Abstract
The testa of higher plant seeds protects the embryo against adverse environmental conditions. Its role is assumed mainly by controlling germination through dormancy imposition and by limiting the detrimental activity of physical and biological agents during seed storage. To analyze the function of the testa in the model plant Arabidopsis, we compared mutants affected in testa pigmentation and/or structure for dormancy, germination, and storability. The seeds of most mutants exhibited reduced dormancy. Moreover, unlike wild-type testas, mutant testas were permeable to tetrazolium salts. These altered dormancy and tetrazolium uptake properties were related to defects in the pigmentation of the endothelium and its neighboring crushed parenchymatic layers, as determined by vanillin staining and microscopic observations. Structural aberrations such as missing layers or a modified epidermal layer in specific mutants also affected dormancy levels and permeability to tetrazolium. Both structural and pigmentation mutants deteriorated faster than the wild types during natural aging at room temperature, with structural mutants being the most strongly affected.
Arabidopsis seeds develop after fertilization within anatropous bitegmic ovules. At maturity, seeds consist of a whitish embryo surrounded by a hyaline layer of remaining central endosperm and a single layer of peripheral endosperm cells (aleurone layer) containing storage reserves and associated with the brown seed coat or testa (Müller, 1963; Vaughan and Whitehouse, 1971; Mansfield and Briarty, 1994; Schneitz et al., 1995). The seed coat derives from ovular tissue and is therefore of maternal origin. The aleurone layer of mature seeds is physiologically active, in contrast to the testa layers, whose cells died during late seed maturation after having encountered considerable developmental changes.
Mature Arabidopsis seeds exhibit primary dormancy when freshly released from the mother plant, which means that seeds are unable to germinate under the appropriate environmental conditions without the help of dormancy-breaking agents such as stratification, after-ripening, or gibberellins (Koornneef and Karssen, 1994). Germination begins with the uptake of water by the dry seed and ends with the elongation of the embryonic axis. The visible consequence of germination is the protrusion of the radicle tip through the seed envelopes. Seed dormancy can be imposed by the embryo, the envelopes (seed coat, endosperm, etc.), or a combination of both factors to an extent that depends on the plant species (Bewley, 1997).
In Arabidopsis, the mutant approach has been very successful in unraveling the role of embryonic abscisic acid and gibberellins in the induction and breaking of dormancy, respectively (Koornneef and Karssen, 1994). Moreover, this approach has indicated that the testa may also interfere with dormancy (Léon-Kloosterziel et al., 1994). However, the nature of the embryo/testa interactions that determine dormancy is still unclear. Moreover, due to the absence of mutants affecting the aleurone layer, the participation of the latter tissue in the control of germination remains to be proven in Arabidopsis.
The seed coat is a multifunctional organ that plays an important role in embryo nutrition during seed development and in protection against detrimental agents from the environment afterward (Mohamed-Yasseen et al., 1994; Weber et al., 1996). Seed coat-imposed dormancy in particular is part of the seed survival strategy of many species (Werker, 1981; Kelly et al., 1992). The seed coat exerts its germination-restrictive action most of the time by being impermeable to water and/or oxygen or by its mechanical resistance to radicle protrusion. These properties have been positively correlated with seed coat color due to phenolic compounds in diverse species.
Red seeds of charlock (Sinapis arvensis L.) exhibit a reduced dormancy compared with black seeds (Duran and Retamal, 1989). In legumes, white seeds imbibe more rapidly than colored seeds and then germinate earlier. White seeds also suffer greater imbibition damage, as measured by higher solute leakage, which affects their vigor and viability (Wyatt, 1977; Werker et al., 1979; Powell, 1989; Kantar et al., 1996). In wheat, the strongest dormancy is associated with a red seed coat color, whereas the lines with white seed coats are non-dormant or weakly dormant and therefore are susceptible to pre-harvest sprouting damage (Gfeller and Svejda, 1960; Mares, 1994). Dark seeds of proso millet (Panicum miliaceum L.) have heavier seed coats, imbibe and germinate more slowly, suffer less imbibition damage, and therefore persist longer in soil than light-colored seeds (Khan et al., 1996a). Weidner and Paprocka (1997) proposed that dormancy of cereal caryopses might be at least partially controlled by the high level of free phenolic acids, through their inhibitory effect on germination and cell division.
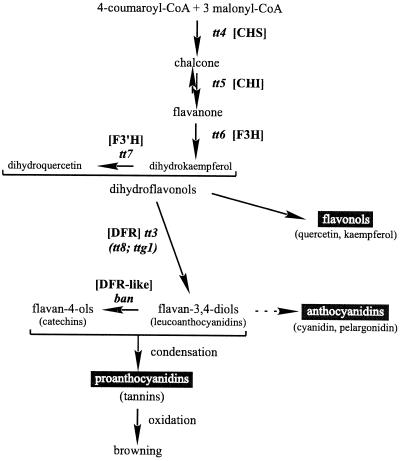
The inner and outer integuments of the Arabidopsis mature ovule are two-layered throughout the micropyle-chalazal extension. The two layers of the inner integument are separated by a third layer that reaches about two-thirds the distance from the chalaza toward the micropyle (Schneitz et al., 1995). After fertilization, these five layers compose the seed integuments. They are still clearly visible in immature seeds at the globular stage of embryo development (Léon-Kloosterziel et al., 1994), but afterward differentiate in such a way that their identification becomes problematic, which has led to some discrepancies in the literature. Vaughan and Whitehouse (1971) described the mature Arabidopsis seed coat as being composed of a mucilaginous epidermal layer, a palisade layer with thickened tangential walls, and a pigmented inner layer. However, Kuang et al. (1996) identified the apparent thickening of the palisade layer as a compressed inner integument layer. The brown pigments of wild-type (WT) Arabidopsis seeds are mainly condensed tannins of the procyanidin type and derivatives of the flavonol quercetin (Chapple et al., 1994), which are end-products of the flavonoid biosynthetic pathway (Fig. 1).
Figure 1.
Flavonoid biosynthetic pathway in Arabidopsis (adapted from Shirley, 1998). The scheme is simplified to show essentially the steps leading to proanthocyanidins, anthocyanins, and flavonols. Only the mutants corresponding to genes of known function are presented. The mutants in parentheses correspond to regulatory genes, the others to structural genes encoding the enzymes chalcone synthase (CHS), chalcone isomerase (CHI), flavonoid 3-hydroxylase (F3H), flavonoid 3′-hydroxylase (F3′H), dihydroflavonol reductase (DFR), and a dihydroflavonol reductase-like (DFR-like), as indicated in square brackets. The dashed arrow represents several steps.
