Abstract
Background:
Studies of haploidentical-related donor (HRD) stem cell transplantation using a combination of peripheral blood stem cells (PBSCs) and bone marrow as the graft have reported encouraging results for patients with hematological diseases. However, few studies specifically reported transplantation of only PBSCs from HRDs among patients with relapsed or refractory acute myeloid leukemia (AML). Here, the long-term outcomes and side effects of unmanipulated HRD PBSC transplantation (HRD-PBSCT) for relapsed/refractory AML were analyzed.
Methods:
We performed a retrospective analysis of the outcomes in relapsed/refractory AML patients who underwent PBSCT from HRDs (n = 36).
Results:
Thirty-one (86.1%) patients in the HRD-PBSCT group achieved platelet recovery. The cumulative incidence of acute graft-versus-host disease (aGVHD) in the HRD-PBSCT group was 40.00%, and the cumulative incidence of grades 2–4 aGVHD in this group was 13.33%. A total of 13 patients in the HRD-PBSCT group had recurrent disease at a median of 183 days after transplantation (range: 10–1700 days), reaching cumulative incidences of relapse of 50.28% at 5 years. On multivariate analysis, donor age and patient age >40 years were independent risk factors for inferior disease-free survival or overall survival (P < 0.05). The results of the present study demonstrate rapid and complete neutrophil engraftment, a low incidence of grade 2–4 aGVHD, and promising survival rates in patients after HRD-PBSCT. Thus, granulocyte colony-stimulating factor–primed PBSCs may be a reliable graft source in unmanipulated HRD-HSCT under myeloablative conditioning when no matched sibling donor is available.
Conclusions:
Our results support the feasibility, effectiveness, and tolerability of PBSCs as a graft source in unmanipulated HRD transplantation under myeloablative conditioning in patients with leukemia.
Keywords: Acute Myeloid Leukemia, Haploidentical Transplantation, Peripheral Blood Stem Cell Transplantation, Recurrence
摘要
背景:
目前的报道关于采用外周血干细胞和骨髓联合输注的单倍体相合移植治疗恶性血液病取得了很好的疗效,而单用外周血干细胞的单倍体相合移植治疗复发难治急性髓系白血病的研究较少。本文分析了单用外周血干细胞单倍体相合移植治疗复发难治急性髓系白血病长期疗效和毒副反应。
方法:
回顾性分析接受单倍体相合外周血干细胞的36例复发难治急性髓系白血病的疗效。
结果:
36例患者中有31例单倍体移植患者血小板植入,植入率为86.7%。急性移植物抗宿主病的累积发生率为40%,2-4度急性移植物抗宿主病的累积发生率为13.33%。移植后13例患者出现疾病复发,平均复发时间为移植后183天(10 -1700天),5年的累积复发率为50.28%。多因素分析显示,供者年龄或患者年龄≥ 40岁是复发难治急性髓系白血病患者单倍体移植预后不良(总生存率和无疾病生存率)的独立危险因素(P < 0.05)。 单倍体相合外周血干细胞移植治疗复发难治急性髓系白血病,移植后中性粒细胞和血小板重建迅速,2-4度急性移植物抗宿主病发生率低,总体生存预后较好。由此,当复发难治急性髓系患者缺乏同胞全合供者时,粒细胞集落刺激因子动员的外周血干细胞单倍体相合移植可以作为治疗该类患者的较好选择。
结论:
本研究证明了粒细胞集落刺激因子动员的外周血干细胞用于单倍体相合清髓移植治疗急性白血病的可行性、有效性和安全性。
INTRODUCTION
New targeted therapy drugs and improved risk stratification strategies have increased the rate of complete remission (CR) among patients with primary acute myeloid leukemia (AML).[1,2,3,4,5,6] However, relapse after CR and refractory leukemia are still major challenges to achieving high cure rates.[7,8,9,10,11,12] Despite aggressive and effective chemotherapeutic strategies, many patients with AML who experience relapse or refractory disease, particularly relapse after a second CR2, succumb to the disease.[13,14,15,16,17,18,19,20,21,22,23] For this reason, researchers have concluded that allogeneic hematopoietic stem cell transplantation (allo-HSCT) should be offered to eligible patients with refractory AML or relapsed AML after the first CR1 whenever feasible.[24,25,26,27,28,29] Unfortunately, many patients are unable to receive an allo-HSCT due to the absence of a matched related or unrelated donor (URD) and the time required to find a phenotypically matched URD as well as the time involved in identifying, typing, and harvesting cells from an URD.[30,31,32,33,34,35] The goal of achieving immediate availability of a suitable haploidentical donor for virtually all patients, particularly those who urgently need transplantation, has led us to focus on haploidentical hematopoietic cell transplantation (HCT) for patients with relapsed/refractory AML.
Unmanipulated haploidentical-related donor (HRD) HCT has yielded encouraging outcomes in the treatment of hematologic malignancies, making it an alternative option for patients who do not have an HLA-matched sibling donor (MSD).[36,37,38,39,40] One study even reported that unmanipulated HRD-HCT from granulocyte colony-stimulating factor (G-CSF)-mobilized bone marrow and peripheral blood stem cells (PBSCs) achieved a stronger graft-versus-leukemia (GVL) effect than MSD-HCT in patients with high-risk acute leukemia.[36] Moreover, PBSC transplantation is less invasive for the donor and easy to perform. Recent studies demonstrated the feasibility of unmanipulated HRD-HCT from G-CSF-mobilized PBSCs for the treatment of hematological malignancy.[41,42] However, the long-term outcomes of unmanipulated HRD-PBSC transplantation (HRD-PBSCT) for relapsed/refractory AML, particularly compared with those achieve with MSD-HCT, remain unclear.
Here, we report the results of the retrospective cohort study on the efficacy and toxicity of HRD-PBSCT for the treatment of relapsed/refractory AML. In addition, we analyzed the results of allo-HSCT from HRDs to illustrate the similar benefits of HSCT from MSDs in this multicenter study.
METHODS
Ethical approval
All study procedures were approved by the Chinese PLA General Hospital Review Board in accordance with the Declaration of Helsinki. Informed consent form was obtained from all the patients.
Patients
This study included all cases diagnosed with AML according to the World Health Organization classification, expect those diagnosed with acute promyelocytic leukemia. Their diagnoses were defined by the French–American–British and World Health Organization criteria.
The study population consisted of patients who received either HRD-PBSCT or MSD-PBSCT at the Chinese PLA General Hospital, Beijing, China, between July 2007 and February 2016. Prior to transplantation, the enrolled patients had been diagnosed with AML beyond CR1 or were in nonremission (NR), regardless of cytogenetics; notably, no patients with t (15;17) were included in this study. The policy of donor choice was based on donor availability. The order of preference for donor selection was MSD, matched URD, and finally HRD. Patients with any uncontrolled infections or with severe pulmonary, renal, hepatic, or cardiac diseases were not eligible for transplantation. For this comparative analysis, we excluded patients who received only chemotherapy (n = 44) and included patients who received HRD and MSD transplantation (n = 62), as shown in Figure 1.
