEC cell line after 72 h. We observed a significant reduction in cell viability at the doses of 150, 450, and 900 (μg/ml) of DCM partition, 450 and 900 (μg/ml) of HEX and BUTOH partitions.
Figure 1.
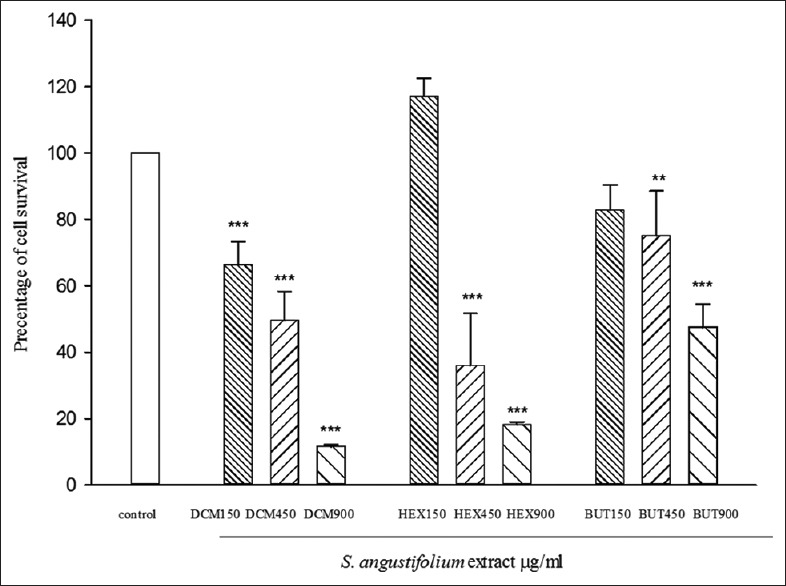
Cytotoxic activity of Sargassum angustifolium partitions on human umbilical vein endothelial cell line. Data represent the mean ± standard error of the mean separate experiments (significant as compared to control, **P < 0.01, ***P < 0.001). Cells viabilities were assessed by mitochondrial tetrazolium test assay. Cells were incubated for 72 h
The results for different concentrations from S. angustifolium partitions on HeLa cell line, after 72 h, are shown in Figure 2. At the base of these results, a significant reduction in cell viability the doses of 450 and 900 (μg/ml) of DCM partition, 150, 450, and 900 (μg/ml) of HEX and 150 and 450 (μg/ml) BUTOH partitions.
Figure 2.
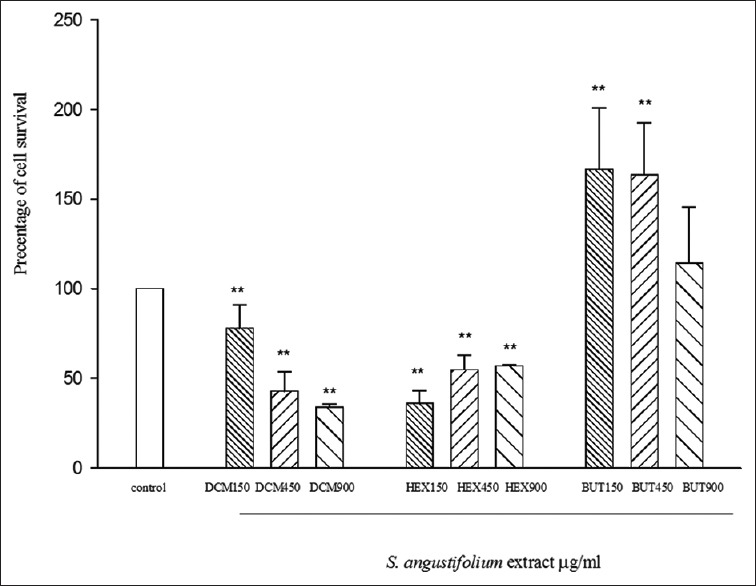
Cytotoxic activity of Sargassum angustifolium partitions on HeLa cell line. Data represent the mean ± standard error of the mean separate experiments (significant as compared to control, **P < 0.01). Cells viabilities were assessed by mitochondrial tetrazolium test assay. Cells were incubated for 72 h
For MCF-7 cell line, after 72 h, results exhibit a significant reduction in cell viability at the doses of 150, 450, and 900 (μg/ml) of DCM partition, 450 and 900 (μg/ml) of HEX, and 900 (μg/ml) BUTOH partitions. These results have been shown in Figure 3.
