Abstract
Background
There is evidence-based consensus for laparoscopic cholecystectomy during index admission for predicted mild gallstone pancreatitis, defined by the absence of organ failure and of local or systemic complications. However, the optimal timing for surgery within that admission is controversial. Early cholecystectomy may shorten hospital length of stay (LOS) and increase patient satisfaction. Alternatively, it may increase operative difficulty and complications resulting in readmissions.
Methods
This trial is a single-center randomized trial of patients with predicted mild gallstone pancreatitis comparing laparoscopic cholecystectomy with intraoperative cholangiogram (IOC) at index admission within 24 hours of presentation versus after clinical resolution on clinical and patient-reported outcomes (PROs). The primary endpoint is 30-day LOS (hours) after initial presentation, which includes the index admission and readmissions. Secondary outcomes are conversion to open, complications, time from admission to cholecystectomy, initial hospital LOS, number of procedures within 30 days, 30-day readmissions, and PROs (change in Gastrointestinal Quality-of-Life Index).
Discussion
The primary goal of this research is to obtain the least biased estimate of effect of timing of cholecystectomy for mild gallstone pancreatitis on clinical and PROs; the results of this trial will be used to inform patient care locally as well as to design future multicenter effectiveness and implementation trials. This trial will provide data regarding PROs including health-related quality of life that can be used in cost-utility and cost-effectiveness analyses.
Trial registration number
NCT02806297, ClinicalTrials.gov.
Keywords: acute pancreatitis, cholecystectomy, randomized clinical trial, postoperative complications
Background
In patients with mild acute gallstone pancreatitis (table 1), evidence-based guidelines recommend cholecystectomy during index admission but do not specify further.1 Based on a randomized trial from the 1980s,2 surgeons have traditionally waited until clinical and laboratory resolution of mild acute gallstone pancreatitis before performing cholecystectomy. This management strategy has recently been challenged.3–5 A systematic review reported that early (within 3 days of admission regardless of whether pain or laboratory values had resolved) versus delayed laparoscopic cholecystectomy for acute gallstone pancreatitis resulted in decreased length of stay (LOS) without increased complications.6 However, the review included only a single randomized trial which was stopped after interim analysis.6
Table 1.
International, consensus-based definitions of severity of acute pancreatitis
| Severity | Definition |
| Mild | No organ failure, local or systemic complications and usually resolves in first week |
| Moderate | Transient organ failure, local complications or exacerbation of comorbid disease |
| Severe | Persistent organ failure (>48 hours) |
Local complications include peripancreatic fluid collections, pancreatic and peripancreatic necrosis (sterile or infected), pseudocyst, and walled-off necrosis.19
Safety-net hospitals perform approximately 12% of emergency general surgery cases nationwide; they have increased LOS and complications.7 A systematic review of the quality of surgical care in safety-net hospitals suggests that interventions to improve timeliness and patient centeredness, among other domains of quality, would have a substantial impact among underserved and vulnerable patients.8
The proposed trial is a pilot randomized trial of early cholecystectomy for predicted mild gallstone pancreatitis within 24 hours of presentation regardless of symptoms or laboratory values versus after clinical resolution during index admission at a safety-net hospital. The objectives are (1) to analyze the feasibility of early cholecystectomy at a safety-net hospital and (2) to obtain unbiased estimates of the effect of early cholecystectomy on hospital LOS, complications, and patient-reported outcomes (PROs). We hypothesized that early cholecystectomy is feasible and results in shorter 30-day total hospital LOS.