Arabidopsis mutants that affect the development of the seed coat without impairing the viability of the seeds are valuable in the investigation of the role of this organ in seed physiology. Seed coat mutants consist of two major groups. One group, affected in flavonoid pigmentation, is represented by the transparent testa (tt) and transparent testa glabra (ttg) mutants (Bürger, 1971; Koornneef, 1981, 1990; Koornneef et al., 1982; Shirley et al., 1995; Shirley, 1998) (Fig. 1). The seed color of tt and ttg mutants ranges from yellow (tt1– tt5; tt8; and ttg1) to pale brown (tt6, tt7, and tt10) or grayish brown (tt9). The ttg1 mutant also lacks testa mucilage and trichomes and is characterized by an aberrant root hair outgrowth due to disturbed epidermal layer structures (Koornneef, 1981; Masucci and Schiefelbein, 1996). A twelfth pigment locus is banyuls (ban). The corresponding mutant accumulates pink flavonoid pigments in the endothelium of immature seeds, and the resulting mature seeds are grayish-green and spotted (Albert et al., 1997; Devic et al., 1999). Additional tt loci have been identified (Focks et al., 1999; I. Debeaujon and M. Koornneef, unpublished data).
The second group is represented by mutants affected in testa structure. The aberrant testa shape (ats) mutant ovules lack two cell layers of the integuments and as a result produce heart-shaped mature seeds (Léon-Kloosterziel et al., 1994). The glabra2 (gl2) mutant has brown seeds but similar defects in mucilage production, testa surface structure, and root hair formation as the ttg1 mutant (Rerie et al., 1994; Masucci and Schiefelbein, 1996). Therefore, ttg1 combines defects of both testa mutant groups. The floral development apetala2 (ap2) mutant has heart-shaped seeds that lack mucilage (Jofuku et al., 1994).
The aim of the present study was to analyze the consequence of mutations affecting the testa on the dormancy, germination, and longevity of Arabidopsis seeds. The availability of a large collection of mutants with related testa defects can be expected to give an indication of how these pigments and structural components of the testa affect germination behavior of seeds. In the present report we relate reduced dormancy to the increased uptake of tetrazolium salts and to a reduced thickness of the testa. These results suggest that the permeability and thickness of the testa are affected by the chemical compounds and structural elements altered in the mutants, which may lead to effects on germination.
MATERIALS AND METHODS
Plant Material
The transparent testa Arabidopsis mutants tt1-1, tt2-1, tt3-1, tt4-1, tt5-1, tt6-1, tt7-1, tt9-1, tt10-1, and transparent testa glabra 1, ttg1-1 were isolated by Koornneef (1981, 1990) and are in the Landsberg erecta (Ler) ecotype background. The tt8-1 mutant is in the Enkheim (En) background. It was described by Bürger (1971) and obtained from Prof. A.R. Kranz (University of Frankfurt). The tt12-1 mutant was isolated from the Feldmann's T-DNA transformant collection during a screening for reduced dormancy and is in the Wassilevskija (Ws) background (Debeaujon et al., 1995). The tt11-2 mutant was generated by ethyl methanesulfonate mutagenesis of Ler ecotype and isolated on the basis of its pale seed color (I. Debeaujon and M. Koornneef, unpublished data). The tt13-1 mutant was obtained by gamma irradiation of Ler and came out of a reduced dormancy screen (Léon-Kloosterziel et al., 1996). The tt14-1 (RR40) mutant, kindly provided by Rebecca Rasoli and Shauna Somerville, was obtained by ethyl methanesulfonate mutagenesis of the Columbia (Col) ecotype. The ban-2 mutant, also obtained from Prof. A.R. Kranz, is in the En background (stock center reference N334). The ats, gl2-1, and ap2-1 mutants have been described by Léon-Kloosterziel et al. (1994), Rerie et al. (1994), and Jofuku et al. (1994), respectively. All mutations are recessive.
For plant culture, seeds were sown on filter paper soaked with demineralized water in 6-cm plastic Petri dishes and incubated for 5 d in a cold room (6°C) to release remaining dormancy. After an incubation in a climate-controlled room at 25°C with a 16-h photoperiod (TL57 bulbs, Philips, Eindhoven, The Netherlands) for 36 h, seeds were planted on a sandy soil in an air-conditioned greenhouse (18°C–23°C) with additional light from October to April (16-h photoperiod with 400 W bulbs, HPI-T, Philips). Seeds were harvested from dry siliques approximately 2 months after planting and stored at room temperature in cellophane bags.
Germination Assays
Seed lots to be compared were harvested on the same day from individual plants grown in identical environmental conditions. Each genotype was sown in triplicate (80–100 seeds from one individual plant per 6-cm Petri dish) on water-soaked filter paper (no. 595, Schleicher & Schuell, Dassel, Germany) and incubated in a climate-controlled room (25°C, 16-h light/day Philips TL57). Germination was scored after a 7-d imbibition, when the radicle had emerged from the testa. The average germination percentages ± se of triplicates were calculated. WT and mutant seeds stored for 4 years at room temperature were used for seed longevity determination on the basis of germination and seedling abnormalities. Seedlings were judged as abnormal when presenting any malformation not present in the 1-year-old seed lots used as a control. Generally, the recorded malformation fell into one of the following categories: no cotyledons, asymmetrical cotyledons, narrow vitrified cotyledons, chlorotic or albino cotyledons, no cauline apex, no root, and short or elongated hypocotyl.
Mucilage Detection
The seeds were incubated for 15 min in an aqueous solution of 0.03% (w/v) ruthenium red at room temperature and rinsed with water before observation under a stereomicroscope (Zeiss, Jena, Germany).
Seed Measurements
The weight of 1-year-old seeds stored at room temperature and coming from a bulk harvest of six individual plants per genotype was determined on a precision balance (UM3, Mettler, Greifensee, Switzerland). The average ± sd of three independent samplings of 100 seeds each are presented. Seed length and width were determined for 100 seeds from the same seed lots after scanning and processing by the ImageTool computer program (Health Science Center at San Antonio, University of Texas; ftp://maxrad6.uthscsa.edu).
Tetrazolium Assay
Intact seeds were incubated in a 1% (w/v) aqueous solution of 2,3,5-triphenyltetrazolium chloride (Merck, Darmstadt, Germany) at 30°C in darkness for 2 d according to the procedure described by Wharton (1955). Tetrazolium salts are metabolically reduced to highly colored end-products called formazans by NADH-dependent reductases of the endoplasmic reticulum (Berridge et al., 1996).
Vanillin Assay
Intact seeds were incubated as described by Aastrup et al. (1984) in a solution of 1% (w/v) vanillin and 6 n HCl at room temperature for 10 min for immature seeds and 1 h for mature seeds. Vanillin turns red upon binding to flavan-3,4-diols (leucoanthocyanidins) and flavan-4-ols (catechins), which are present either as monomers or as terminal subunits of proanthocyanidins (Deshpande et al., 1986).