Figure 1.
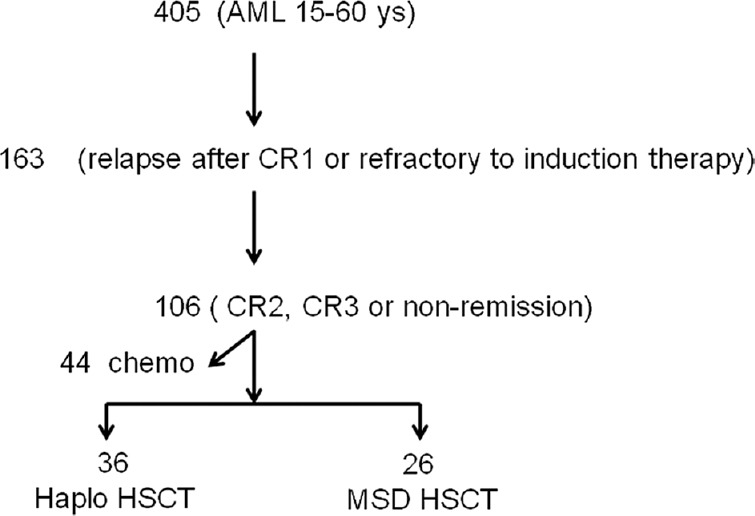
Patient selection for analysis. AML: Acute myeloid leukemia; CR: Complete remission; PBSCT: Peripheral blood stem cell transplantation; HRD: Haploidentical-related donor; MSD: Matched sibling donor; MUD: Matched unrelated donor.
Transplantation procedures
Note that numbers of days before the transplantation are preceded by “−” and numbers of days after the last stem cell infusion day are preceded by “+.”
High-resolution DNA techniques were used to evaluate the HLA-A, B, DRB1, DQB1, and C loci. Donors were preferentially ranked according to HLA-matched loci, male sex, younger age, and better performance status. Donors were subcutaneously treated with recombinant human G-CSF (5 μg·kg−1·d−1; Filgrastim, Kirin, Japan) for 5–6 consecutive days starting on day 4. PBSCs were collected with a COBE Blood Cell Separator (Spectra LRS; COBEBCT Inc., Lakewood, CO, USA), and on the same day, the unmanipulated PBSCs were infused into the recipients. The target mononuclear cell count and CD34+ cell count were 5–15 × 108/kg and 2–10 × 106/kg of recipient weight, respectively.
All patients were given myeloablative conditioning. The conditioning regimen for HRD-PBSCT consisted of busulfan (Otsuka Pharmaceutical Company in China, 3.2 mg·kg−1·d−1 intravenously [IV], days −10 to −8), carmustine (Jinyao Tianjin Pharmaceutical Company, China, 250 mg/m2, day 5), cytarabine (Pfizer Pharmaceutical Company, USA, 4 g·m−2· d−1, days −7 to −6), cyclophosphamide (Baxter Pharmaceutical Company, USA, 60 mg·kg−1·d−1, days −4 to −3), and antithymocyte globulin (ATG; thymoglobuline, rabbit; Genzyme Pharmaceutical Company, USA, 2.5 mg·kg−1·d−1, days −5 to −2). Patients receiving MSD-PBSCT received the same conditioning regimen but without ATG.
All transplant recipients received cyclosporine A (CsA, Novartis Pharmaceutical Company, Switzerland), mycophenolate mofetil (Roche Pharmaceutical Company, Switzerland), and short-term methotrexate (MTX, Pfizer Pharmaceutical Company, USA) for GVHD prophylaxis. CsA (3 mg/kg, every 12 h, IV) was used from day 9, and the concentration was adjusted to 180–200 ng/ml. IV CsA was switched to oral administration when the patient's bowel function recovered. From day 9, 0.5 g mycophenolate mofetil was administered orally every 12 h and was withdrawn on day +45 for HRD-PBSCT or day +30 for MSD-PBSCT. After graft infusion, MTX was given to all patients at 15 mg/m2 on day +1 and 10 mg/m2 on days +3, +6, and +11. Patients who relapsed after transplantation received modified donor lymphocyte infusion (DLI) for the prevention of relapse if they showed no signs of acute GVHD. G-CSF–primed PBSCs were used for DLI, and the modified DLI regimen was described previously.[43]
Definitions and endpoints
Patients were included if they fulfilled at least one of the following criteria defining relapsed/refractory AML: (1) primary induction failure after 2 or more cycles of chemotherapy, (2) first early relapse after a remission duration of fewer than 6 months, (3) first relapse after a remission duration of more than 6 months and refractory to salvage combination chemotherapy that was useful in the first induction therapy, (4) second or subsequent relapse, and (5) extramedullary leukemia. Acute GVHD was graded according to modified Glucksberg criteria. The endpoint of the last follow-up for all surviving patients was February 1, 2016. Engraftment, GVHD, overall survival (OS), disease-free survival (DFS), transplantation-related mortality (TRM), and relapse were calculated from day +1. The primary endpoint was DFS. Only patients with successful engraftment were included in the analysis of aGVHD. Patients who survived at least 100 days were analyzed for chronic GVHD (cGVHD). TRM was defined as death from any cause without evidence of disease recurrence in the first 28 days post-HSCT or death beyond day +28. The date of neutrophil recovery was defined as the first of 3 consecutive days with an absolute neutrophil count of 0.5 × 109/L. The date of platelet recovery was defined as the first of 7 consecutive days with an absolute platelet count of 20 × 109/L without the aid of transfusion. For OS, patients were considered to have an event at the time of death from any cause. DFS was defined as the time to either relapse or death from any other causes. Relapse included hematologic, molecular, and cytogenetic relapse.
Statistical analysis
The incidences of aGVHD, cGVHD, leukemia relapse, engraftment, and TRM were evaluated with cumulative incidence curves, taking into account competing risks. Assessments of risk factors for outcomes were calculated by multivariate analysis using Cox proportional hazards regression. The prognostic value of the following variables was investigated: treatment strategy (HRD vs. MSD), patient or donor age (<40 vs. ≥40 years), CD34-positive (+) cells in the graft, mononuclear cells in the graft, Eastern Cooperative Oncology Group (ECOG) score, cytogenetics risk status, molecular abnormalities risk status, CR duration time after the first induction therapy, blasts in peripheral blood before transplantation, blasts in bone marrow before transplantation, donor–recipient sex match, and occurrence of GVHD. Probabilities of survival and DFS were calculated via the Kaplan–Meier method using log-rank tests. Multivariate analyses were conducted to identify independent predictors of NRM, relapse, DFS, and OS. The SAS statistical software package version 8.2 (SAS Institute, Cary, NC, USA) was used for all analyses.