Figure 3.
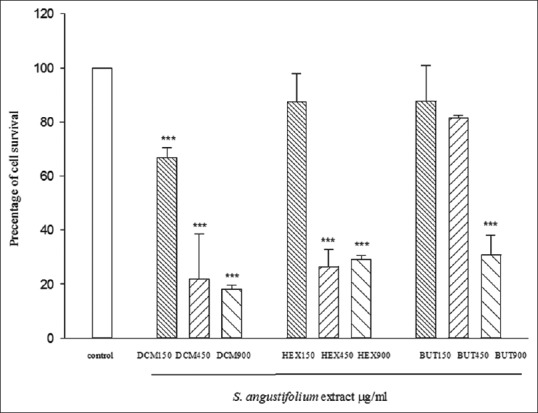
Cytotoxic activity of Sargassum angustifolium partitions on MCF-7 cell line. Data represent the mean ± standard error of the mean separate experiments (significant as compared to control, **P < 0.01, ***P < 0.001). Cells viabilities were assessed by mitochondrial tetrazolium test assay. Cells were incubated for 72 h
Nowadays, investigating biological activities and structure elucidation of compounds isolated from marine sources have become the focus of much attention.[20] Sea algae containing different genuses and species produce various secondary metabolites with attractive biological and physiological activities. Several literatures report the potential cytotoxic activity of marine seaweeds.[24] Our study documents the potential influence of the seaweed S. angustifolium extract against HeLa, MCF-7, and HUVEC cell lines using MTT assay. The intensity of HeLa and MCF-7 cell density were decreased by increasing the concentration of extracts from 150 μg/ml to 900 μg/ml. This infers the existence of dose-dependent properties of extracts against cancer cell lines which was found effective. The IC50 value of HEX, dichloromethane, and BUTOH fractions against HeLa and MCF-7 were 200 μg/ml and 250 μg/ml, respectively. The study reveals that dichloromethane showed the highest activity, especially against MCF-7 cell line. In addition, partitions were more effective against MCF-7 cell line than HeLa ones.
Marine organisms produce several types of metabolites. Metabolites such as terpenoids, bromophenols, carotene, phlorotannins, sulfated polysaccharides, steroids, and halogenated compounds were isolated and purified from some algae.[25] Fucoidans are antitumor polysaccharide isolated from Sargassum polycystum and some other algae. They seem to exhibit important role against some human cancer cell lines.[26] Steroids are the other compounds in seaweeds, especially in brown algae with antitumor activity. They have been reported in several Sargassum species.[27] In a study by Tannoury et al. both water: ethanol extract and chloroform: ethanol extract of Sargassum vulgare showed cytotoxic activity against Jurkat cancer cell line with IC50 values of 136.907 μg/ml and 49.056 μg/ml, respectively, after 72 h of treatment but another research shows that HEX fraction of Sargassum thunbergii was the most cytotoxic partition against HL-60 tumor cell lines. In addition, HEX partition of S. angustifolium showed cytotoxicity against T47D and HT-29 cell lines (IC50 166.42 ± 26.7 and 190.24 ± 52.8 μg/ml), respectively, in the other study. Fucosterol may be the active component in HEX partitions.[22] Yagdaneh et al. have analyzed S. angustifolium phytochemically and biologically in a study and reported that tannins, saponins, sterols, and triterpenes were the most abundant compounds in this seaweed. Partitioning pattern of compounds into different solvents depends mainly on their structure. Nonpolar compounds will tend to solvents such as HEX; polar compounds are found in butanolic partition while dichloromethane fraction contains semipolar compounds. We did not investigate fractions phytochemically, but it seems that semipolar terpenoids and steroids are the main components of dichloromethane fraction. Bioassay-guided isolation of secondary metabolites in dichloromethane fraction is needed to find the active metabolites. In summary, these results show that S. angustifolium decreased cell viability in carcinoma.
