Abstract
Background:
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening clinical syndrome. Central nervous system (CNS) involvement is a severe complication, which can lead to rapid disease development and higher morality. However, this has not been given enough attention in adult HLH. Therefore, we carried out this study to analyze the clinical features, laboratory findings, treatment outcomes, and other characteristics of adult HLH with CNS involvement.
Methods:
A retrospective analysis of 96 adult patients with HLH combined with CNS involvement between June 2003 and December 2016 was conducted. Clinical features, cerebrospinal fluid (CSF) features, image changes, and therapeutic outcomes were analyzed.
Results:
Among the 96 patients, 86 had various CNS symptoms and 33 (38.4%) had already presented symptoms before the HLH diagnosis was confirmed. A total of 59 patients received CSF examinations and showed abnormalities in 23 patients (39.0%). Seventy patients received imaging examinations and the results showed fifty patients with imaging changes (71.4%). Fifty-seven patients received multiple rounds of repeated intrathecal injection therapy and 35 patients improved (61.4%). As for the multiple analyses of effective factors on survival time, the results showed that the effects of combined Epstein–Barr virus (EBV) infection (P = 0.026, Exp(B) = 2.309, 95% confidence interval [CI] [1.108, 4.823) and intrathecal injection therapy (P = 0.013, Exp(B) = 0.422, 95% CI [0.214, 0.831]) on the survival time of the CNS-HLH patients were significant.
Conclusions:
Complication with EBV infection is a risk factor, and intrathecal injection is a protective factor. CNS involvement in HLH is not rare, which can result in a poor prognosis. Multiple rounds of repeated intrathecal injection therapy can improve the prognosis of CNS-HLH patients.
Keywords: Central Nervous System, Hemophagocytic Lymphohistiocytosis, Neuroimaging, Treatment
摘要
背景:
噬血细胞淋巴组织增多症(hemophagocytic lymphohistiocytosis,HLH)是一类威胁生命的临床综合征。中枢神经系统(central nervous system,CNS)受累是其一种严重的并发症,进展较快,死亡率增加,但在成人HLH中未得到广泛重视。因此,我们通过观察这类患者,来分析成人HLH伴CNS受累的临床表现、实验室检查、治疗预后等特点。
方法:
回顾性分析2003年6月至2016年12月我中心96例HLH伴CNS受累成人患者的临床资料、脑脊液变化、影像学异常及治疗、转归。
结果:
在收录的96名患者中,86名存在神经系统症状,其中33名(38.4%)在HLH确诊前即出现神经系统症状。59名患者接受脑脊液检查,其中23名(39.0%)存在异常。70名患者接受影像学检查,其中50名(71.4%)出现影像学改变。共57名患者接受多次鞘内注射治疗,其中35名(61.4%)患者情况改善。对于影响预后的多因素分析发现,合并EB病毒(Epstein-Barr virus,EBV)感染(P=0.026,Exp(B)=2.309,95% CI [1.108,4.823])及接受鞘内注射治疗(P=0.013, Exp(B)=0.422,95% CI [0.214,0.831])对生存时间的影响有统计学意义。
结论:
对于成人HLH合并CNS受累的患者,存在EB病毒感染提示不良预后,而接受鞘内注射治疗则可改善预后。成人当中的HLH合并CNS受累并不少见,并且一旦出现CNS受累提示预后较差。多疗程的鞘内注射治疗可以有效改善这类CNS-HLH患者的预后。
INTRODUCTION
Hemophagocytic lymphohistiocytosis (HLH) is a severe or even fatal inflammatory status caused by a hereditary or acquired immunoregulatory abnormality, nonmalignant proliferation of lymphocytes and tissue cells, and secretion of a large amount of inflammatory cytokines. In addition to basic presentations, such as fever, splenomegaly, and hemophagocytic phenomenon, the clinical manifestations of HLH sometimes also include end-organ involvement. The final pathology of this organ involvement is inflammatory cell infiltration with/without combined tissue necrosis.[1] Although organ involvement is rare, involvement of the central nervous system (CNS) as an end organ in the HLH disease process is often observed in the clinic. Current reports have shown that CNS involvement in HLH is mainly presented as nervous system symptoms/signs and abnormal relevant examination results. The definite diagnosis still relies on pathology as the reference. However, current reports and case summaries have mostly focused on pediatric HLH patients to the detriment of adult patients, and single-center, comprehensive, large-scale reports are lacking due to the limited number of cases. This study collected the data of 96 adult HLH patients with CNS involvement (CNS-HLH) at our center between November 2009 and December 2016. The possible pathogenic mechanisms and specific clinical presentations of adult CNS-HLH are summarized based on analyses of the clinical symptoms, examination indicators, and imaging presentations of these patients. Furthermore, although a review of the literature showed that the presence of CNS involvement in HLH strongly suggested a poor prognosis,[2] clear and effective measures for CNS-HLH treatment are lacking. Therefore, we investigated factors in CNS-HLH that were associated with a poor prognosis and effective measures for the prolongation of survival of CNS-HLH patients through comprehensive analyses of the treatment and patient prognosis to provide opinions concerning CNS-HLH clinical treatment.
METHODS
Ethical approval
The study was approved by the Ethical Committee of the Beijing Friendship Hospital. Written informed consent was obtained from the participating patients.