RESULTS
Characteristics of patients and transplantations
The final study population consisted of the 36 patients who received HRD-PBSCT and the 26 patients who received MSD-PBSCT [Figure 1]. The median follow-up times were 2177 days for the HRD group and 2673 days for the MSD group. The characteristics of patients and donors in these transplantation cases are listed in Table 1. No significant differences were observed in patient characteristics between the two groups, and for both groups, donor age was distributed evenly above and below 40 years old. There were also no significant differences in terms of underlying disease, time from diagnosis to PBSCT, disease status at PBSCT, or conditioning regimen between the two groups. In the HRD-PBSCT cohort, patients received PBSCs from a direct family member for whom more than 3 of 10 HLA-A,-B,-C,-DRB1, and-DQB1 allele loci (>3/10) were mismatched from the patient.
Table 1.
Patient and donor characteristics at the time of allogeneic-HCT
| Characteristics | HRD-HPBSCT (n = 36) | MSD-HPBSCT (n = 26) | P |
|---|---|---|---|
| Gender, n (%) | |||
| Receipt (male) | 26 (73.3) | 15 (57.6) | 0.372 |
| Donor (male) | 26 (73.3) | 14 (53.8) | 0.239 |
| Donor, n (%) | |||
| Mother | 2 (5.5) | ||
| Father | 13 (36.1) | ||
| Brother | 7 (19.4) | 14 (53.8) | |
| Sister | 5 (13.8) | 12 (46.1) | |
| Son | 4 (11.1) | ||
| Daughter | 3 (8.3) | ||
| Other relative | 2 (5.5) | ||
| Age, n (%) | |||
| Patient <40 years | 25 (69.4) | 14 (53.8) | 0.382 |
| Donor <40 years | 16 (44.4) | 13 (50.0) | 0.779 |
| Hematologic malignancy, n (%) | |||
| AML | |||
| CR2 | 6 (16.7) | 4 (15.4) | 0.665 |
| CR3 | 1 (2.8) | 2 (7.7) | |
| NR | 29 (80.5) | 20 (76.9) | |
| Percentage of bone marrow blasts before stem cell transplantation (%) | 13.7 | 15.2 | |
| Cytogenetic risk group, n (%) | |||
| Favorable | 4 (11.1) | 3 (11.5) | |
| Intermediate | 26 (72.2) | 21 (58.3) | |
| Poor | 4 (11.1) | 1 (2.8) | |
| No results | 2 (5.6) | 1 (2.8) | |
| Molecular abnormalities, n (%) | |||
| Favorable | 4 (11.1) | 3 (8.3) | |
| Intermediate | 29 (80.6) | 18 (50) | |
| Poor | 3 (8.33) | 3 (8.3) | |
| Not determined | 2 (5.6) | ||
| Time to transplantation (from diagnosis), n (%) | |||
| <7 months | 19 (52.8) | 13 (50) | 0.829 |
| >7 months | 17 (47.2) | 13 (50) | |
| Conditioning regimen, n (%) | |||
| BuCy | 26 (72.2) | 17 (65.4) | 0.664 |
| TBIcy | 5 (13.9) | 3 (11.5) | |
| FB | 5 (13.9) | 6 (23.1) | |
| HCT graft | |||
| CD34+ | |||
| <4.77 × 106/kg | 15 (41.7) | 17 (65.4) | 0.171 |
| >4.77 × 106/kg | 21 (58.3) | 9 (34.6) |
HCT: Hematopoietic cell transplantation; HRD-PBSCT: Unmanipulated haploidentical-related donor peripheral blood stem cell transplantation; MSD-PBSCT: Matched sibling donors peripheral blood stem cell transplantation; CR: Complete remission; NR: Nonremission; AML: Acute myeloid leukemia.
Engraftment
Sustained myeloid engraftment was achieved in both groups (100%) at a median of 16 (10–26) days. During the follow-up period, 31 patients (86.1%) in the HRD-PBSCT group achieved platelet recovery in a median of 25 (10–90) days, and all patients in the MSD-PBSCT group achieved platelet recovery in a median of 20 (9–36) days.
Graft-versus-host disease
By day +100, 15 patients in the HRD-PBSCT group and 5 patients in the MSD-PBSCT group had experienced aGVHD at a median of 42 (10–100) days after transplantation. The cumulative incidence of aGVHD in the HRD-PBSCT group was little higher than that in the MDS-PBSCT group [40.00% vs. 18.18%, respectively; Table 2 and Figure 2]. The cumulative incidence of grades 2–4 aGVHD was 13.33% in the HRD-PBSCT group and 9.091% in the MDS-PBSCT group. On univariate analysis, no risk factors were found for the occurrence of grades 2–4 aGVHD.
Table 2.
Cumulative incidence of GVHD after HRD-PBSCT or MSD-PBSCT in patients with relapsed/refractory AML
| Items | HRD-PBSCT (n = 36) | MSD-PBSCT (n = 26) | ||
|---|---|---|---|---|
| n | Percentage | n | Percentage | |
| Acute GVHD | ||||
| Grade 1–4 | 14 | 40.00 ± 9.09 | 6 | 18.18 ± 8.44 |
| Grade 2–4 | 5 | 13.33 ± 6.33 | 3 | 9.091 ± 6.68 |
| Chronic GVHD | 6 | 17.90 ± 7.47 | 5 | 33.78 ± 14.03 |
HRD-PBSCT: Unmanipulated haploidentical-related donor peripheral blood stem cell transplantation; MSD-PBSCT: Matched sibling donors peripheral blood stem cell transplantation; GVHD: Graft-versus-host disease; AML: Acute myeloid leukemia.
Figure 2.
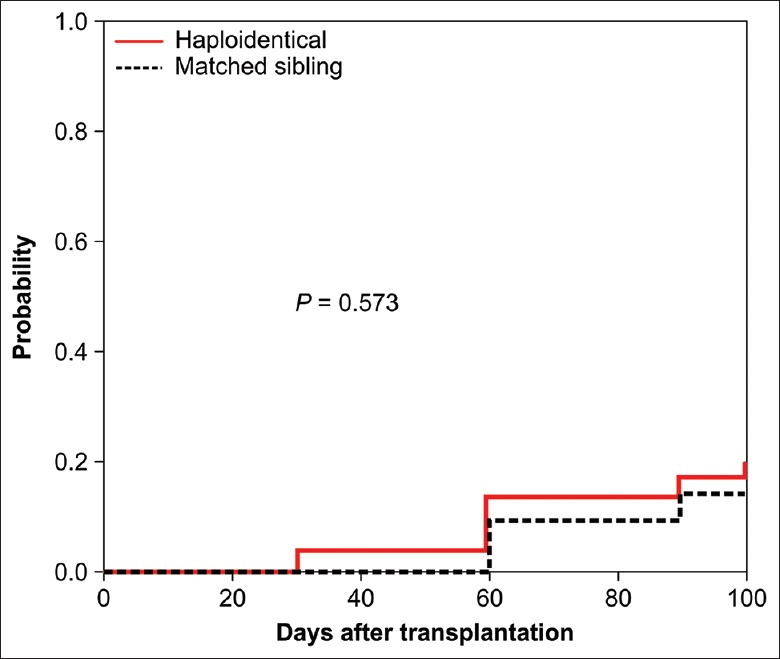
Cumulative incidence of grades 2–4 acute GVHD after HRD-PBSCT or MSD-PBSCT. PBSCT: Peripheral blood stem cell transplantation; MSD: Matched sibling donor; HRD: Haploidentical-related donor; GVHD: Graft-versus-host disease.