Microscopy
Mature seeds imbibed for 30 min in water were fixed during 24 h at 4°C in 5% (v/v) glutaraldehyde before embedding in Technovit 7100 historesin (Heraeus-Kulzer, Wehrheim, Germany). Sections (2 μm thick) obtained on a microtome (Leica Microsystems, Wetzlar, Germany) were stained for 1 min with 1% (w/v) toluidine blue O in 0.1 m phosphate buffer at pH 7.2. Toluidine blue stains pecto-cellulose pink and phenolic compounds blue-green (O'Brien et al., 1964). Observations and photographs were done on a light microscope (Optiphot, Nikon, Tokyo).
RESULTS
Description of the Seed Phenotypes
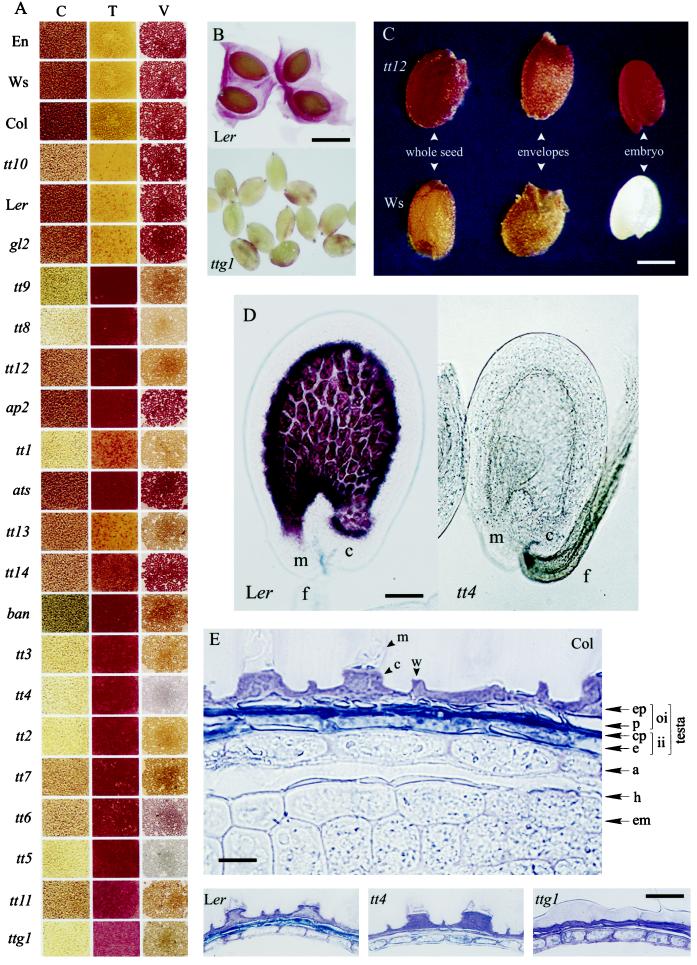
Seed colors (Fig. 2A, column C) varied from brown in the WTs to pale yellow for tt4, with intermediate pigmentation such as pale brown, grayish-brown, or grayish-green. The pale yellow color of tt4 seeds, which are completely devoid of any flavonoids, is conferred by carotenoids localized in the aleurone layer and the embryo (data not shown). In tt10 and tt14 mutants, the seeds were pale brown at harvest but exhibited progressive browning during storage until becoming nearly identical to the WT after a few months. Several characteristics of WT and mutant seeds are presented in Table I. Most mutants exhibited a reduced seed size and weight. This was particularly evident for tt9. An exception to this observation was tt14, in which the seeds were much heavier than the corresponding Col WT, and gl2, which was similar to the Ler WT. ats and ap2 were both heart-shaped but ats had lighter seeds and ap2 heavier seeds than Ler. Mucilage release upon imbibition (Fig. 2B) could be observed in all genotypes except in ttg1, ap2, and gl2.
Figure 2.
Characterization of the Arabidopsis seed coat. A, The permeability of the testa to tetrazolium salts (T) and the presence of catechins an proanthocyanidins in mature seeds determined by the vanillin assay (V) are compaired with the original color of untreated seeds (C); the genotypes are arranged from the top to the bottom in the direction of the more to the less dormant, as assessed in Figure 3B; the rectangles represent portions of multiarray wells filled with seeds. B, Visualization of the mucilage in Ler and its absence in ttg1 as shown by ruthenium red staining. Bar = 450 μm. C, Color (Legend continues on facing page.)of whole seed, envelopes, and embryo after tetrazolium treatment of tt12 and Ws. Bar = 225 μm. D, Immature seeds treated with vanillin showing the presence of catechins and proanthocyanidins in the endothelium of the WT Ler and their absence in the mutant tt4. m, Micropyle; c, chalazal area; f, funiculus. Bar = 50 μm. E, Cytochemical localization of phenolic compounds in the testa of Col mature seeds after staining with toluidine blue O. Bar = 15 μm. The flavonoid pigments, stained in blue-green, are localized in the endothelium layer (e) and the crushed parenchymatic layers (cp); these pigments are totally absent in tt4 and ttg1, compared with their WT Ler. Bar = 30 μm. a, Aleurone layer; c, columella; em, embryo; ep, epidermis; h, hyaline layer; ii, inner integument; m, mucilage; oi, outer integument; p, palisade layer; w, cell wall remnants.
Table I.