Subject eligibility criteria
Patients who enrolled in this study fulfilled the following criteria: (1) met HLH-2004 diagnostic criteria;[3] (2) were older than 18 years; and (3) suffered from CNS involvement. The definition of CNS involvement has not been standardized. In this study, we defined CNS involvement as at least meeting one of these three criteria: (a) the presence of neurological signs/symptoms, (b) neuroimaging abnormalities (including cerebral computed tomography [CT] scan and/or magnetic resonance imaging [MRI]), and (c) elevated level of protein in cerebrospinal fluid (CSF) (>450 mg/L) or cell count (>8 cells/μl).[4] Besides, eliminate tumor infiltration in CNS of these patients by searching for tumors cells in CSF. Between November 2009 and December 2016, 96 patients met these criteria at Beijing Friendship Hospital.
Observed indicators and evaluation criteria
The observational indicators included: (1) the age of onset, the gender, and the cause of HLH; (2) the neurological signs/symptoms and the onset time of the CNS involvement; (3) the pressure, cell count, and protein of CSF; (4) neuroimage and the Epstein–Barr virus (EBV)-DNA copies in peripheral blood and CSF (which were detected by quantitative polymerase chain reaction and were considered positive if the copies were >500/L);[5] and (5) the therapeutic methods and prognosis.
The assessment of the efficacy of treatment of CNS-HLH included two aspects: the evaluation of HLH itself and the evaluation of CNS involvement. HLH itself was assessed by the criteria proposed by Marsh et al.[6,7] Complete response (CR) was defined as the normalization of all quantifiable symptoms and laboratory markers of HLH, including levels of soluble CD25, ferritin, and triglyceride; hemoglobin levels; neutrophil and platelet counts; and alanine aminotransferase (ALT) levels. Partial response (PR) was defined as improvement in two or more of the following quantifiable symptoms and laboratory markers by 2 weeks: 1.5-fold decrease in soluble CD25 response; ferritin and triglyceride decreases of at least 25%; an increase of at least 100% to >0.5 × 109/L in patients with an initial neutrophil count of <0.5 × 109/L; an increase by at least 100% to >2.0 × 109/L in patients with an initial neutrophil count of 0.5 to 2.0 × 109/L; and a decrease of at least 50% in patients with initial ALT levels >400 U/L. In addition, the patient's body temperature had to have reverted to normal ranges for either CR or PR to be diagnosed. Failure to achieve PR was defined as no response. As for the CNS involvement, there is no consensus so far, and we defined it as the improvement of the neurological signs/symptoms, neuroimaging abnormalities, and/or CSF abnormalities.
Survival time
Survival times were calculated from the date of onset of HLH. All patients were followed up until death or February 28, 2017, whichever occurred first.
Statistical analysis
SPSS 22.0 (IBM, USA) statistical software was adopted, and data that did not fit a normal distribution were presented as median and range. Kaplan–Meier survival curves were used to analyze the patients’ survival and the log-rank test was used to evaluate survival time. Multiple-factor analysis was done using COX risk analysis regression model, and the factors included the age of onset, the severity of CNS symptoms, EBV infection, the intrathecal injection, and the allogeneic-hematopoietic stem cell transplantation (allo-HSCT). P < 0.05 was considered to denote a significant difference and P < 0.01 was considered very significant.
RESULTS
General conditions
There were 96 cases of CNS-HLH in our center between November 2009 and December 2016, and the occurrence rate was 20.7% (96/463). The median age of HLH onset was 34 (range: 18–79) years and there were 48 men and 48 women.
Causative factor of hemophagocytic lymphohistiocytosis
The most common type of HLH was infection-associated HLH (53/96, 55.2%), followed by tumor-associated HLH (18/96, 18.8%) and autoimmune-associated HLH (6/96, 6.3%). Most of the pathogens of infection-associated HLH were EBV (48/53, 90.6%). The number of HLH cases associated with other disease causes was lower, and most cases were sporadic. Two cases were confirmed to have primary hemophagocytic syndrome based on gene detection.
Clinical symptoms and signs
A total of 86 patients (89.6%) presented nervous system symptoms/signs. Disturbance of consciousness was the most common sign (39.6%), followed by headache/dizziness (24.0%), seizure (17.7%), and psychiatric symptoms (16.7%). The detailed characteristics of our patients are presented in Table 1. Disturbance of consciousness and seizure were considered as severe symptoms. Among the patients with nervous symptoms/signs, 33 patients (38.4%) had already presented nervous symptoms/signs before the HLH diagnosis was confirmed.
Table 1.
Clinical symptoms and signs of CNS-HLH patients
| Group | CNS symptoms and signs | Number of cases |
|---|---|---|
| 1 | Disturbance of consciousness | 38 |
| 2 | Headache/dizziness | 23 |
| 3 | Seizure | 17 |
| 4 | Psychiatric symptoms | 13 |
| 5 | Irritability | 16 |
| 6 | Ataxia | 9 |
| 7 | Hypotonia | 9 |
| 8 | Meningeal irritation | 8 |
| 9 | Cranial nerve palsies | 7 |
CNS: Central nervous system; HLH: Hemophagocytic lymphohistiocytosis.