By 2-year post-HCT, recurrent or late-onset aGVHD had developed in 13.5% (n = 6) of HCT recipients, including 4 patients in the HRD-PBSCT group and 2 patients in the MSD-PBSCT group. The median time to onset was 5.8 months (range, 0.9–24.0 months) and was similar in both groups.
After a median of 586 (90–2010) days, the cumulative incidences of cGVHD were 17.90% (n = 6) and 33.78% (n = 5) after HRD-PBSCT and MSD-PBSCT, respectively [Table 2 and Figure 3]. According to NIH criteria, 2 (33.3%) of the HRD-PBSCT recipients had mild cGVHD, 3 (50%) had moderate cGVHD, and 1 (16.7%) had severe cGVHD. Among the MSD-PBSCT recipients, 1 (33%) had moderate cGVHD and 2 (67%) had severe cGVHD. The mouth was the most common site involved (n = 7, 63.6%), followed by skin (n = 6, 54.5%) and liver (n = 4, 36.4%).
Figure 3.
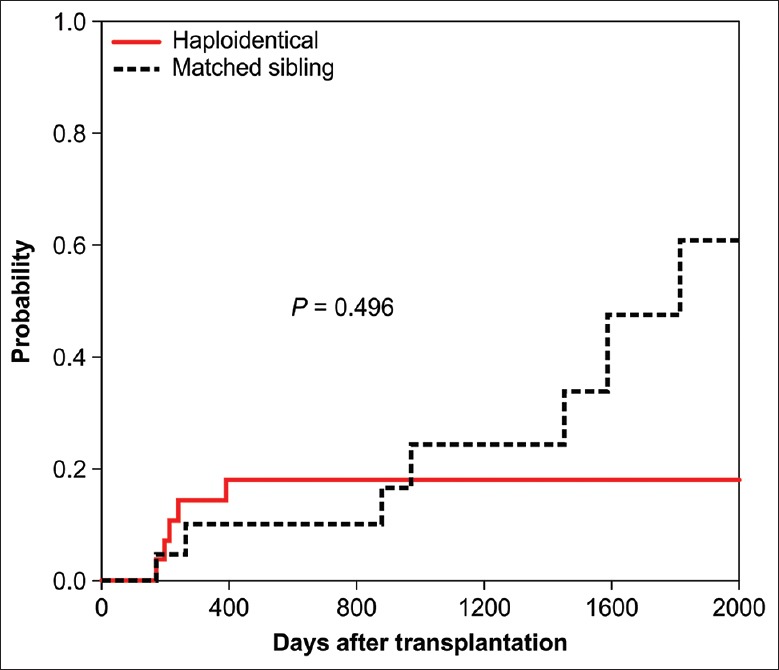
Cumulative incidence of chronic GVHD after HRD-PBSCT or MSD-PBSCT. PBSCT: Peripheral blood stem cell transplantation; MSD: Matched sibling donor; HRD: Haploidentical-related donor; GVHD: Graft-versus-host disease.
Relapse
As of February 1, 2016, a total of 13 patients in the HRD-PBSCT group and 10 patients in the MSD-PBSCT group had recurrent disease after a median of 183 days (range: 10–1700 days), reaching cumulative incidences of relapse of 50.28% and 33.79% at 5 years, respectively [Figure 4]. Six patients in the HRD-PBSCT group and 4 patients in the MSD-PBSCT group relapsed within 100 days after transplantation. The incidences of relapse after transplantation from HRDs or MSDs in AML patients in NR and CR2 as well as in primary refractory patients are shown in Table 3.
Figure 4.
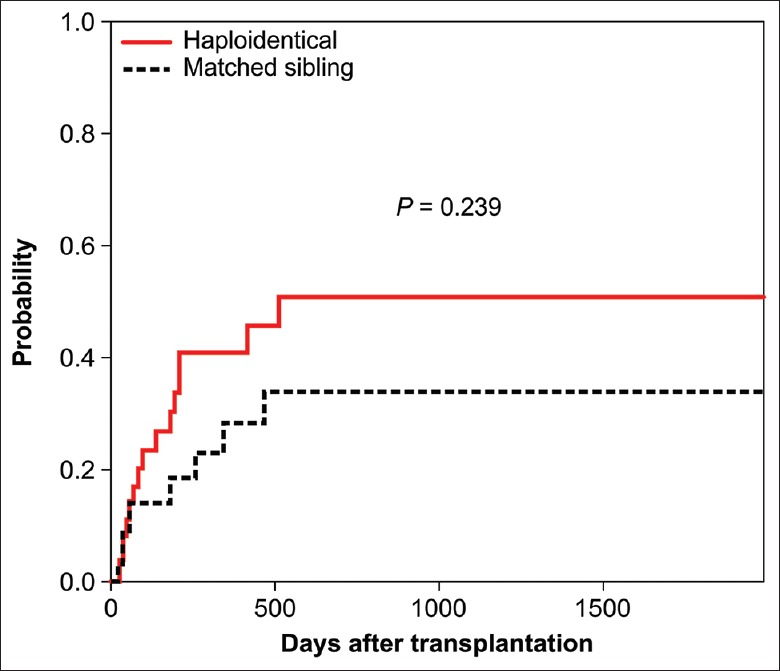
Cumulative incidence of relapse after HRD-PBSCT or MSD-PBSCT. PBSCT: Peripheral blood stem cell transplantation; MSD: Matched sibling donor; HRD: Haploidentical-related donor.
Table 3.
Cumulative incidence of relapse after HRD-PBSCT or MSD-PBSCT in patients with refractory/relapsed AML
| Relapse | HRD-PBSCT | MSD-PBSCT | ||
|---|---|---|---|---|
| n/n | Percentage | n/n | Percentage | |
| NR | 6/13 | 60.0 | 2/4 | 50.0 |
| CR2/3 | 3/7 | 33.3 | 2/6 | 33.3 |
| Primary refractory | 4/16 | 36.4 | 6/16 | 37.5 |
HRD-PBSCT: Unmanipulated haploidentical-related donor peripheral blood stem cell transplantation; MSD-PBSCT: Matched sibling donors peripheral blood stem cell transplantation; AML: Acute myeloid leukemia; CR: Complete remission; NR: Nonremission.