Comparison of Arabidopsis wild-type and testa mutant seeds
| Genotype | Color | Weight | Length | Width | |||
|---|---|---|---|---|---|---|---|
| mg/100 seeds | μm | ||||||
| Col | Brown | 1.91 ± 0.02 | 446.1 ± 35.2 | 258.3 ± 24.7 | |||
| En | Brown | 2.05 ± 0.01 | 465.6 ± 32.2 | 271.4 ± 20.2 | |||
| Ler | Brown | 1.78 ± 0.04 | 433.6 ± 36.0 | 272.0 ± 22.4 | |||
| Ws | Brown | 1.75 ± 0.02 | 444.3 ± 34.9 | 254.8 ± 22.1 | |||
| tt1-1 | Yellow | 1.47 ± 0.01 | *a | 393.4 ± 40.6 | * | 253.2 ± 27.2 | * |
| tt2-1 | Yellow | 1.52 ± 0.01 | * | 390.3 ± 28.8 | * | 271.0 ± 25.2 | n.s. |
| tt3-1 | Yellow | 1.53 ± 0.04 | * | 414.4 ± 33.7 | * | 257.8 ± 21.5 | * |
| tt4-1 | Yellow | 1.66 ± 0.01 | * | 389.8 ± 33.1 | * | 269.8 ± 28.8 | n.s. |
| tt5-1 | Yellow | 1.43 ± 0.01 | * | 376.6 ± 28.9 | * | 257.9 ± 30.9 | * |
| tt6-1 | Pale brown | 1.74 ± 0.01 | n.s.b | 415.4 ± 32.4 | * | 247.6 ± 20.2 | * |
| tt7-1 | Pale brown | 1.54 ± 0.01 | * | 388.3 ± 31.4 | * | 237.2 ± 24.0 | * |
| tt8-1 | Yellow | 2.08 ± 0.03 | n.s. | 460.1 ± 39.6 | n.s. | 288.2 ± 26.0 | * |
| tt9-1 | Grayish-brown | 1.25 ± 0.04 | * | 367.8 ± 32.0 | * | 227.1 ± 21.0 | * |
| tt10-1 | Pale brown | 1.64 ± 0.02 | * | 428.8 ± 32.1 | n.s. | 260.2 ± 19.9 | * |
| ttg1-1 | Yellow | 1.74 ± 0.01 | n.s. | 434.9 ± 34.3 | n.s. | 263.8 ± 25.5 | * |
| tt11-2 | Pale brown | 2.00 ± 0.01 | * | 447.8 ± 28.9 | * | 268.0 ± 22.6 | n.s. |
| tt12-1 | Pale brown | 1.77 ± 0.02 | n.s. | 427.2 ± 30.7 | * | 245.3 ± 21.5 | * |
| tt13-1 | Pale brown | 1.49 ± 0.01 | * | 393.5 ± 29.3 | * | 244.2 ± 23.7 | * |
| tt14-1 | Pale brown | 2.60 ± 0.01 | * | 512.6 ± 43.9 | * | 283.9 ± 26.5 | * |
| ban-2 | Grayish-green | 1.69 ± 0.01 | * | 410.5 ± 35.4 | * | 262.5 ± 22.1 | * |
| ats-1 | Brown | 1.51 ± 0.02 | * | 328.8 ± 42.2 | * | 296.2 ± 33.7 | * |
| ap2-1 | Brown | 2.16 ± 0.02 | * | 402.6 ± 43.9 | * | 309.3 ± 36.3 | * |
| gl2-1 | Brown | 1.81 ± 0.02 | n.s. | 424.5 ± 33.9 | n.s. | 269.6 ± 2.0 | n.s. |
Values are means ± sd (n = 3 for seed weight and 100 for seed length and width).
*, Significant difference between the mutant and the corresponding WT at P = 0.05, according to Student's t-test.
n.s., No significant difference between the mutant and the corresponding WT at P = 0.05, according to Student's t-test.
Dormancy and Germination Characteristics
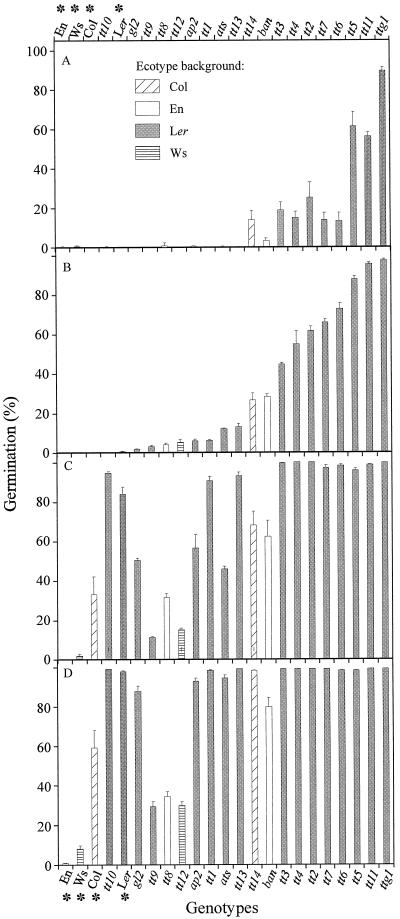
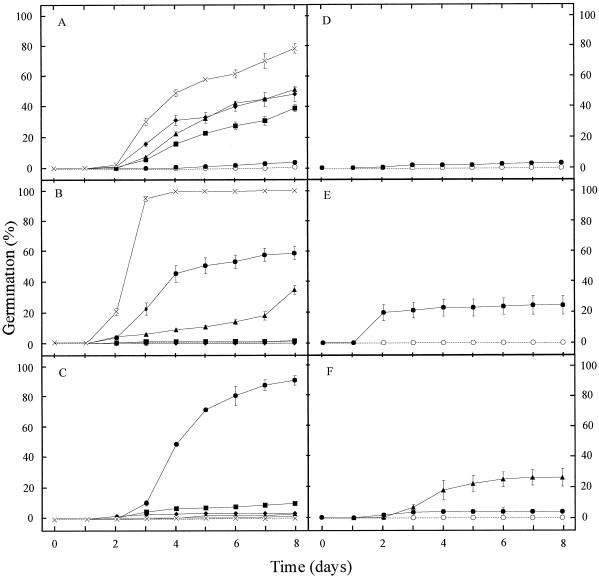
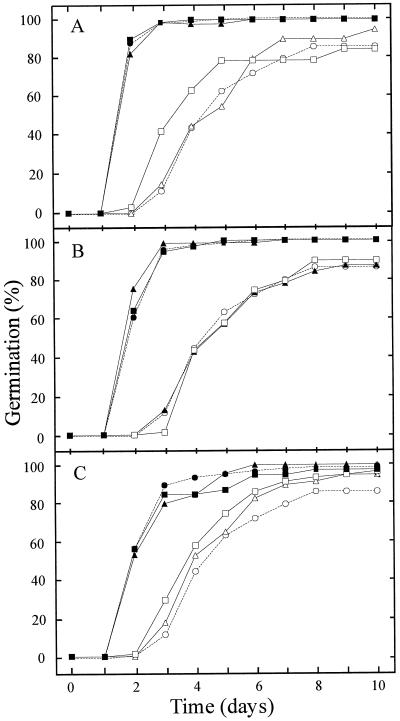
The degree of dormancy was assessed by determining the germination percentage of the seed lots at different times after seed harvest, which indicates the after-ripening requirement to reach 100% germination (Fig. 3). Most of the pigmentation mutants exhibited a reduced dormancy compared with the corresponding WT. After a cold treatment, all the genotypes reached 100% germination, which means that the lack of germination observed for some of the genotypes after 27 d of storage (Fig. 3D) was due to remaining dormancy. The present data confirm earlier observations (van der Schaar et al., 1997) that the freshly harvested seeds of commonly used WT strains Ler and Col are dormant when germination is tested in light, but that this dormancy has disappeared after approximately 1 month of after-ripening. However, compared with the Ler and Col ecotypes, the Ws and En ecotypes are substantially more dormant. The ttg1 mutant is particularly non-dormant because it germinates at nearly 100% at 2 d after harvesting of the seeds. The structural mutants ats and ap2 show a slightly reduced dormancy, and gl2 is slightly more dormant than Ler. Dormancy reduction is also expressed by higher germination rates, which are particularly visible at 9 d after harvest (Fig. 4). Therefore, pigmentation mutants appear to be more drastically affected in their germination pattern than structural mutants, in the sense of reduced seed dormancy and a higher germination rate. They also exhibit a higher capacity to germinate in darkness (data not shown). The reduced dormancy of the testa pigmentation mutants can be related to the degree of paleness, as shown in Figure 2A (column C). However, there was considerable variation in germination behavior among seeds belonging to a similar color group.