Laboratory and imaging findings
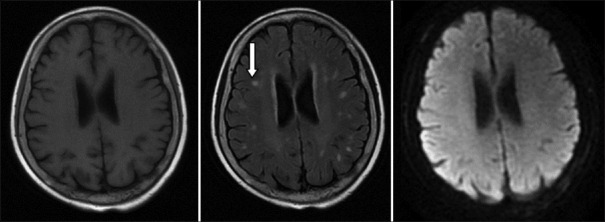
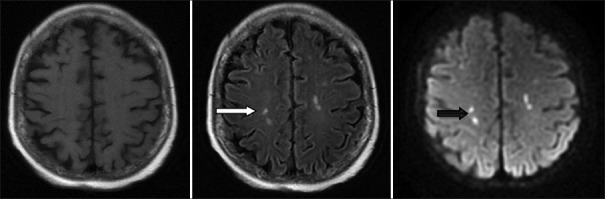
Among the 96 patients, 70 patients received imaging examinations, including cranial CT and/or cranial MRI. The results showed 50 patients (52.1%) with imaging changes. The most common location of the imaging changes was the bilateral white matter with a total of 18 patients. The next popular location was the basal ganglia region with a total of 13 patients; additionally, 6 patients exhibited widening of the ventricle and sulcus and 3 patients had meningeal involvement. The most common change in the cranial CT was the presence of low-density punctate lesions in the brain. The most common change in the MRI was a white matter demyelination-like change, which had a T1-weighted image (T1WI) low-intensity signal, T2WI, and FLARE high-intensity signals and no obviously abnormal diffusion-WI (DWI) signal [Figure 1]. In addition, the enhanced scan showed that three patients had meningeal enhancement [Figure 2] and four patients had DWI high-intensity signals [Figure 3].
Figure 1.
In both sides of the bilateral ventricle and in the deep white area of the brain, spotted abnormal signal is shown on T1-weighted image or lower signal, with high signal on T2-weighted image and fluid-attenuated inversion recovery (white arrow), and the boundary is clear. No abnormal high signal is seen on diffusion-weighted image.
Figure 2.
The left picture is from normal magnetic resonance imaging and the right picture is from enhanced magnetic resonance imaging. The leptomeninges are enhanced on the right picture (white arrow).
Figure 3.
The double-sided radial crown and the deep white matter of the brain with multiple spotted abnormal signals are slightly lower on T1-weighted image, high signal on T2-weighted image (white arrow), and high signal on diffusion-weighted image (black arrow).
Fifty-nine patients received CSF examinations; the other patients did not receive CSF examinations due to lumbar puncture contraindications (such as rash) or refusal by family members. The CSF examinations showed abnormalities in 23 patients (39.0%), of which 10 patients had elevated white blood cell counts and 13 patients had elevated protein contents. Twenty-two of the 48 EBV-associated HLH patients received an EBV-DNA examination in CSF and 63.6% were positive (14/22).
Treatment and efficacy evaluation
For the systemic treatment, 77 patients received initial therapy (including 62 HLH-94/04 regimens, 5 glucocorticoids alone, 5 DEP [liposomal doxorubicin, etoposide, and methylprednisolone] regimens, and other therapy) and 39 patients achieved a CR/PR (50.6%). The other 19 patients just had supportive treatment. There were 38 patients who did not get response or those who suffered relapse received the DEP salvage regimen treatment and 30 patients achieved CR/PR (78.9%). The overall response rate of HLH for systemic treatment was 67.7% (65/96). Twenty patients received allo-HSCT after remission, of which 13 patients obtained remission (65.0%). Fifty-seven patients received multiple rounds of repeated intrathecal injection therapy after the discovery of CNS involvement signs (the injected drugs were methotrexate and dexamethasone; the injection method was once every week for a total of 8 weeks). The CNS involvement signs of 35 patients improved (61.4%).
Survival analysis
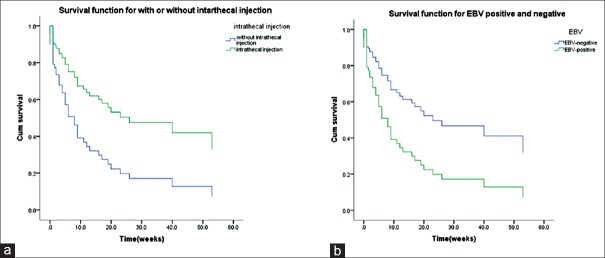
(1) Analysis of overall survival: The statistical analysis of the survival times ended on February 28, 2017. The overall mortality of the 96 CNS-HLH patients was 56.3%. The 4-week survival rate was 78.6%, the 12-week survival rate was 57.5%, and the 53-week was 23.7%. The survival curves of CNS-HLH patients and HLH patients without CNS are shown in [Figure 4]. It is clear that CNS-HLH patients’ long-term survival was worse than those without CNS involvement (log-rank test χ2 = 4.004, P = 0.045) (2) In 65 patients who got remission, analysis of the effects of allo-HSCT on the survival rate showed no differences between patients who received allo-HSCT and those without transplantation (log-rank test χ2 = 3.144, P = 0.076), but the survival curves are clearly different [Figure 5]. Comparison between HLH with CNS involvement in remission who had allo-SCT and HLH without CNS involvement in remission who had allo-SCT showed no differences (log-rank test χ2 = 0.014, P = 0.906) [Figure 6]. For the patients who did not receive allo-HSCT (76 patients), the COX proportional hazards regression model was used to calculate the effects of the factors on the survival time. The results showed that the effects of combined EBV infection (P = 0.026, Exp(B) = 2.309, 95% confidence interval [CI] [1.108, 4.823]) and intrathecal injection therapy (P = 0.013, Exp(B) = 0.422, 95% CI [0.214, 0.831]) on the survival time of the CNS-HLH patients were significant [Figure 7]. Combination with EBV infection is a risk factor and intrathecal injection is a protective factor. Conversely, the effects of age (P = 0.588), whether the CNS involvement was new onset (P = 0.093), and the severity of CNS symptoms (P = 0.091) did not significantly affect the survival time of the CNS-HLH patients. Interestingly, with those patients who had the positive EBV-DNA and had not get allo-HSCT, intrathecal injection cannot improve the outcome (P = 0.112).