On univariate analysis, the incidence of relapse was 36.1% in the HRD-PBSCT group and 38.4% in the MSD-PBSCT group. The incidence of relapse in patients who were beyond CR2 (10/20, 50.0%) before transplantation was higher than that among those in CR2 (3/10, 33%; P = 0.328). The incidence of relapse was lower in recipients of PBSCT from a donor aged <40 years (relative risk = 0.25, 95% confidence interval: 0.067–0.937; P = 0.04).
Transplantation-related mortality and causes of death
The incidence of 3-year TRM was 16.7% after HRD-PBSCT and 4.5% after MSD-PBSCT. In the HRD-PBSCT group, three patients died of infection (one of cytomegalovirus, one of diffuse alveolar hemorrhage, and one of septic shock), and two patients died of severe cGVHD. In the MSD-PBSCT group, one patient died of secondary graft failure. A low number of CD34+ cells (<4.77 × 106/kg infused in the graft) was identified as a risk factor for TRM [P = 0.083, Table 4].
Table 4.
Death after HRD-PBSCT or MSD-PBSCT in patients with relapsed/refractory AML
| Cause | HRD-PBSCT (n = 36) | MSD-PBSCT (n = 26) |
|---|---|---|
| Cases, n (%) | Cases, n (%) | |
| Relapse | 12 (33.3) | 8 (30.8) |
| Infection | 5 (13.9) | |
| cGVHD | 1 (2.8) | 1 (3.8) |
HRD-PBSCT: Unmanipulated haploidentical-related donor peripheral blood stem cell transplantation; MSD-PBSCT: Matched sibling donors peripheral blood stem cell transplantation; GVHD: Graft-versus-host disease; cGVHD: Chronic GVHD; AML: Acute myeloid leukemia.
Survival
The probabilities of DFS at 5 years after HRD-PBSCT and MSD-PBSCT were 29.0% and 50.0%, respectively. The 5-year probability of OS in the HRD-PBSCT group tended to be lower than that in the MSD-PBSCT group, but the difference was not significant [39% vs. 55%; P = 0.125; Figure 5]. To assess the prognostic significance of the factors, we focused on treatment strategy (HRD vs. MSD) and considered factors including patient or donor age (<40 vs. ≥40 years), CD34+ cells in the graft, mononuclear cells in the graft, ECOG score, cytogenetics risk status, molecular abnormalities risk status, CR duration time after the first induction therapy, blasts in peripheral blood before transplantation, blasts in bone marrow before transplantation, donor–recipient sex match, and occurrence of GVHD. Univariate analysis identified a CD34+ cell count <4.77 × 106/kg (P = 0.046), patient age > 40 years (P = 0.035), and donor age >40 years (P = 0.004) as risk factors for lower DFS or OS [Table 5]. On multivariate analysis, donor age or patient age >40 years was an independent risk factor for inferior DFS or OS [P < 0.05, Table 5].
Figure 5.
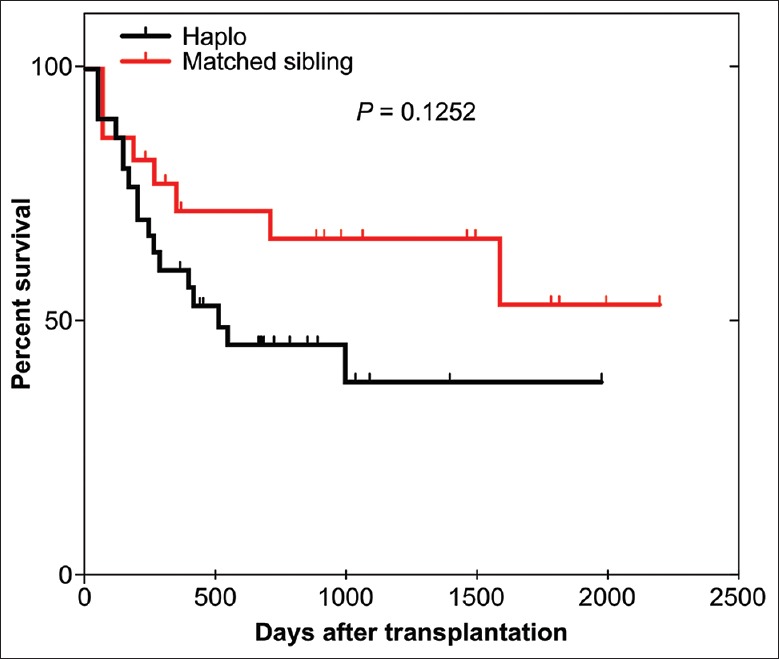
Overall survival after HRD-PBSCT or MSD-PBSCT. PBSCT: Peripheral blood stem cell transplantation; MSD: Matched sibling donor; HRD: Haploidentical-related donor.
Table 5.
Univariate and multivariate analyses of OS and PFS in 62 AML patients (n = 62)
| Items | Univariate | Multivariate | ||
|---|---|---|---|---|
| OS (HR, 95% CI) | DFS (HR, 95% CI) | OS (HR, 95% CI) | DFS (HR, 95% CI) | |
| Age (≥40 years) | 2.417 (1.079–5.413) | – | 5.873 (1.872–18.428) | – |
| Blast cells in bone marrow pretransplantation (≥20%) | – | 2.442 (1.073–5.557) | – | 2.868 (1.238–6.643) |
| CD34+ cells (<4.77 × 106/kg) infused in the graft | – | 2.141 (1.028–5.455) | – | – |
| Donor age (≥40 years) | 5.347 (1.790–15.976) | 6.068 (2.043–18.029) | 10.359 (2.735–39.236) | 6.702 (2.222–12.21) |
AML: Acute myeloid leukemia; OS: Overall survival; DFS: Disease-free survival; HR: Hazard ratio; CI: Confidence interval; PFS: Progression-free survival; –: No significance.
DISCUSSION
The current study provides a retrospective analysis of HRD-PBSCT and MSD-PBSCT in an unselected disease-specific population of patients with relapsed/refractory AML, particularly those in the NR phase.[11,14,16,17,18,20] The results of the present study demonstrate the clinical potential of HRD-PBSCT for the treatment of relapsed/refractory AML.
HRD-PBSCT has become increasingly attractive as an alternative therapy in the past few years; however, it remains unclear whether HRDs should be used when MSDs are available.[6,11,14] Our results support four important conclusions regarding HRD-PBSCT. First, for patients with high-risk leukemia, HRD-PBSCT provided almost the same GVL effect as MSD-PBSCT. Second, PBSCT from HRDs was equally efficacious as that from suitably matched MSDs, resulting in comparable rates of grades 2–4 and severe aGVHD, TRM, OS, and DFS. Although the TRM did not differ significantly between HRD and MSD, this result may need more patients to be confirmed. Third, compared with MSD-PBSCT, patients receiving PBSCs from an HRD and a total dose of 10 mg/kg ATG were less likely to suffer grades 2–4 aGVHD and severe cGVHD. Finally, donor age >40 years was an independent risk factor for inferior DFS, particularly in the HRD-PBSCT group.