Methods/design
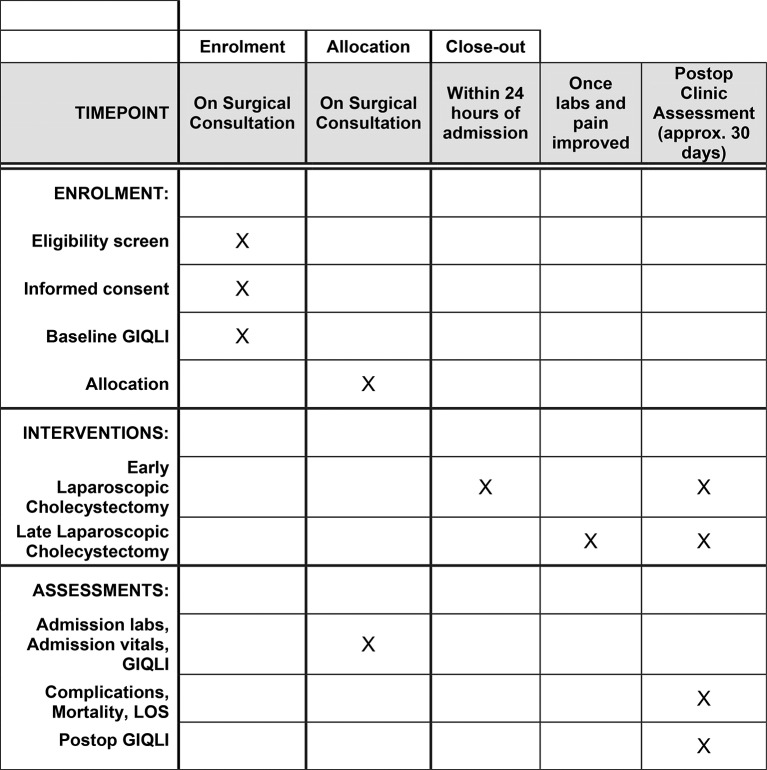
This trial is a single-center, parallel-group randomized trial that complies with the Standard Protocol Items: Recommendations for Interventional Trials statement (figure 1).9 The trial is registered on ClinicalTrials.gov(NCT02806297). The first patient was enrolled on June 27, 2016.
Figure 1.
Schedule of enrollment, interventions, and assessments.
Setting
The setting is Lyndon B. Johnson General Hospital (LBJGH), a 235-bed safety-net hospital in Houston, Texas, USA, that performs approximately 1000 elective and non-elective cholecystectomies annually. In a prior analysis of 356 non-elective cholecystectomies performed during 8 months, 14% (n=48) of patients had a diagnosis of gallstone pancreatitis.10
Outcomes
The primary outcome is total 30-day hospital LOS (hours), which accounts for both the potential benefits (ie, shorter initial hospital LOS3 4) and harms (ie, readmissions due to complications) of early cholecystectomy. Secondary clinical outcomes include conversion to open; overall and individual 30-day complications including transfusions, surgical site infection, pneumonia, bile duct injury, retained stone, and bowel injury; transfer to the intensive care unit (ICU); readmissions and development of local or systemic complications of pancreatitis. Additional outcomes include time to cholecystectomy, initial hospital LOS, number of procedures, and night-time cholecystectomy. Change in the validated Gastrointestinal Quality-of-Life Index (GIQLI) will be assessed between admission and 1 month postoperatively.11 12 The GIQLI can detect clinically significant changes after cholecystectomy13 and has been validated in Spanish.14
Study population
Patients are considered to have gallstone pancreatitis if they have: (1) upper abdominal pain, nausea, vomiting, and epigastric tenderness, (2) the absence of ethanol abuse, (3) elevated plasma lipase level above the upper limit of normal (≥370 U/L), and (4) imaging confirming gallstones or sludge.15 This definition is consistent with guidelines recommending the combination of clinical features with elevation of plasma concentrations of pancreatic enzymes (lipase over amylase) for diagnosis.16 Only patients with predicted mild pancreatitis based on the Bedside Index of Severity in Acute Pancreatitis (BISAP) score (0–2 points,<2% mortality) are screened for enrollment.17 Inclusion criteria include: patients scheduled for laparoscopic cholecystectomy prior to discharge, age ≥18 years, lack of any very strong indicator for choledocholithiasis based on the American Society for Gastrointestinal Endoscopy (ASGE) guidelines (table 2),18 and clinical stability as denoted by admission to a non-monitored floor bed. Exclusion criteria include: pregnancy, severe preexisting medical comorbidities precluding surgery, organ failure, local or systemic complications of acute pancreatitis,19 chronic pancreatitis, native language other than English and Spanish, and refusal to participate.
Table 2.