Figure 4.
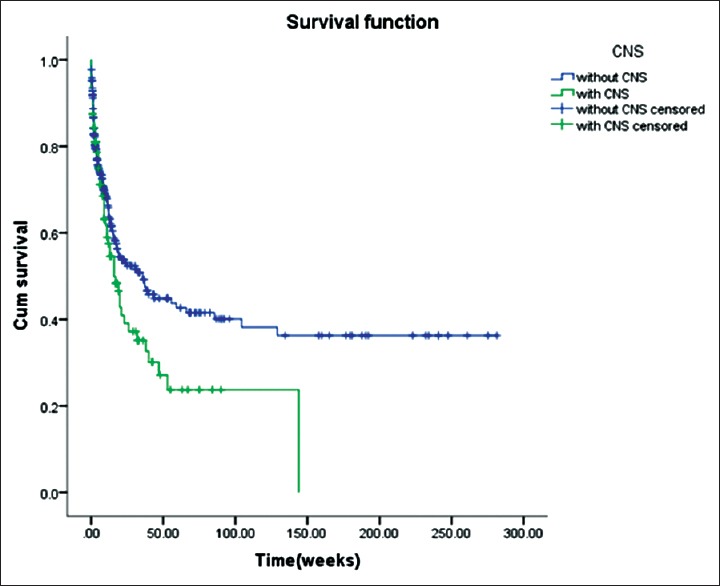
Relationships between central nervous system involvement and survival.
Figure 5.
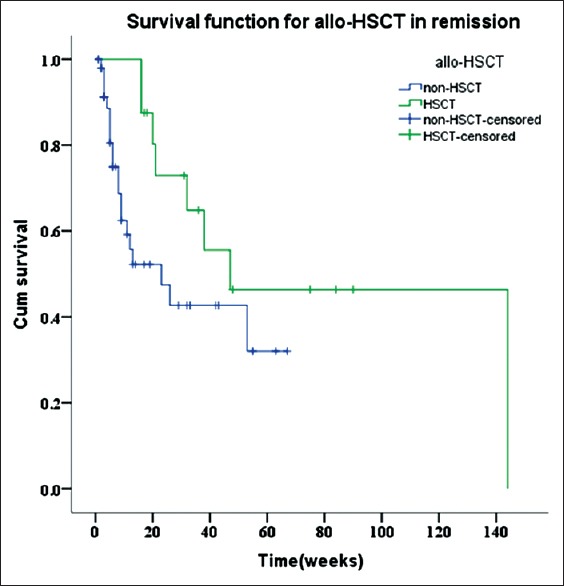
Relationships between allogeneic-hematopoietic stem cell transplantation and survival.
Figure 6.

Comparison between central nervous system-hemophagocytic lymphohistiocytosis in remission who had allogeneic hematopoietic stem cell transplantation and the hemophagocytic lymphohistiocytosis without central nervous system involvement in remission who had allogeneic hematopoietic stem cell transplantation.
Figure 7.
(a) COX analysis of multiple factors in patients without allogeneic-hematopoietic stem cell transplantation (including the age of onset, severity of central nervous system symptoms, Epstein–Barr virus infection, and intrathecal injection). (b) The survival curve of intrathecal injection factor and Epstein–Barr virus infection is shown.
DISCUSSION
HLH is a group of inflammatory reaction syndromes with similar clinical manifestations that result from many disease causes. HLH was characterized as observed hemophagocytosis in bone marrow, perinatal blood, spleen and so on, sometimes [Figure 8]. An inflammatory cytokine storm caused by the excessive proliferation and activation of T-lymphocytes and macrophages has been generally accepted as the pathogenic mechanism. The presence of CNS involvement signs has been observed during the HLH disease course. Currently, the incidence of CNS-HLH reported by different institutions is not completely consistent and varies between 10% and 73%.[8,9] However, these data are mostly from pediatric patients. Studies in our center showed that the occurrence of CNS-HLH in adult HLH patients was 20.7%, which was lower than the incidence reported in pediatric patients in most reports.[10] It is obvious that the development of the blood–brain barrier was more complete in adults; thus, inflammatory cells had more difficultly in passing through the blood–brain barrier.
Figure 8.