Recently, a promising approach to HRD-PBSCT was developed in which ATG is used to achieve depletion of infused donor T lymphocytes in vivo.[15,19,24,35] For URD-PBSCT, the recommended dose of ATG is typically 6–8 mg/kg. For ATG-F (Fresenius), a dose of 30–60 mg/kg is recommended. Recommended doses of ATG for HRD-PBSCT need to be established. Kharfan et al.[24,35,36,37,40] reported that in patients with leukemia receiving PBSCs and a dose of 10 mg/kg ATG-F (Fresenius), HRD resulted in an inferior OS (60.8%) and DFS (58.3%) compared with HCT from an MSD (OS, 77.2% and DFS, 63.6%). The cumulative incidences of grades 2–4 aGVHD at 3 months were 42.4% and 15.6% for recipients of PBSCs from HRDs and MSDs, respectively. The incidences of cGVHD at 2 years were 41.4% and 24.3% in patients receiving transplants from HRDs and MSDs, respectively. Song et al.[41] used a total dose of 10 mg/kg ATG (Genzyme, rabbit) in patients receiving haploidentical bone marrow plus PBSCs and observed rates of 43% for grades 2–4 aGVHD and 53% for 2-year cGVHD. Our data suggest that HRD-PBSCT with in vivo T-cell depletion (10 mg/kg ATG, Genzyme) for relapsed/refractory AML could yield improved OS and DFS with acceptable incidences of grades 2–4 aGVHD and cGVHD, superior to the findings of the previous two studies. The incidence of cGVHD was slightly lower in HRD-PBSCT patients than in MSD-PBSCT patients.
The good outcomes of HRD-PBSCT in the present study may be explained by several factors. First, we used a myeloablative conditioning regimen that efficiently reduced the number of leukemia cells and achieved full immune suppression in the recipient to prevent graft rejection. Second, we used 10 mg/kg ATG (Genzyme) to balance GVHD toxicity and the risk of relapse and infections and found similar incidences of relapse but low rates of aGVHD and cGVHD compared with those obtained using low doses of ATG.[24,35,36,37,40,41] Different doses of ATG exhibit different immunomodulatory potencies; very low doses may have reduced immunosuppressive effects, whereas very high doses of ATG may aggravate the delay in immune recovery and increase the risk of relapse and infection. Patients in our study and the study by Holtick et al. received PBSCT;[19,24,35,36,37,40,41] however, we used a higher dose of ATG and obtained better outcomes. Although future clinical studies are needed to determine the possibility of further improvement in HRD-PBSCT outcomes with appropriate doses of ATG, our results showed that patients with relapsed/refractory AML receiving HRD-PBSCT with 10 mg/kg ATG experienced statistically similar outcomes as patients receiving MSD-PBSCT.
HRD-HSCT may potentially exert a strong GVL effect. However, comparative clinical studies to confirm the potential beneficial effects of HRD-HSCT have not yet been performed. Kanate et al. reported that the incidence of relapse was dramatically decreased after one-locus-mismatched HSCT compared with matched HSCT for high-risk diseases.[22] Yu et al. reported that the relapse rate for high-risk patients after HRD-HSCT (26%) was lower than that after MSD-HSCT (49%). Other studies have shown that the 5-year incidence of relapse is significantly affected by donor type (34.0% in a MSD cohort vs. 14.2% in a HRD cohort).[19,24,35,36,37,40,41] Given that many factors, such as disease type, remission status before HSCT, infused T-cell number, conditioning regimen, GVHD prophylaxis regimen, presence of aGVHD and cGVHD, patient age, and other factors, can influence the relapse rate after HSCT, the GVL effects in these previous studies are difficult to interpret because of the heterogeneity of the included diseases, e.g., AML and ALL.[19,24,35,36,37,40,41] In the present study, the disease type was relapsed/refractory AML, primarily in the NR phase, which resulted in relatively uniform subpopulations. In addition, all patients in this study were treated with same conditioning regimen, and the occurrence of grade 2–4 aGVHD was similar in both groups. The only disparity in the GVHD prophylaxis schedule was that patients receiving HRD-PBSCT were given ATG for additional immune suppression. Although this is a distinguishing feature between the groups, we believe that this did not affect the conclusions of this study. The requirement for more intense immune suppression in patients undergoing HRD-HSCT is an integral requirement for the prevention of GVHD and facilitation of engraftment. For patients with relapsed/refractory AML, HRD-PBSCT carried a similar probability of GVL as MSD-PBSCT.
The results of the present study demonstrate rapid and complete neutrophil engraftment, a low incidence of grade 2–4 aGVHD, and promising survival rates in patients after HRD-PBSCT. Thus, G-CSF–primed PBSCs may be a reliable graft source in unmanipulated HRD-HSCT under myeloablative conditioning when no MSD is available.