American Society for Gastrointestinal Endoscopy (ASGE) guidelines
| Likelihood | Predictors |
| Very strong | Common bile duct stone on transabdominal ultrasound |
| Clinical ascending cholangitis | |
| Bilirubin >4 mg/dL | |
| Strong | Dilated common bile duct (>6 mm) on ultrasound |
| Bilirubin level 1.8–4 mg/dL | |
| Moderate | Abnormal liver biochemical test other than bilirubin |
| Age older than 55 years | |
| Clinical gallstone pancreatitis |
Presence of any strong or both strong predictors suggests a high likelihood of choledocholithiasis. No predictors suggest a low likelihood, and all other patients have an intermediate likelihood.18 By definition, all patients enrolled in this trial will have at least a moderate likelihood because of the clinical diagnosis of gallstone pancreatitis.
Using these criteria, patients were enrolled and randomized from June 27, 2016 to May 18, 2017. Subsequently, due to concerns regarding the accuracy of BISAP to predict mild acute gallstone pancreatitis, the criteria were changed to prevent potentially operating on a patient who had progression of their pancreatitis. Starting on August 16, 2017, patients were enrolled but not randomized until there was no evidence of clinical deterioration after at least 12 hours after enrollment. Clinical deterioration is defined by new onset tachycardia, hypotension requiring fluids or vasopressors, decreased urine output, indication for further imaging to rule out necrotizing pancreatitis, transfer to the ICU, or clinical judgement of the treating physician.
Randomization, allocation concealment, and blinding
Consolidated Standards of Reporting Trials guidelines are being followed.20 Patients are randomized using variable permuted blocks using a computer-generated random sequence. Patients are randomized by a research coordinator not involved in the study using sequentially numbered, opaque, sealed envelopes. Patients are stratified based on the ASGE risk for choledocholithiasis (intermediate vs high likelihood, table 2). Data analysts, but not patients and healthcare providers, are blinded. A blinded outcome adjudication committee will review the outcomes.
Intervention and control
The intervention is laparoscopic cholecystectomy with intraoperative cholangiogram (IOC) at admission within 24 hours of presentation, whether pain or tenderness is present or laboratory values are elevated. Patients in whom pancreatitis severity worsens or who no longer fit trial criteria will not receive surgical intervention until it is deemed safe and clinically appropriate by the surgical team. The control is laparoscopic cholecystectomy with IOC once the patient has: (1) a score of less than 2 on the Visual Analogue Pain Scale or a decrease in score by 5, (2) no tenderness on physical examination, and (3) decreased lipase to either less than half of the peak value or within normal range (73–370 U/L). Since patients with any very strong predictor of choledocholithiasis are excluded, patients do not undergo preoperative endoscopic retrograde cholangiopancreatography (ERCP). If a retained common bile duct stone is identified on IOC, postoperative ERCP is obtained within 30 days. Patients receive standardized postoperative care in both arms. Patients receive a regular diet postoperatively unless a complication has occurred, and they are discharged based on: (1) normal vital signs, (2) regular diet, (3) adequate pain control, and (4) clearance of the common bile duct based on IOC or ERCP or plans for outpatient ERCP.
Sample size calculation
An intention-to-treat analysis will be performed. The sample size necessary to achieve a 1-day reduction in LOS (two-sided α=0.05, β=0.80) from 3 to 2 with 10% crossover in each group is 50, or a total of 100 patients. Based on this sample size and an annual volume of approximately 60 patients with an approximately 60% consent rate, we expect to be able to enroll this sample size in 1–2 years. A Poisson regression will be used to compare 30-day LOS between the two groups including the stratifying variable as a covariate. Secondary outcomes that are binary will be analyzed using the Cochran-Mantel-Haenszel test. In addition to the frequentist analysis, a Bayesian analysis will also be performed.21 Using a neutral prior probability, we can estimate the probability of reduction in LOS as well as that of an increase in complications.
Data monitoring
The Data Safety Monitoring Board (DSMB) consists of a pediatric surgeon, epidemiologist/statistician, and PhD investigator, all of whom have significant clinical research expertise. The DSMB has already met once (May 5, 2017) at the request of the investigators due to concerns about the accuracy of BISAP or any other scoring system to predict mild acute gallstone pancreatitis at admission. The DSMB approved the study to continue with modification to the inclusion criteria to include a second evaluation at 12 hours after initial presentation.