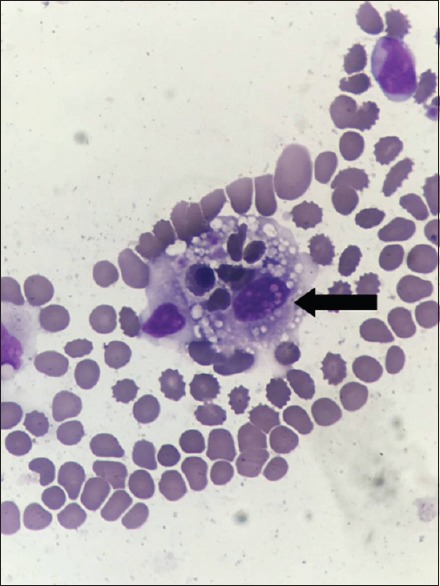
Perinatal blood smear reveals activated macrophages with phagocytosis of hematopoietic cells (black arrow).
The pathogenic mechanism of CNS-HLH is not clear. The mainstream view is that HLH is induced by an inflammatory cytokine storm which was secreted by inflammatory cells and these cells can also cause CNS involvement through crossing the blood–brain barrier.[11] Of course, this effect requires the accumulation of inflammatory reactions in HLH disease to a certain extent. Therefore, CNS involvement mostly occurs during the HLH disease course, especially when HLH exhibits clinical aggravation, which is similar to the findings observed in the majority of cases in our center. However, we also observed that signs of CNS involvement were present at the same time with HLH onset. It turns out that almost half of these patients suffered an EBV infection. Is it possible that in these cases, it is the virus that induces the CNS manifestation, so it does not need to wait until HLH progress? The EBV-DNA detection in CSF may be helpful to the different diagnosis. Regrettably, because of the technology limitation, we did not get the specific data and more studies are needed.
The comparison of disease causes of all HLH patients showed that infection is the most common, especially EBV infection. EBV-HLH is one of the most common secondary HLH.[12] Our survival analyses showed that the presence of peripheral EBV infection was a factor for a poor CNS-HLH prognosis. This may be related to the poor prognosis of EBV-HLH. EBV infection is usually silent, and only a small number of acute infections cause infectious mononucleosis.[13] EBV infects mostly B-cells in infectious mononucleosis but mostly cytotoxic T-cells or NK cells in EBV-HLH.[14] Interestingly, 1–5% of patients with infectious mononucleosis caused by EBV infection have nervous system symptoms.[15] Combined with the high rate of CSF EBV positive in our center, the presence of EBV infection itself was a high-risk factor for the presence of CNS involvement regardless of which cells were infected by EBV. In the future, the detection of EBV-DNA in the CSF may provide an indication of CNS involvement for an early diagnosis of CNS-HLH.
The differences in CNS clinical symptoms in HLH patients are relatively large. Some patients only have a headache or dizziness. However, severe patients can develop epilepsy and disturbance of consciousness. A comparison between imaging results and clinical symptoms showed that the clinical symptoms were usually more severe and disturbance of consciousness and seizures were common when the cranial CT/MRI displayed multiple lesions or the degree of lesion involvement was higher (i.e., the presence of an obvious DWI high-intensity signal in the MRI or the presence of large patches of lesions). Furthermore, a few patients did not present CNS symptoms/signs. Most of the patients who did not suffer from CNS symptoms/signs, were found of CNS involvement from the regular examination before allo-HSCT. CSF examination and neuroimaging were carried out regularly before allo-HSCT to exclude the CNS involvement. Hence, we suggest that, even without the clinical symptoms, CSF examination and/or cranial CT/MRI examinations should be performed in early stage, especially when these patients were considered of allo-HSCT.
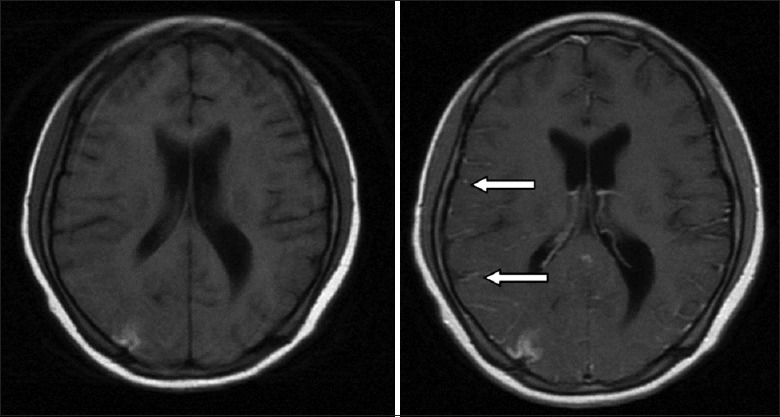
The imaging presentations had a strong correlation with the brain tissue biopsies in CNS-HLH patients in previous reports. The pathological results divided the degrees of CNS involvement into three stages: Stage I involved lymphocyte and tissue cell infiltration into the meninges; Stage II included brain parenchyma and perivascular space infiltration in addition to the meninges; and Stage III involved infiltration of the brain parenchyma, especially diffuse infiltration of the white matter, and might have the presentation of multifocal necrosis of the brain tissues and demyelination.[1,16] In the study at our center, only three patients who received a cranial MRI enhancement examination had meningeal enhancement, which suggests that early-stage CNS involvement is hardly seen in clinical settings. Bilateral white matter involvement was most common; however, this did not indicate that HLH was more likely to involve brain parenchyma. The more likely explanation was that abnormal CNS presentations more easily occurred when the disease involved the brain parenchyma. The involvement locations were mainly bilateral, which differed from other cerebrovascular disease lesions that were mostly unilateral, and could also be used as a point of differentiation between HLH with CNS involvement and other cerebrovascular diseases. In addition, four patients had DWI high-intensity signals in the cranial MRI and they all died. DWI high intensity may indicate destruction of blood vessels and necrosis, so it may be a poor prognosis factor for CNS-HLH. Furthermore, some patients with abnormal imaging had lesion absorption at the imaging re-examination after treatment remission. Our center observed one patient with abnormal imaging who exhibited significant improvement of imaging after 8 rounds of intrathecal injection therapy [Figure 8]. The details of this patient are described in the section below. In addition to assisting with the CNS-HLH diagnosis, imaging also has excellent suggestion functions concerning the severity of CNS-HLH and can be used as one observation indicator for the evaluation of efficacy.