Figure 3.
Effect of dry storage on dormancy release. Germination was scored 2 d (A), 9 d (B), 18 d (C), and 27 d (D) after seed harvest. The WT ecotypes are indicated with an asterisk. The genotypes are ranked from the left to the right in the direction of the more to the less dormant at d 9 of storage (B) and are grouped under the motif corresponding to their ecotype background, as presented in Figure 3A.
Figure 4.
Time course of germination for freshly harvested seed lots (d 9 after harvest) (compare with Fig. 3B). In A, B, and C, ○, Ler; ●, tt1; ▴, tt2; ▪, tt3; ♦, tt4; ×, tt5. In D, ○, Ws and ●, tt12. In E, ○, Col and ●, tt14. In F, ○, En, ●, tt8, and ▴, ban.
The genetic determinism, either by maternal or zygotic tissue, of altered germination behavior can be analyzed with reciprocal crosses. As an example, the germination curves for three tt mutants are presented in Figure 5. All members of the F1 generation behaved as their maternal parent, which indicates that the testa defect is responsible for the higher germination rate. Cytoplasmic inheritance can be discarded, because a segregation for seed dormancy is observed among F3 lines, which correlates strictly with the monogenic seed color or testa structural defect (data not shown).
Figure 5.
Genetic determinism of the high germination rate encountered in the mutants tt2, tt4, and tt7. The time course of germination after 16 d of dry storage is presented. The parent mentioned first was used as female parent and the second as pollen parent. Seeds from a bulk of nine siliques derived from crosses were used. In A, ○, Ler; ▵, Ler × Ler; □, Ler × tt2; ●, tt2; ▴, tt2 × tt2; ▪, tt2 × Ler. In C, ○, Ler; ▵, Ler × Ler; □, Ler × tt4; ●, tt4; ▴, tt4 × tt4; ▪, tt4 × Ler. In D, ○, Ler; ▵, Ler × Ler; □, Ler × tt7; ●, tt7; ▴, tt7 × tt7; ▪, tt7 × Ler.
Seed Longevity
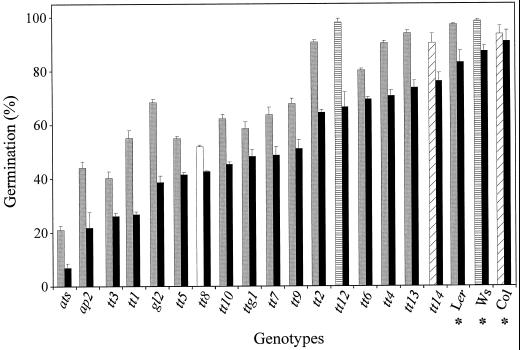
Seed lots stored for 4 years at room temperature were compared for their ability to germinate and produce normal seedlings. Testa mutants generally showed a reduced germination capacity and a higher rate of seedling abnormalities than their WTs (Fig. 6). The structural mutants ats and ap2 were particularly affected. The degree of seed deterioration was not strictly correlated with dormancy characteristics or with seed size and weight.
Figure 6.
Effect of dry storage on seed longevity. The germination of seed lots after 45 months of dry storage at room temperature was assessed. The WTs are indicated by an asterisk. The genotypes are ranked from left to right as the smallest to the highest percentage of normal seedlings (black bars). For total germination percentage, they are grouped under the motif corresponding to their ecotype background, as presented in Figure 3A.
Permeability of the Seed Coat
In Arabidopsis, it is difficult to monitor water uptake because of the water-holding capacity of the mucilage layer. Therefore, we used the uptake of tetrazolium salts by the embryo to assess the permeability of the testa. The embryo and the aleurone layer stain red upon entry of the tetrazolium solution into the viable seed, but stay whitish when the dye does not penetrate. This pattern of staining is shown in Figure 2C, with tt12 as an example of a genotype taking up the dye and and Ws as one that does not. None of the genotypes with a brown seed coat (such as the WTs, tt10, and gl2) were stained with tetrazolium, leading to dark yellow seeds at the end of the assay (Fig. 2A, column T). In contrast, the pigmentation mutants and those exhibiting a structural testa abnormality (such as ats and ap2) reacted positively; their seeds developed a light red to dark red color depending on the genotype. The onset of impermeability of the WT seeds to tetrazolium during the later phases of seed development was concomitant with the appearance of the brown pigments during seed desiccation (data not shown). This impermeability decreased very slowly during after-ripening at a rate much slower than overall dormancy release by after-ripening. Moreover, it was not affected by cold treatments. The occurrence of germination indicates that the permeability to tetrazolium salts cannot be used to monitor the permeability of Arabidopsis seeds to water. From this assay, we cannot conclude that the testa imposes dormancy in wild-type seeds by limiting water entry.
Detection of Proanthocyanidins in the Seed Coat
Uncolored proanthocyanidins were detected in WT immature seeds by their dark red staining with vanillin (Fig. 2D) as early as at the 2-cell embryo stage. These polymers progressively become colored through oxidative browning (Fig. 1), which starts around 10 d after pollination (cotyledonary stage) concomitantly with the onset of seed desiccation. The browning could be observed earlier in the case of aborted seeds upon precocious desiccation. WT seeds have a tendency to darken with time of storage. The proanthocyanidins fill completely the large vacuole of the endothelium cells. Vanillin staining did not take place in the tt4 mutant (Fig. 2D), which confirms the specificity of the vanillin assay for flavonoids with our material. Mature seeds with tannins also stained dark red (Fig. 2A, column V), and the different colors probably reveal various differences in flavonoid composition.
Structure of the Mature Seed Envelopes
Cross-sections of mature seeds were studied for all genotypes. This microscopic analysis is illustrated here by the WT Col and the tt4 and ttg1 mutants (Fig. 2E). The WT mature seed coat appears to be composed of four cell layers (from outside to inside the seed): an epidermis and a palisade layer composing the outer integument followed by a crushed layer and an endothelium layer forming the inner integument. Considering that the immature testa is composed of five layers (Schneitz et al., 1995), a layer must have been crushed in late seed development. The two cell layers between the endothelium and the outer integument are parenchymatic and thin-walled (Léon-Kloosterziel et al., 1994). These layers are therefore more likely to be crushed during maturation and probably form the third “layer” of the mature testa. The phenolic impregnation of the endothelium layer and of the two neighboring parenchymatic layers was not observed in the tt4 and ttg1 mutants, which therefore had a testa thinner than the WT Ler testa. The thick aleurone layer appeared to be closely associated with the testa in the intact seed, but both tissues were nevertheless easily separable upon microscopic dissection. A thin hyaline layer that stayed unstained with toluidine blue was found to follow intimately the contours of the embryo.