Conclusion
Different partitions of the seaweed S. angustifolium from Persian Gulf, Iran were screened for their cytotoxic analysis by MTT assay against HeLa and MCF-7 cell lines. The cell survivals of both cell lines were decreased by increasing the concentration of extracts. Hexane, dicholoromethane and butanol extracts of this seaweed and their active components could emerge as natural and alternative cytotoxic agents or serve as starting points for synthesizing more effective cytotoxic drugs.
Financial support and sponsorship
This study was financially supported by the Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This study was supported by the Isfahan University of Medical Sciences.
References
- 1.Senthil A, Mamatha BS, Vishwanath P, Bhat KK, Ravishankar GA. Studies on development and storage stability of instant spice adjunct mix from seaweed (Eucheuma) J Food Sci Technol. 2011;48:712–7. doi: 10.1007/s13197-010-0165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mine I. Biological interactions during the life history of seaweed – A microscopic review. Kuroshio Science. 2008;2:35–40. [Google Scholar]
- 3.Patra JK, Rath SK, Jena K, Rathod VK, Thatoi H. Evaluation of antioxidant and antimicrobial activity of seaweed (Sargassum sp.) extract: A study on inhibition of glutathione-S-transferase activity. Turk J Biol. 2008;32:119–25. [Google Scholar]
- 4.Kim YH, Kim EH, Lee C, Kim MH, Rho JR. Two new monogalactosyl diacylglycerols from brown alga Sargassum thunbergii. Lipids. 2007;42:395–9. doi: 10.1007/s11745-007-3035-7. [DOI] [PubMed] [Google Scholar]
- 5.Yegdaneh A, Ghannadi A, Dayani L. Chemical constituents and biological activities of two Iranian Cystoseira species. Res Pharm Sci. 2016;11:311. doi: 10.4103/1735-5362.189307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ermakova S, Sokolova R, Kim SM, Um BH, Isakov V, Zvyagintseva T. Fucoidans from brown seaweeds Sargassum hornery, Eclonia cava, Costaria costata: Structural characteristics and anticancer activity. Appl Biochem Biotechnol. 2011;164:841–50. doi: 10.1007/s12010-011-9178-2. [DOI] [PubMed] [Google Scholar]
- 7.Zhu W, Ooi VE, Chan PK, Ang PO., Jr Isolation and characterization of a sulfated polysaccharide from the brown alga Sargassum patens and determination of its anti-herpes activity. Biochem Cell Biol. 2003;81:25–33. doi: 10.1139/o02-169. [DOI] [PubMed] [Google Scholar]
- 8.Hoshino T, Hayashi T, Hayashi K, Hamada J, Lee JB, Sankawa U. An antivirally active sulfated polysaccharide from Sargassum horneri (TURNER) C. AGARDH. Biol Pharm Bull. 1998;21:730–4. doi: 10.1248/bpb.21.730. [DOI] [PubMed] [Google Scholar]
- 9.Na HJ, Moon PD, Ko SG, Lee HJ, Jung HA, Hong SH, et al. Sargassum hemiphyllum inhibits atopic allergic reaction via the regulation of inflammatory mediators. J Pharmacol Sci. 2005;97:219–26. doi: 10.1254/jphs.fp0040326. [DOI] [PubMed] [Google Scholar]
- 10.Kang JY, Khan MN, Park NH, Cho JY, Lee MC, Fujii H, et al. Antipyretic, analgesic, and anti-inflammatory activities of the seaweed Sargassum fulvellum and Sargassum thunbergii in mice. J Ethnopharmacol. 2008;116:187–90. doi: 10.1016/j.jep.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 11.Lee HJ, Kim YA, Ahn W.J, Na H.J, Kim H.M, Seo Y.J, Na HJ, Kim HM, Seo Y. Screening of Korean marine plants for their inhibitory effect on histamine release from RPMC in vitro. Biotechnol Bioprocess Eng. 2006;11:80–3. [Google Scholar]