Currently, there is a lot of attention on CSF abnormalities worldwide. It has been reported that the CSF abnormality rate in HLH patients was pretty high.[17] Hirst et al. reported that 76.47% of patients had abnormal CSF.[18] However, the rate of CSF abnormity of our study was lower. Analysis of medical records showed that one explanation might be the timing of the CSF examination. At our center, the CSF examinations were mainly performed at the stable condition stage and the changes might disappear with the control of the disease condition.[19] In future studies, these data should be improved.
The prognosis of patients who develop CNS development during the HLH disease course is significantly poorer than those without CNS involvement. Current treatment for CNS-HLH patients lacks prospective studies. The general treatment opinion is to target HLH itself in combination with intrathecal drug administration based on the conditions. In this study, the overall response rate of HLH disease for systemic treatment was 67.7%. The presence of the blood–brain barrier restricts the drug effects of the systemic therapy; therefore, intrathecal injection of methotrexate + glucocorticoid was included in the standard HLH-04 regimen.[3] However, intrathecal injection in the HLH-04 regimen has only targeted children, and currently, no large-scale report has investigated the use of intrathecal injection in adults with CNS-HLH. In the study at our center, the response rate of CNS symptoms/signs who received intrathecal injection reached 61.4%, indicating that intrathecal injection should also be considered in adult CNS-HLH.
Regarding the CNS-HLH prognosis, current views generally consider that intrathecal injection does not have a significantly active function on the final outcome of CNS-HLH and that more often allo-HSCT is required.[4,20] Even though in our study, the P value is not significant, which may be related to the limited number of cases, it is also very clear in the survival curve that allo-HSCT significantly improved the prognosis of CNS-HLH patients and effectively prolonged their survival times. As for the comparison between allo-HSCT patients with and without CNS involvement, it may be suggested that allo-HSCT can improve CNS-HLH patients’ survival to the level of patients without CNS involvement. However, the survival analysis results obtained for the patients who did not receive transplantation showed that the intrathecal injection therapy could improve their survival significantly. In addition, the patients we observed included one case of a 20-year-old young male patient. This patient was confirmed to have primary HLH after gene screening (c.172T>C, heterozygous missense mutation, serine-proline (p.S58P) mutation)[21] and experienced a recurrence with CNS involvement after transplantation. The presentation of symptoms was status epilepticus and elevated CSF pressure. Cranial imaging examination showed abnormal signals in the bilateral frontotemporal lobes and an occipital dorsal thalamus. After 8 rounds of the intrathecal injection therapy, the CNS symptoms/signs of this patient relieved and CSF returned to normal. The cranial imaging showed significant absorption [Figure 9]. This patient had survived for more than 2 years until now. Therefore, combined with the above aspects, multiple rounds of intrathecal injection may be an alternative treatment method for CNS-HLH patients who cannot receive allo-HSCT. Intrathecal injection therapy can even be attempted for patients who experience a CNS-HLH recurrence after transplantation. Another interesting finding is that intrathecal injection seems to have no significant effects on the prognosis of HLH patients with EBV infection. The current international EBV-HLH treatment regimens all recommend performing allo-HSCT after remission. Obviously, for these patients, simple intrathecal injection therapy cannot achieve the treatment intensity and control the storm of inflammatory factor successfully. This leads to a bigger picture on what kind of situation that simple intrathecal injection can lead to a better outcome and on what kind of situation that using intrathecal injection alone may not be good enough. Further prospective clinical studies are required to provide a more precise evaluation system. Overall, intrathecal injection is an effective therapeutic measure for CNS-HLH and under specific condition it can be used alone and the high-risk caused by allo-HSCT can be avoided.
Figure 9.