The central parts of epidermal cells were shown to contain a columnar structure, or columella, in which mucilage is synthesized and stored. Upon imbibition, the top part of the columella is removed and the mucilage is excreted. In the ttg1 mutant, which is affected in the columnar structure of the epidermis, very little mucilage was present (Fig. 2B) and was distributed as a thin layer over the surface of the seed coat (Fig. 2E).
DISCUSSION
The data presented here confirm that structural and/or pigmentation defects of the Arabidopsis seed integuments can affect dormancy, germination, and longevity of seeds, together with seed morphology (size and weight).
The morphological defects that we observed were a slight reduction in size and weight encountered both with most tt mutants and ats, with the exception of the tt14 seeds, which were much larger and heavier than Col seeds. The developmental basis of this size difference is unknown. The hypothesis of tetraploidy can be ruled out, because the progeny from crosses of tt14 with diploid genotypes were totally fertile (data not shown). All mutant seeds had a normal shape, except ats and ap2 seeds, which were reported previously to be heart-shaped (Jofuku et al., 1994; Léon-Kloosterziel et al., 1994).
Most testa mutants showed reduced seed dormancy, as ascertained by a lower requirement for after-ripening and a higher germination rate. In our conditions, the ban seeds exhibited reduced dormancy together with an increased permeability to tetrazolium, which differs from the results of Albert et al. (1997), who showed that ban is more dormant than the WT. These authors postulated that the overaccumulation of pigments in the seed coat is an obstacle to germination. Our vanillin and tetrazolium assays showed that there may not be an overaccumulation of pigments, but rather a replacement of the proanthocyanidin polymers by anthocyanins, which may lead to an increased permeability to tetrazolium. This hypothesis is supported by further analysis of the mutant (Devic et al., 1999).
As an attempt to correlate the germination behavior of Arabidopsis WT and mutants with precise testa characteristics, we performed a histological analysis of mature testas. For WT testas four distinct layers could be distinguished after toluidine blue staining, the last two being impregnated with phenolic compounds. Our observations confirm the mature seed coat model proposed by Kuang et al. (1996), particularly on the interpretation of the crushed parenchymatic layers. Vaughan and Whitehouse (1971) interpreted these layers as a phenolic thickening of the tangential wall of the palisade layer. However, this explanation denies the fact that the immature testa is composed of five layers. This does not rule out the possibility that the tangential walls may be impregnated, but we may not distinguish this detail due to the fact that the underlying layers are also stained. In crucifers, the presence or absence of phenolic impregnation and the thickness of the palisade layer vary according to the species and have therefore been used as a taxonomic criterion, together with the pigmentation of the endothelium (Vaughan and Whitehouse, 1971). In this respect, Arabidopsis differs from Brassica, Raphanus, Sinapis, and Capsella species, which have a thick palisade layer generally heavily impregnated in such a way that the cells have a typical U shape.
The main defect we could detect in the testa of pigmentation mutants was a reduction or absence of phenolics in the endothelium and the crushed parenchymatic layers. The tt4 and ttg1 mutant testas appeared thinner as a consequence of a complete lack of pigments. Similarly, yellow Brassica campestris seeds were also reported to have thinner testas than their brown counterparts, but this characteristic was associated with a heavier embryo and a lower fiber content, without any increase in seed size (Stringam et al., 1974). The reduced thickness of the testa may enable the embryo to occupy more space in the seed. The situation may be different in Arabidopsis, in which most pigmentation mutants show reduced seed weight and size. Colored seeds of snap bean were also reported to have greater seed coat dry weight and thickness than white-seeded lines (Wyatt, 1977).
Precocious germination of a Chinese cabbage mutant (Brassica rapa subsp. pekinensis) was related to a reduction or absence of secondary cell wall deposition on the radial and basal walls of the palisade (Ren and Bewley, 1998).
The structural ttg1, ap2, and gl2 mutants have a very reduced or absent mucilage due to a malformation of the epidermal cell structures (columella) producing it. The mucilage was proposed to influence moisture relations and seed dispersal during seed germination (Young and Evans, 1973). Witztum et al. (1969) proposed that mucilage acts as an oxygen barrier during germination of Blepharis persica. If it was the case in Arabidopsis, we would expect the gl2 mutant to germinate earlier than Ler. However, this was not observed here, showing that mucilage does not have a significant restrictive effect on Arabidopsis seed germination.
The germination behavior of testa mutants is related to a better penetration of tetrazolium salts in the seed. A better permeability to either an endogenous inhibitor (e.g. abscisic acid) or to an exogenous stimulant of germination (e.g. water or oxygen) may explain the reduced dormancy exhibited by testa mutants. In legumes, water-impermeable seed dormancy was extensively studied and has been attributed to the presence of flavonoid compounds in the seed coat (Wyatt, 1977; Werker et al., 1979; Kantar et al., 1996). It was hypothesized that during dehydration of seeds, an enzymatic oxidation of phenolic compounds in the presence of oxygen might render the seed coat impermeable to water (Marbach and Mayer, 1974, 1975). In our study, because the non-dormant germinable seed lots of Arabidopsis did not stain red, it can be concluded that the tetrazolium assay is not appropriate to assess water entry.
The observation that, unlike WT testas, mutant testas were permeable to tetrazolium salts in a manner related to a reduction or absence of proanthocyanidins (condensed tannins) demonstrates that testa pigmentation and structural integrity play a major role on the imposition of impermeability to tetrazolium solution. Genetic variation for staining by tetrazolium has been described before in Brassica, where B. napus and B. napobrassica seeds appeared to be impermeable, as deduced from the pink color of the embryo. However, non-aged cabbage (Brassica oleracea L. var capitata) seeds were permeable to tetrazolium chloride. This difference was explained by the absence of a semipermeable layer in the embryo envelopes of the latter species (Beresniewicz et al., 1995).
The important restrictive role of the seed envelopes in the germination of dormant WT seeds could be demonstrated by the germination of embryos when these envelopes were removed (data not shown). Since microscopic analysis did not detect any consistent defects at the level of the aleurone and hyaline layer (data not shown), this restriction may be imposed essentially by the testa itself, which is in agreement with the maternal inheritance of both testa defects and germination behavior. Several mechanisms may explain how the chemical and structural composition of the testa determine the germination capacity of the seeds. The oxidized flavonoid polymers may play a major role in limiting not only water entry, as seen in legumes, but also oxygen supply to the embryo, for example, as reported by Corbineau and Côme (1993) for cereals, and by contributing to the mechanical resistance of the testa. They may also inhibit the leaching of germination inhibitors out of the seed, as proposed for charlock (Edwards, 1968, 1969). Whether one or all of these phenomena participates in the control of Arabidopsis seed germination remains to be investigated.