Financial support and sponsorship
This work was partially supported by grants from the Beijing Nova Program (2011114), the National Natural Science Foundation of China (No. 30800482, 30971297, 81102242, 81000221, 81270610, 81470010, 81170518, 81370666, and 90919044), the Beijing Natural Science Foundation of China (No. 7102147, 7172200, and 7132217), the High and New Technology Program of the PLA (2010gxjs091), the Capital Medical Development Scientific Research Fund (No. 2007-2040), the National Public Health Grand Research Foundation (No 201202017), the Public Health Project (Z111107067311070), and the National 973 Project of China (No. 2005CB522400).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Ethier MC, Blanco E, Lehrnbecher T, Sung L. Lack of clarity in the definition of treatment-related mortality: Pediatric acute leukemia and adult acute promyelocytic leukemia as examples. Blood. 2011;118:5080–3. doi: 10.1182/blood-2011-07-363333. doi:10.1182/blood-2011-07-363333. [DOI] [PubMed] [Google Scholar]
- 2.Forman SJ, Rowe JM. The myth of the second remission of acute leukemia in the adult. Blood. 2013;121:1077–82. doi: 10.1182/blood-2012-08-234492. doi:10.1182/blood-2012-08-234492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dou L, Zheng D, Li J, Li Y, Gao L, Wang L, et al. Methylation-mediated repression of microRNA-143 enhances MLL-AF4 oncogene expression. Oncogene. 2012;31:507–17. doi: 10.1038/onc.2011.248. doi:10.1038/onc.2011.248. [DOI] [PubMed] [Google Scholar]
- 4.Lazarus HM, Litzow MR, Gale RP. Improving survival in acute myeloid leukemia: Pick the best subjects? J Clin Oncol. 2013;31:3854–6. doi: 10.1200/JCO.2013.52.0296. doi:10.1200/jco.2013.52.0296. [DOI] [PubMed] [Google Scholar]
- 5.Sengsayadeth S, Savani BN, Blaise D, Malard F, Nagler A, Mohty M, et al. Reduced intensity conditioning allogeneic hematopoietic cell transplantation for adult acute myeloid leukemia in complete remission – A review from the acute leukemia working party of the EBMT. Haematologica. 2015;100:859–69. doi: 10.3324/haematol.2015.123331. doi:10.3324/haematol.2015.123331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sierra J, Storer B, Hansen JA, Martin PJ, Petersdorf EW, Woolfrey A, et al. Unrelated donor marrow transplantation for acute myeloid leukemia: An update of the seattle experience. Bone Marrow Transplant. 2000;26:397–404. doi: 10.1038/sj.bmt.1702519. doi:10.1038/sj.bmt.1702519. [DOI] [PubMed] [Google Scholar]
- 7.Bashey A, Zhang X, Jackson K, Brown S, Ridgeway M, Solh M, et al. Comparison of outcomes of hematopoietic cell transplants from T-replete haploidentical donors using post-transplantation cyclophosphamide with 10 of 10 HLA-A, -B, -C, -DRB1, and -DQB1 allele-matched unrelated donors and HLA-identical sibling donors: A Multivariable analysis including disease risk index. Biol Blood Marrow Transplant. 2016;22:125–33. doi: 10.1016/j.bbmt.2015.09.002. doi:10.1016/j.bbmt.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Breems DA, Van Putten WL, Huijgens PC, Ossenkoppele GJ, Verhoef GE, Verdonck LF, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23:1969–78. doi: 10.1200/JCO.2005.06.027. doi:10.1200/jco.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 9.Burnett AK, Goldstone A, Hills RK, Milligan D, Prentice A, Yin J, et al. Curability of patients with acute myeloid leukemia who did not undergo transplantation in first remission. J Clin Oncol. 2013;31:1293–301. doi: 10.1200/JCO.2011.40.5977. doi:10.1200/jco.2011.40.5977. [DOI] [PubMed] [Google Scholar]
- 10.Burnett AK, Russell NH, Hills RK, Hunter AE, Kjeldsen L, Yin J, et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: Results of the medical research council AML15 trial. J Clin Oncol. 2013;31:3360–8. doi: 10.1200/JCO.2012.47.4874. doi:10.1200/jco.2012.47.4874. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Xie H, Wood BL, Walter RB, Pagel JM, Becker PS, et al. Relation of clinical response and minimal residual disease and their prognostic impact on outcome in acute myeloid leukemia. J Clin Oncol. 2015;33:1258–64. doi: 10.1200/JCO.2014.58.3518. doi:10.1200/jco.2014.58.3518. [DOI] [PubMed] [Google Scholar]
- 12.Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28:3730–8. doi: 10.1200/JCO.2010.28.8852. doi:10.1200/jco.2010.28.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eriksson A, Lennartsson A, Lehmann S. Epigenetic aberrations in acute myeloid leukemia: Early key events during leukemogenesis. Exp Hematol. 2015;43:609–24. doi: 10.1016/j.exphem.2015.05.009. doi:10.1016/j.exphem.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara F, Schiffer CA. Acute myeloid leukaemia in adults. Lancet. 2013;381:484–95. doi: 10.1016/S0140-6736(12)61727-9. doi:10.1016/s0140-6736(12)61727-9. [DOI] [PubMed] [Google Scholar]
- 15.Fu H, Xu L, Liu D, Zhang X, Liu K, Chen H, et al. Late-onset hemorrhagic cystitis after haploidentical hematopoietic stem cell transplantation in patients with advanced leukemia: Differences in ATG dosage are key. Int J Hematol. 2013;98:89–95. doi: 10.1007/s12185-013-1350-8. doi:10.1007/s12185-013-1350-8. [DOI] [PubMed] [Google Scholar]
- 16.Gao L, Li Y, Zhang Y, Chen X, Gao L, Zhang C, et al. Long-term outcome of HLA-haploidentical hematopoietic SCT without in vitro T-cell depletion for adult severe aplastic anemia after modified conditioning and supportive therapy. Bone Marrow Transplant. 2014;49:519–24. doi: 10.1038/bmt.2013.224. doi:10.1038/bmt.2013.224. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton BK, Copelan EA. Concise review: The role of hematopoietic stem cell transplantation in the treatment of acute myeloid leukemia. Stem Cells. 2012;30:1581–6. doi: 10.1002/stem.1140. doi:10.1002/stem.1140. [DOI] [PubMed] [Google Scholar]
- 18.Hemmati PG, Terwey TH, Na IK, Jehn CF, le Coutre P, Vuong LG, et al. Allogeneic stem cell transplantation for refractory acute myeloid leukemia: A single center analysis of long-term outcome. Eur J Haematol. 2015;95:498–506. doi: 10.1111/ejh.12522. doi:10.1111/ejh.12522. [DOI] [PubMed] [Google Scholar]
- 19.Holtick U, Shimabukuro-Vornhagen A, Chakupurakal G, Theurich S, Leitzke S, Burst A, et al. FLAMSA reduced-intensity conditioning is equally effective in AML patients with primary induction failure as well as in first or second complete remission. Eur J Haematol. 2016;96:475–82. doi: 10.1111/ejh.12615. doi:10.1111/ejh.12615. [DOI] [PubMed] [Google Scholar]
- 20.Huang XJ, Zhu HH, Chang YJ, Xu LP, Liu DH, Zhang XH, et al. The superiority of haploidentical related stem cell transplantation over chemotherapy alone as postremission treatment for patients with intermediate- or high-risk acute myeloid leukemia in first complete remission. Blood. 2012;119:5584–90. doi: 10.1182/blood-2011-11-389809. doi:10.1182/blood-2011-11-389809. [DOI] [PubMed] [Google Scholar]