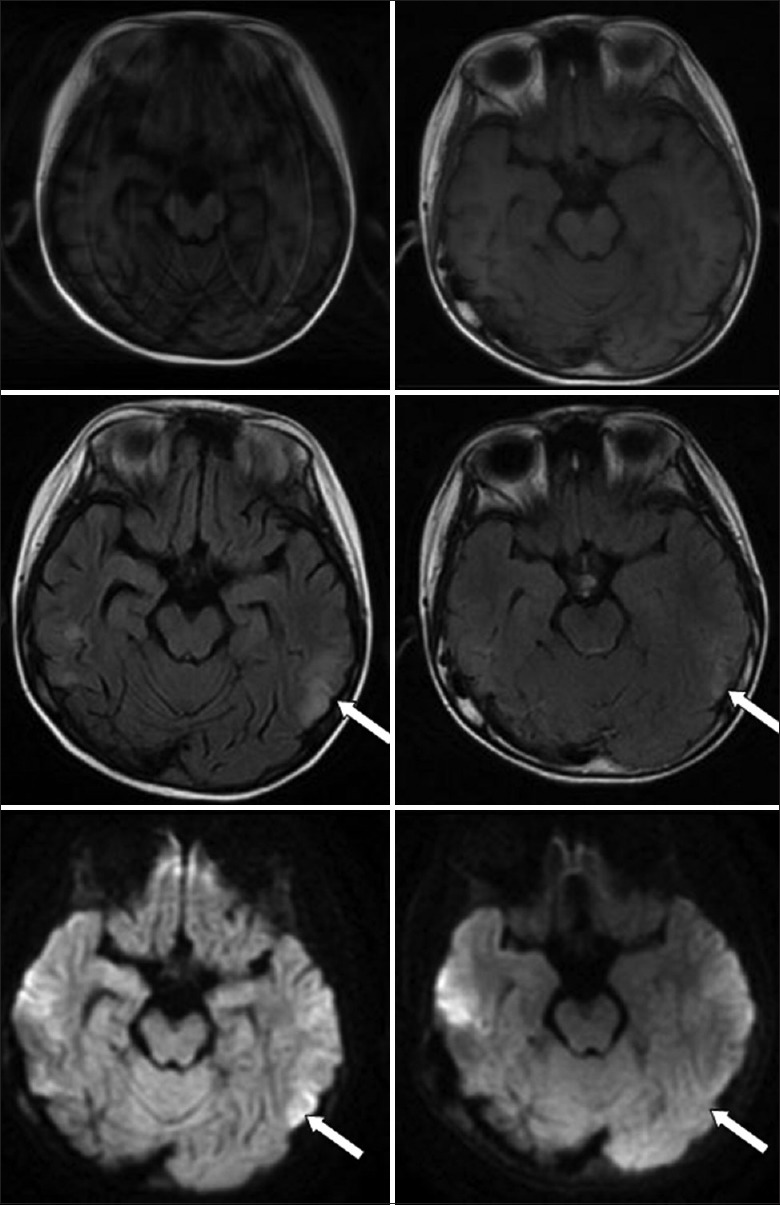
There was an abnormal signal of the lateral thalamus of the frontotemporal and occipital lobes, and the lesion was obviously absorbed in 8 intrathecal injection (left: before treatment, right: after treatment) (white arrow).
In summary, CNS involvement in HLH is not rare in adults. Of all the disease causes of HLH, infection-associated HLH is most common, and a positive EBV-DNA in the peripheral blood is associated with a poor prognosis. CNS symptoms/signs are diverse and should receive clinical attention. HLH patients without CNS symptoms/signs should receive CSF and/or imaging examinations. Imaging changes may prompt the course of CNS involvement. CNS-HLH patients have a poor prognosis and high mortality. Currently, allo-HSCT is the effective measure. Multiple rounds of repeated intrathecal injection therapy can improve the prognosis of CNS-HLH patients who cannot receive allo-HSCT or experience CNS recurrence after HSCT, especially in non-EBV-HLH patients.
Financial support and sponsorship
This work was supported by the grants from the Beijing Science and Technology Plan (No. Z151100004015172), National Science Fund for Distinguished Young Scholars (No. 81401627), and the Capital's Funds for Health Improvement and Research (No. 2016-2-2027).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
REFERENCES
- 1.Henter JI, Nennesmo I. Neuropathologic findings and neurologic symptoms in twenty-three children with hemophagocytic lymphohistiocytosis. J Pediatr. 1997;130:358–65. doi: 10.1016/s0022-3476(97)70196-3. doi:10.1016/S0022-3476(97)70196-3. [DOI] [PubMed] [Google Scholar]
- 2.Goo HW, Weon YC. A spectrum of neuroradiological findings in children with haemophagocytic lymphohistiocytosis. Pediatr Radiol. 2007;37:1110–7. doi: 10.1007/s00247-007-0569-z. doi:10.1007/s00247-007-0569-z. [DOI] [PubMed] [Google Scholar]
- 3.Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–31. doi: 10.1002/pbc.21039. doi:10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 4.Horne A, Wickström R, Jordan MB, Yeh EA, Naqvi A, Henter JI, et al. How to treat involvement of the central nervous system in hemophagocytic lymphohistiocytosis? Curr Treat Options Neurol. 2017;19:3. doi: 10.1007/s11940-017-0439-4. doi:10.1007/s11940-017-0439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imashuku S. Clinical features and treatment strategies of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Crit Rev Oncol Hematol. 2002;44:259–72. doi: 10.1016/s1040-8428(02)00117-8. doi:10.1016/S1040-8428(02)00117-8. [DOI] [PubMed] [Google Scholar]
- 6.Marsh RA, Allen CE, McClain KL, Weinstein JL, Kanter J, Skiles J, et al. Salvage therapy of refractory hemophagocytic lymphohistiocytosis with alemtuzumab. Pediatr Blood Cancer. 2013;60:101–9. doi: 10.1002/pbc.24188. doi:10.1002/pbc.24188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Huang W, Hu L, Cen X, Li L, Wang J, et al. Multicenter study of combination DEP regimen as a salvage therapy for adult refractory hemophagocytic lymphohistiocytosis. Blood. 2015;126:2186–92. doi: 10.1182/blood-2015-05-644914. doi:10.1182/blood-2015-05-644914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jovanovic A, Kuzmanovic M, Kravljanac R, Micic D, Jovic M, Gazikalovic S, et al. Central nervous system involvement in hemophagocytic lymphohistiocytosis: A single-center experience. Pediatr Neurol. 2014;50:233–7. doi: 10.1016/j.pediatrneurol.2013.10.014. doi:10.1016/j.pediatrneurol.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Weisfeld-Adams JD, Frank Y, Havalad V, Hojsak JM, Posada R, Kaicker SM, et al. Diagnostic challenges in a child with familial hemophagocytic lymphohistiocytosis type 3 (FHLH3) presenting with fulminant neurological disease. Childs Nerv Syst. 2009;25:153–9. doi: 10.1007/s00381-008-0744-z. doi:10.1007/s00381-008-0744-z. [DOI] [PubMed] [Google Scholar]