Seed aging defines the time-dependent deterioration of seed metabolism, leading to the loss of vigor and eventually viability (Walters, 1998). Testa pigmentation was seen to confer a better resistance to solute leakage, to imbibition damage, and to attack by soil-born fungi, thereby improving seed vigor and germination in legumes (Powell, 1989; Kantar et al., 1996). Oxidative stress may also be involved in the aging process. Membrane damage through lipid peroxidation and free radical accumulation were reported to play a major role in this degradation process (Khan et al., 1996b). The antioxidant properties of phenolic compounds, particularly flavonols, are well established (Rice-Evans et al., 1997; Yamasaki, 1997). In Arabidopsis, mutants deficient in flavonoid biosynthesis exhibit a 60% higher level of lipid peroxidation than WT plants when exposed to UVB (Landry et al., 1995). Therefore, it is very likely that seed flavonoids play a protective role against solute leakage, imbibition damage, and oxidative stress. The results presented here, showing that pigmentation mutants exhibit more deterioration than their WTs, are in agreement with this hypothesis. Moreover, the poor storability of ats demonstrates that a drastic structural defect can also be very detrimental for seed viability.
ACKNOWLEDGMENTS
We thank Dr. Hans de Jong for his help in photograph scanning, Dr. Iris van Recklingshausen for assistance in seed scanning, and Dr. Peter Wittich for advice on cytology.
Footnotes
This research was financially supported by the European Community Human Capital and Mobility program (grant no. ERB4001GT930753 to I.D.) and Bridge program (to K.M.L.-K.).
LITERATURE CITED
- Aastrup S, Outtrup H, Erdal K. Location of the proanthocyanidins in the barley grain. Carlsberg Res Commun. 1984;49:105–109. [Google Scholar]
- Albert S, Delseny M, Devic M. BANYULS, a novel negative regulator of flavonoid biosynthesis in the Arabidopsis seed coat. Plant J. 1997;11:289–299. doi: 10.1046/j.1365-313x.1997.11020289.x. [DOI] [PubMed] [Google Scholar]
- Beresniewicz MM, Taylor AG, Goffinet MC, Terhune BT. Characterization and location of a semipermeable layer in seed coats of leek and onion (Liliaceae), tomato and pepper (Solanaceae) Seed Sci Technol. 1995;23:123–134. [Google Scholar]
- Berridge MV, Tan AS, McCoy KD, Wang R. The biochemical and cellular basis of cell proliferation assays that use tetrazolium salts. Biochemica. 1996;4:15–20. [Google Scholar]
- Bewley JD. Seed germination and dormancy. Plant Cell. 1997;9:1055–1066. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürger D. Die morphologischen Mutanten des Göttinger Arabidopsis-Sortiment, einschliesslich der Mutanten mit abweichender Samenfarbe. Arabidopsis Inf Serv. 1971;8:36–42. [Google Scholar]
- Chapple CCS, Shirley BW, Zook M, Hammerschmidt R, Somerville SC. Secondary metabolism in Arabidopsis. In: Meyerowitz EM, Somerville CR, editors. Arabidopsis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 989–1030. [Google Scholar]
- Corbineau F, Côme D (1993) The concept of dormancy in cereal seeds. Proceedings of the 4th International Workshop on Seeds, Basic and Applied Aspects of Seed Biology, Angers, France, July 20–24
- Debeaujon IJ, Léon-Kloosterziel KM, Peeters AJM, Koornneef M. Abstracts of 6th International Conference on Arabidopsis Research, Madison, WI, June 7–11, 1995. 1995. Phenotypic, genetic and molecular analysis of tt12, a new transparent testa mutant of Arabidopsis thaliana. [Google Scholar]
- Deshpande SS, Cheryan M, Salunkhe DK. Tannin analysis of food products. Crit Rev Food Sci Nutr. 1986;24:401–449. doi: 10.1080/10408398609527441. [DOI] [PubMed] [Google Scholar]
- Devic M, Guilleminot J, Debeaujon I, Bechtold N, Bensaude E, Koornneef M, Pelletier G, Delseny M. The Banyuls gene encodes a DFR-like protein and is a marker of early seed coat development. Plant J. 1999;19:387–398. doi: 10.1046/j.1365-313x.1999.00529.x. [DOI] [PubMed] [Google Scholar]
- Duran JM, Retamal N. Coat structure and regulation of dormancy in Sinapis arvensis L. seeds. J Plant Physiol. 1989;135:218–222. [Google Scholar]
- Edwards MM. Dormancy in seeds of Charlock: III. Occurrence and mode of action of an inhibitor associated with dormancy. J Exp Bot. 1968;19:601–610. [Google Scholar]
- Edwards MM. Dormancy in seeds of Charlock: IV. Interrelationships of growth, oxygen supply and concentration of inhibitor. J Exp Bot. 1969;20:876–894. [Google Scholar]
- Focks N, Sagasser M, Weisshaar B, Benning C. Characterization of tt15, a novel transparent testa mutant of Arabidopsis thaliana. Planta. 1999;208:352–357. doi: 10.1007/s004250050569. [DOI] [PubMed] [Google Scholar]
- Gfeller F, Svejda F. Inheritance of post-harvest seed dormancy and kernel colour in spring wheat lines. Can J Plant Sci. 1960;40:1–6. [Google Scholar]
- Jofuku KD, den Boer BGW, Van Montagu M, Okamuro JK. Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell. 1994;6:1211–1225. doi: 10.1105/tpc.6.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M, Cavers PB, Kane M, Thompson K. Role of the pigmented seed coat of proso millet (Panicum miliaceum L.) in imbibition, germination and seed persistence. Seed Sci Res. 1996a;7:21–25. [Google Scholar]
- Kahn MM, Hendry GAF, Atherton NM, Vertucci-Walters CW. Free radical accumulation and lipid peroxidation in testas of rapidly aged soybean seeds: a light-promoted process. Seed Sci Res. 1996b;6:101–107. [Google Scholar]
- Kantar F, Pilbeam CJ, Hebblethwaite PD. Effect of tannin content of faba bean (Vicia faba) seed on seed vigour, germination and field emergence. Ann Appl Biol. 1996;128:85–93. [Google Scholar]
- Kelly KM, Van Staden J, Bell WE. Seed coat structure and dormancy. Plant Growth Regul. 1992;11:201–209. [Google Scholar]
- Koornneef M. The complex syndrome of ttg mutants. Arabidopsis Inf Serv. 1981;18:45–51. [Google Scholar]
- Koornneef M. Mutations affecting the testa colour in Arabidopsis. Arabidopsis Inf Serv. 1990;27:1–4. [Google Scholar]
- Koornneef M, Karssen CM. Seed dormancy and germination. In: Meyerowitz EM, Somerville CR, editors. Arabidopsis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 313–334. [Google Scholar]