- 21.Johny A, Song KW, Nantel SH, Lavoie JC, Toze CL, Hogge DE, et al. Early stem cell transplantation for refractory acute leukemia after salvage therapy with high-dose etoposide and cyclophosphamide. Biol Blood Marrow Transplant. 2006;12:480–9. doi: 10.1016/j.bbmt.2005.12.031. doi:10.1016/j.bbmt.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 22.Kanate AS, Pasquini MC, Hari PN, Hamadani M. Allogeneic hematopoietic cell transplant for acute myeloid leukemia: Current state in 2013 and future directions. World J Stem Cells. 2014;6:69–81. doi: 10.4252/wjsc.v6.i2.69. doi:10.4252/wjsc.v6.i2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanda Y, Chiba S, Hirai H, Sakamaki H, Iseki T, Kodera Y, et al. Allogeneic hematopoietic stem cell transplantation from family members other than HLA-identical siblings over the last decade (1991-2000) Blood. 2003;102:1541–7. doi: 10.1182/blood-2003-02-0430. doi:10.1182/blood-2003-02-0430. [DOI] [PubMed] [Google Scholar]
- 24.Kharfan-Dabaja MA, Labopin M, Bazarbachi A, Socie G, Kroeger N, Blaise D, et al. Higher busulfan dose intensity appears to improve leukemia-free and overall survival in AML allografted in CR2: An analysis from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Leuk Res. 2015;39:933–7. doi: 10.1016/j.leukres.2015.04.009. doi:10.1016/j.leukres.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Liu DH, Xu LP, Liu KY, Wang Y, Chen H, Han W, et al. Long-term outcomes of unmanipulated haploidentical HSCT for paediatric patients with acute leukaemia. Bone Marrow Transplant. 2013;48:1519–24. doi: 10.1038/bmt.2013.99. doi:10.1038/bmt.2013.99. [DOI] [PubMed] [Google Scholar]
- 26.Mori T, Aisa Y, Watanabe R, Yamazaki R, Kato J, Shimizu T, et al. Long-term follow-up of allogeneic hematopoietic stem cell transplantation for de novo acute myelogenous leukemia with a conditioning regimen of total body irradiation and granulocyte colony-stimulating factor-combined high-dose cytarabine. Biol Blood Marrow Transplant. 2008;14:651–7. doi: 10.1016/j.bbmt.2008.03.006. doi:10.1016/j.bbmt.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Othus M, Appelbaum FR, Petersdorf SH, Kopecky KJ, Slovak M, Nevill T, et al. Fate of patients with newly diagnosed acute myeloid leukemia who fail primary induction therapy. Biol Blood Marrow Transplant. 2015;21:559–64. doi: 10.1016/j.bbmt.2014.10.025. doi:10.1016/j.bbmt.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips GL. Allogeneic hematopoietic stem cell transplantation (HSCT) for high-risk acute myeloid leukemia (AML)/myelodysplastic syndrome (MDS): How can we improve outcomes in the near future? Leuk Res. 2012;36:1490–5. doi: 10.1016/j.leukres.2012.08.004. doi:10.1016/j.leukres.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Przepiorka D, Deisseroth A, Farrell AT. Acute myeloid leukemia response measures other than complete remission. J Clin Oncol. 2015;33:3675–6. doi: 10.1200/JCO.2015.62.0864. doi:10.1200/jco.2015.62.0864. [DOI] [PubMed] [Google Scholar]
- 30.Ruggeri A, Labopin M, Sanz G, Piemontese S, Arcese W, Bacigalupo A, et al. Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29:1891–900. doi: 10.1038/leu.2015.98. doi:10.1038/leu.2015.98. [DOI] [PubMed] [Google Scholar]
- 31.Sasine JP, Schiller GJ. Emerging strategies for high-risk and relapsed/refractory acute myeloid leukemia: Novel agents and approaches currently in clinical trials. Blood Rev. 2015;29:1–9. doi: 10.1016/j.blre.2014.07.002. doi:10.1016/j.blre.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D, et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood. 2006;108:1092–9. doi: 10.1182/blood-2005-10-4165. doi:10.1182/blood-2005-10-4165. [DOI] [PubMed] [Google Scholar]
- 33.Stelljes M, Krug U, Beelen DW, Braess J, Sauerland MC, Heinecke A, et al. Allogeneic transplantation versus chemotherapy as postremission therapy for acute myeloid leukemia: A prospective matched pairs analysis. J Clin Oncol. 2014;32:288–96. doi: 10.1200/JCO.2013.50.5768. doi:10.1200/jco.2013.50.5768. [DOI] [PubMed] [Google Scholar]
- 34.Takami A, Yano S, Yokoyama H, Kuwatsuka Y, Yamaguchi T, Kanda Y, et al. Donor lymphocyte infusion for the treatment of relapsed acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation: A retrospective analysis by the Adult Acute Myeloid Leukemia Working Group of the Japan Society for Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2014;20:1785–90. doi: 10.1016/j.bbmt.2014.07.010. doi:10.1016/j.bbmt.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Tang X, Song YH, Sun A, Zhu X, Ruan C, Wu D, et al. Successful treatment of relapsed acute myeloid leukemia without chemotherapy. J Clin Oncol. 2016;34:e117–9. doi: 10.1200/JCO.2012.48.0442. doi:10.1200/JCO.2012.48.0442. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Liu DH, Liu KY, Xu LP, Zhang XH, Han W, et al. Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: Nine years of experience at a single center. Cancer. 2013;119:978–85. doi: 10.1002/cncr.27761. doi:10.1002/cncr.27761. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Liu DH, Xu LP, Liu KY, Chen H, Chen YH, et al. Superior graft-versus-leukemia effect associated with transplantation of haploidentical compared with HLA-identical sibling donor grafts for high-risk acute leukemia: An historic comparison. Biol Blood Marrow Transplant. 2011;17:821–30. doi: 10.1016/j.bbmt.2010.08.023. doi:10.1016/j.bbmt.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical vs identical-sibling transplant for AML in remission: A multicenter, prospective study. Blood. 2015;125:3956–62. doi: 10.1182/blood-2015-02-627786. doi:10.1182/blood-2015-02-627786. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Wu DP, Liu QF, Qin YZ, Wang JB, Xu LP, et al. In adults with t(8;21)AML, posttransplant RUNX1/RUNX1T1-based MRD monitoring, rather than c-KIT mutations, allows further risk stratification. Blood. 2014;124:1880–6. doi: 10.1182/blood-2014-03-563403. doi:10.1182/blood-2014-03-563403. [DOI] [PubMed] [Google Scholar]
- 40.Wong R, Shahjahan M, Wang X, Thall PF, De Lima M, Khouri I, et al. Prognostic factors for outcomes of patients with refractory or relapsed acute myelogenous leukemia or myelodysplastic syndromes undergoing allogeneic progenitor cell transplantation. Biol Blood Marrow Transplant. 2005;11:108–14. doi: 10.1016/j.bbmt.2004.10.008. doi:10.1016/j.bbmt.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Song KW, Lipton J. Is it appropriate to offer allogeneic hematopoietic stem cell transplantation to patients with primary refractory acute myeloid leukemia? Bone Marrow Transplant. 2005;36:183–91. doi: 10.1038/sj.bmt.1705038. doi:10.1038/sj.bmt.1705038. [DOI] [PubMed] [Google Scholar]
- 42.Luo Y, Xiao H, Lai X, Shi J, Tan Y, He J, et al. T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood. 2014;124:2735–43. doi: 10.1182/blood-2014-04-571570. doi:10.1182/blood-2014-04-571570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan CH, Liu DH, Liu KY, Xu LP, Liu YR, Chen H, et al. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood. 2012;119:3256–62. doi: 10.1182/blood-2011-09-380386. doi:10.1182/blood-2011-09-380386. [DOI] [PubMed] [Google Scholar]