Discussion
Increasing evidence, including from a multicenter randomized controlled trial,6 22 has led to evidence-based guideline recommendations for cholecystectomy during the initial index admission for mild gallstone pancreatitis.1 However, the optimal timing for surgery during index admission has not been rigorously studied. Despite recommendations for early cholecystectomy, there remain concerns unsubstantiated by prospective data regarding increased risk of surgical complications due to severity of inflammation or unrecognized pancreatic necrosis. In a small, single-center study, 46 patients were identified during 16 years that had unrecognized necrosis at the time of same admission cholecystectomy.23 These patients had an increased risk of persistent organ failure, infected necrosis, and LOS as compared with others with necrosis that did not have cholecystectomy.
One of the challenges in selecting the appropriate cohort of patients to study is the difficulty in predicting the severity of acute pancreatitis. Several studies have compared both clinical (ie, Ranson’s criteria and Acute Physiology and Chronic Health Evaluation II) and radiologic scoring systems (ie, Modified CT Severity Index) for the prediction of severity of pancreatitis.24–27 These studies suggest that BISAP is accurate for risk stratification, is easy to use, and correlates with mortality and intensive care unit admission.24 25 However, despite a high specificity (91%), a BISAP score ≥3 has only moderate sensitivity (51%) suggesting that it is useful in ruling in severe pancreatitis but not in ruling it out.28 Given the lack of certainty in predicting mild acute pancreatitis using BISAP, the trial protocol was changed to include a 12-hours observation window.
Although surgeons are concerned most about postoperative complications, patients with acute gallstone abnormality are primarily concerned with quality of life after surgery.29 Quality of life does appear to be impaired after acute pancreatitis, but there is no standardized reporting, and short-term and long-term follow-up are lacking.30 There are very few studies evaluating the effect of cholecystectomy on quality of life and other PROs overall31 or after acute pancreatitis.
The high proportion of Hispanic patients in our population is a unique aspect of this trial. Prior studies suggest that Hispanic patients with gallstone pancreatitis may present at an earlier age and with milder disease32 and are slightly more likely to undergo cholecystectomy than whites.33 On the other hand, Hispanic patients are more likely to require ERCP34 and have increased delays to cholecystectomy after ERCP for choledocholithiasis.35 These data suggest that Hispanic patients may be an ideal population in whom to study this question as they may be most likely to benefit.
Limitations of the trial include lack of generalizability to other hospitals without an acute care surgery service. Although the setting is a safety-net hospital with limited resources, there is capacity for performing cholecystectomies at any time during the day or night. Second, 30-day LOS is not the most clinically important outcome. Since the trial is underpowered to detect rare complications such as common bile duct injury, the primary outcome of 30-day LOS is intended to serve as a surrogate for the balance between potential benefits and harms. Third, only short-term clinical outcomes and PROs are being measured. However, the trial will provide unbiased estimates of outcomes on which to base a larger multicenter trial that should include long-term outcomes.
In summary, this is a randomized trial evaluating the timing of laparoscopic cholecystectomy during index admission for predicted mild acute gallstone pancreatitis in a safety-net hospital. Interventions are needed to improve quality of care at safety-net hospitals after emergency surgery—particularly timeliness, efficacy, and patient centeredness. The proposed trial will evaluate feasibility and provide estimates of the probability of improved clinical and patient-reported outcomes. If the results are favorable, they would be used to improve local care and to inform a multicenter pragmatic trial.
Acknowledgments
Richard Escamilla, BA, Debbie Lew, MPH, Karla Bernardi, MD, Louis Carrillo, MD, Mitchell J George, MD, Margaret L Jackson, MD, and Akshita Kumar, MD, provided assistance with screening, consent, and enrollment of patients.
Footnotes
Contributors: All of the authors made substantial contributions to the concept and design of the randomized trial, were involved in the drafting and revision of the manuscript, gave final approval of the manuscript to be published, and agreed to be accountable for all aspects of the work.
Competing interests: None declared.
Ethics approval: Institutional Review Board (December 2, 2015).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There is no available unpublished data from the trial as it is currently enrolling.