- 10.Yang S, Zhang L, Jia C, Ma H, Henter JI, Shen K, et al. Frequency and development of CNS involvement in Chinese children with hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2010;54:408–15. doi: 10.1002/pbc.22239. doi:10.1002/pbc.22239. [DOI] [PubMed] [Google Scholar]
- 11.Gratton SM, Powell TR, Theeler BJ, Hojsak JM, Posada R, Kaicker SM, et al. Neurological involvement and characterization in acquired hemophagocytic lymphohistiocytosis in adulthood. J Neurol Sci. 2015;357:136–42. doi: 10.1016/j.jns.2015.07.017. doi:10.1016/j.jns.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Imashuku S. Treatment of Epstein-Barr virus-related hemophagocytic lymphohistiocytosis (EBV-HLH); update 2010. J Pediatr Hematol Oncol. 2011;33:35–9. doi: 10.1097/MPH.0b013e3181f84a52. doi:10.1097/MPH.0b013e3181f84a52. [DOI] [PubMed] [Google Scholar]
- 13.Maeda E, Akahane M, Kiryu S, Kato N, Yoshikawa T, Hayashi N, et al. Spectrum of Epstein-Barr virus-related diseases: A pictorial review. Jpn J Radiol. 2009;27:4–19. doi: 10.1007/s11604-008-0291-2. doi:10.1007/s11604-008-0291-2. [DOI] [PubMed] [Google Scholar]
- 14.Smith MC, Cohen DN, Greig B, Yenamandra A, Vnencak-Jones C, Thompson MA, et al. The ambiguous boundary between EBV-related hemophagocytic lymphohistiocytosis and systemic EBV-driven T cell lymphoproliferative disorder. Int J Clin Exp Pathol. 2014;7:5738–49. [PMC free article] [PubMed] [Google Scholar]
- 15.Dunmire SK, Hogquist KA, Balfour HH. Infectious mononucleosis. Curr Top Microbiol Immunol. 2015;390(Pt 1):211–40. doi: 10.1007/978-3-319-22822-8_9. doi:10.1007/978-3-319-22822-8_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ju HY, Hong CR, Kim SJ, Lee JW, Kim H, Kang HJ, et al. Hemophagocytic lymphohistiocytosis diagnosed by brain biopsy. Korean J Pediatr. 2015;58:358–61. doi: 10.3345/kjp.2015.58.9.358. doi:10.3345/kjp.2015.58.9.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horne A, Trottestam H, Aricò M, Egeler RM, Filipovich AH, Gadner H, et al. Frequency and spectrum of central nervous system involvement in 193 children with haemophagocytic lymphohistiocytosis. Br J Haematol. 2008;140:327–35. doi: 10.1111/j.1365-2141.2007.06922.x. doi:10.1111/j.1365-2141.2007.06922.x. [DOI] [PubMed] [Google Scholar]
- 18.Hirst WJ, Layton DM, Singh S, Mieli-Vergani G, Chessells JM, Strobel S, et al. Haemophagocytic lymphohistiocytosis: Experience at two U.K. centres. Br J Haematol. 1994;88:731–9. doi: 10.1111/j.1365-2141.1994.tb05111.x. doi: 10.1111/j.1365.2141.2007.06922.x. [DOI] [PubMed] [Google Scholar]
- 19.Anderson TL, Carr CM, Kaufmann TJ. Central nervous system imaging findings of hemophagocytic syndrome. Clin Imaging. 2015;39:1090–4. doi: 10.1016/j.clinimag.2015.07.013. doi:10.1016/j.clinimag.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Sparber-Sauer M, Hönig M, Schulz AS, zur Stadt U, Schütz C, Debatin KM, et al. Patients with early relapse of primary hemophagocytic syndromes or with persistent CNS involvement may benefit from immediate hematopoietic stem cell transplantation. Bone Marrow Transplant. 2009;44:333–8. doi: 10.1038/bmt.2009.34. doi:10.1038/bmt.2009.34. [DOI] [PubMed] [Google Scholar]
- 21.Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis. Annu Rev Med. 2012;63:233–46. doi: 10.1146/annurev-med-041610-134208. doi:10.1146/annurev-med-041610-134208. [DOI] [PubMed] [Google Scholar]