- 12.Gany SA, Tan SC, Gan SY. Antioxidative, anticholinesterase and anti-neuroinflammatory properties of Malaysian brown and green seaweeds. World Acad Sci Eng Technol. 2015;8:1269–75. [Google Scholar]
- 13.Vinayak RC, Sabu AS, Chatterji A. Bio-prospecting of a few brown seaweeds for their cytotoxic and antioxidant activities. Evid Based Complement Alternat Med. 2011;2011:673083. doi: 10.1093/ecam/neq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zandi K. In vitro antitumor activity of Gracilaria corticata (a red alga) against Jurkat and molt-4 human cancer cell lines. Afr J Biotechnol. 2010;9:6787–90. [Google Scholar]
- 15.Mayer AM, Gustafson KR. Marine pharmacology in 2003-2004: Anti-tumour and cytotoxic compounds. Eur J Cancer. 2006;42:2241–70. doi: 10.1016/j.ejca.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Mabeau S, Fleurence J. Seaweed in food products: Biochemical and nutritional aspects. Trends Food Sci Technol. 1993;4:103–7. [Google Scholar]
- 17.Gupta S, Abu-Ghannam N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov Food Sci Emerg Technol. 2011;12:600–9. [Google Scholar]
- 18.Kwon HJ, Kwona HJ, Baea SY, Kima KJ, Hanb CH, Choc SH. Induction of apoptosis in HeLa cells by ethanolic extract of Corallina pilulifera. Food Chem. 2007;104:196–201. [Google Scholar]
- 19.Ahn GN, Kim KN, Cha SH, Song CB, Lee J, Heo MS. Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur Food Res Technol. 2007;226:71–9. [Google Scholar]
- 20.Mehdinezhad N, Ghannadi A, Yegdaneh A. Phytochemical and biological evaluation of some Sargassum species from Persian Gulf. Res Pharm Sci. 2016;11:243–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Kupchan SM, Tsou G, Sigel CW. Datiscacin, a novel cytotoxic cucurbitacin 20-acetate from Datisca glomerata. J Org Chem. 1973;38:1420–1. doi: 10.1021/jo00947a041. [DOI] [PubMed] [Google Scholar]
- 22.Khanavi M, Gheidarloo R, Sadati N, Ardekani MR, Nabavi SM, Tavajohi S, et al. Cytotoxicity of fucosterol containing fraction of marine algae against breast and colon carcinoma cell line. Pharmacogn Mag. 2012;8:60–4. doi: 10.4103/0973-1296.93327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geran RI, Greenberg NH, Macdonald MM, Shumacher AM, Abbott BJ. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chem Rep. 1972;3:1–103. [Google Scholar]
- 24.Rocha FD, Soares AR, Houghton PJ, Pereira RC, Kaplan MA, Teixeira VL. Potential cytotoxic activity of some Brazilian seaweeds on human melanoma cells. Phytother Res. 2007;21:170–5. doi: 10.1002/ptr.2038. [DOI] [PubMed] [Google Scholar]
- 25.Xu N, Fan X, Yan X, Tseng CK. Screening marine algae from China for their antitumor activities. J Appl Phycol. 2004;16:451–6. [Google Scholar]
- 26.Bui LM, Buu N.Q, Nhut ND, Thinh PD, Thanh Van TT. Studies on fucoidan and its production from Vietnamese brown seaweeds. ASEAN J Sci Technol Dev. 2007;22:371–80. [Google Scholar]
- 27.Tang HF, Yang-Hua Y, Yao XS, Xu QZ, Zhang SY, Lin HW. Bioactive steroids from the brown alga Sargassum carpophyllum. JAsian Nat Prod Res. 2002;4:95–101. doi: 10.1080/10286020290027362. [DOI] [PubMed] [Google Scholar]