- Koornneef M, Luiten W, de Vlaming P, Schram AW. A gene controlling flavonoid 3′ hydroxylation in Arabidopsis. Arabidopsis Inf Serv. 1982;19:113–115. [Google Scholar]
- Kuang A, Xiao Y, Musgrave ME. Cytochemical localization of reserves during seed development in Arabidopsis under spaceflight conditions. Ann Bot. 1996;78:343–351. doi: 10.1006/anbo.1996.0129. [DOI] [PubMed] [Google Scholar]
- Landry LG, Chapple CCS, Last RL. Arabidopsis mutants lacking phenolic sunscreens exhibit enhanced ultraviolet-B injury and oxidative damage. Plant Physiol. 1995;109:1159–1166. doi: 10.1104/pp.109.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léon-Kloosterziel KM, Keijzer CJ, Koornneef M. A seed shape mutant of Arabidopsis that is affected in integument development. Plant Cell. 1994;6:385–392. doi: 10.1105/tpc.6.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léon-Kloosterziel KM, van de Bunt GA, Zeevaart JAD, Koornneef M. Arabidopsis mutants with a reduced seed dormancy. Plant Physiol. 1996;110:233–240. doi: 10.1104/pp.110.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield SG, Briarty LG. Endosperm development. In: Bowman J, editor. Arabidopsis, an Atlas of Morphology and Development. New York: Springer-Verlag; 1994. [Google Scholar]
- Marbach I, Meyer AM. Permeability of seed coats to water as related to drying conditions and metabolism of phenolics. Plant Physiol. 1974;54:817–820. doi: 10.1104/pp.54.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbach I, Meyer AM. Changes in catechol oxidase and permeability to water in seed coats of Pisum elatius during seed development and maturation. Plant Physiol. 1975;56:93–96. doi: 10.1104/pp.56.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mares DJ. Proceedings 1st International Symposium on Plant Dormancy, Corvallis, OR, August 4–6, 1994. 1994. Mechanism and genetic control of dormancy in wheat (symposium abstract 39) [Google Scholar]
- Masucci JD, Schiefelbein JW. Hormones act downstream of TTG and GL2 to promote root hair outgrowth during epidermis development in the Arabidopsis root. Plant Cell. 1996;8:1505–1517. doi: 10.1105/tpc.8.9.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed-Yasseen Y, Barringer SA, Splittstoesser WE, Costanza S. The role of seed coats in seed viability. Bot Rev. 1994;60:426–439. [Google Scholar]
- Müller AJ. Embryonentest zum nachweis rezessiver letal faktoren bei Arabidopsis thaliana. Biol Zentralblatt. 1963;82:133–163. [Google Scholar]
- O'Brien TP, Feder N, McCully ME. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma. 1964;59:367–373. [Google Scholar]
- Powell AA. The importance of genetically determined seed coat characteristics to seed quality in grain legumes. Ann Bot. 1989;63:169–195. [Google Scholar]
- Ren C, Bewley JD. Seed development, testa structure and precocious germination of chinese cabbage (Brassica rapa subsp. pekinensis) Seed Sci Res. 1998;8:385–397. [Google Scholar]
- Rerie WG, Feldmann KA, Marks MD. The GLABRA2 gene encodes a homeodomain protein required for normal trichome development in Arabidopsis. Genes Dev. 1994;8:1388–1399. doi: 10.1101/gad.8.12.1388. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. [Google Scholar]
- Schneitz K, Hülskamp M, Pruitt RE. Wild-type ovule development in Arabidopsis thaliana: a light microscope study of cleared whole-mount tissue. Plant J. 1995;7:731–749. [Google Scholar]
- Shirley BW. Flavonoids in seeds and grains: physiological function, agronomic importance and the genetics of biosynthesis. Seed Sci Res. 1998;8:415–422. [Google Scholar]
- Shirley BW, Kubasek WL, Storz G, Bruggemann E, Koornneef M, Ausubel FM, Goodman HM. Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J. 1995;8:659–671. doi: 10.1046/j.1365-313x.1995.08050659.x. [DOI] [PubMed] [Google Scholar]
- Stringam GR, McGregor DI, Pawlowski SH. Proceedings of the 4th International Rapeseed Congress, Giessen, Germany, June 4–8, 1974. 1974. Chemical and morphological characteristics associated with seed coat color in rapeseed. [Google Scholar]
- van der Schaar W, Alonso-Blanco C, Léon-Kloosterziel KM, Jansen RC, van Ooijen JW, Koornneef M. QTL analysis of seed dormancy in Arabidopsis using recombinant inbred lines and MQM mapping. Heredity. 1997;79:190–200. doi: 10.1038/hdy.1997.142. [DOI] [PubMed] [Google Scholar]
- Vaughan JG, Whitehouse JM. Seed structure and the taxonomy of the Cruciferae. Bot J Linn Soc. 1971;64:383–409. [Google Scholar]
- Walters C. Understanding the mechanisms and kinetics of seed aging. Seed Sci Res. 1998;8:223–244. [Google Scholar]
- Weber H, Borisjuk L, Wobus U. Controlling seed development and seed size in Vicia faba: a role for seed coat-associated invertases and carbohydrate state. Plant J. 1996;10:823–834. [Google Scholar]
- Weidner S, Paprocka J. Proceedings of COST 828 Workgrou. 1997. Preharvest sprouting as related to change in concentration of phenolic compounds in cereal grain and embryo sensitivity to phenolic acids during seed development; p. 2. Meeting; Barcelona, November 10, 1997. [Google Scholar]
- Werker E. Seed dormancy as explained by the anatomy of embryo envelopes. Israel J Bot. 1981;29:22–44. [Google Scholar]
- Werker E, Marbach I, Mayer AM. Relation between the anatomy of the testa, water permeability and the presence of phenolics in the genus Pisum. Ann Bot. 1979;43:765–771. [Google Scholar]
- Wharton MJ. The use of tetrazolium test for determining the viability of seeds of the genus Brassica. Proc Int Seed Test Assoc. 1955;20:81–88. [Google Scholar]
- Witztum A, Gutterman Y, Evenari M. Integumentary mucilage as an oxygen barrier during germination of Blepharis persica (Burm) Kuntze. Bot Gaz. 1969;130:238–241. [Google Scholar]
- Wyatt JE. Seed coat and water absorption properties of seed of near-isogenic snap bean lines differing in seed coat color. J Am Soc Hortic Sci. 1977;102:478–480. [Google Scholar]
- Yamasaki H. A function of colour. Trends Plant Sci. 1997;2:7–8. [Google Scholar]
- Young JA, Evans RA. Mucilaginous seed coats. Weed Sci Soc Am. 1973;21:52–54. [Google Scholar]