References
- 1.Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 2013;13:e1–15. doi:10.1016/j.pan.2013.07.063 [DOI] [PubMed] [Google Scholar]
- 2.Kelly TR, Wagner DS. Gallstone pancreatitis: a prospective randomized trial of the timing of surgery. Surgery 1988;104:600–5. [PubMed] [Google Scholar]
- 3.Aboulian A, Chan T, Yaghoubian A, Kaji AH, Putnam B, Neville A, Stabile BE, de Virgilio C. Early cholecystectomy safely decreases hospital stay in patients with mild gallstone pancreatitis: a randomized prospective study. Ann Surg 2010;251:615–9. doi:10.1097/SLA.0b013e3181c38f1f [DOI] [PubMed] [Google Scholar]
- 4.Rosing DK, de Virgilio C, Yaghoubian A, Putnam BA, El Masry M, Kaji A, Stabile BE. Early cholecystectomy for mild to moderate gallstone pancreatitis shortens hospital stay. J Am Coll Surg 2007;205:762–6. doi:10.1016/j.jamcollsurg.2007.06.291 [DOI] [PubMed] [Google Scholar]
- 5.Taylor E, Wong C. The optimal timing of laparoscopic cholecystectomy in mild gallstone pancreatitis. Am Surg 2004;70:971–5. [PubMed] [Google Scholar]
- 6.Gurusamy KS, Nagendran M, Davidson BR. Early versus delayed laparoscopic cholecystectomy for acute gallstone pancreatitis. Cochrane Database Syst Rev 2013;9:CD010326 doi:10.1002/14651858.CD010326.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shahan CP, Bell T, Paulus E, Zarzaur BL. Emergency general surgery outcomes at safety net hospitals. J Surg Res 2015;196:113–7. doi:10.1016/j.jss.2015.02.044 [DOI] [PubMed] [Google Scholar]
- 8.Mouch CA, Regenbogen SE, Revels SL, Wong SL, Lemak CH, Morris AM. The quality of surgical care in safety net hospitals: a systematic review. Surgery 2014;155:826–38. doi:10.1016/j.surg.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 9.Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, Dickersin K, Hróbjartsson A, Schulz KF, Parulekar WR, et al. . SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586 doi:10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phatak UR, Chan WM, Lew DF, Escamilla RJ, Ko TC, Wray CJ, Kao LS. Is nighttime the right time? Risk of complications after laparoscopic cholecystectomy at night. J Am Coll Surg 2014;219:718–24. doi:10.1016/j.jamcollsurg.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 11.Carraro A, Mazloum DE, Bihl F. Health-related quality of life outcomes after cholecystectomy. World J Gastroenterol 2011;17:4945–51. doi:10.3748/wjg.v17.i45.4945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eypasch E, Williams JI, Wood-Dauphinee S, Ure BM, Schmülling C, Neugebauer E, Troidl H. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg 1995;82:216–22. doi:10.1002/bjs.1800820229 [DOI] [PubMed] [Google Scholar]
- 13.Shi HY, Lee HH, Chiu CC, Chiu HC, Uen YH, Lee KT. Responsiveness and minimal clinically important differences after cholecystectomy: GIQLI versus SF-36. J Gastrointest Surg 2008;12:1275–82. doi:10.1007/s11605-008-0526-7 [DOI] [PubMed] [Google Scholar]
- 14.Quintana JM, Cabriada J, López de Tejada I, Varona M, Oribe V, Barrios B, Perdigo L, Bilbao A. Translation and validation of the gastrointestinal Quality of Life Index (GIQLI). Rev Esp Enferm Dig 2001;93:693–706. [PubMed] [Google Scholar]
- 15.Toouli J, Brooke-Smith M, Bassi C, Carr-Locke D, Telford J, Freeny P, Imrie C, Tandon R. Working Party of the Program Commitee of the Bangkok World Congress of Gastroenterology 2002. Guidelines for the management of acute pancreatitis. J Gastroenterol Hepatol 2002;17:S15–39. [DOI] [PubMed] [Google Scholar]
- 16.Working Party of the British Society of Gastroenterology, Association of Surgeons of Great Britain and Ireland, Pancreatic Society of Great Britain and Ireland, Association of Upper GI Surgeons of Great Britain and Ireland. UK guidelines for the management of acute pancreatitis. Gut 2005;549:iii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008;57:1698–703. doi:10.1136/gut.2008.152702 [DOI] [PubMed] [Google Scholar]
- 18.Maple JT, Ben-Menachem T, Anderson MA, Appalaneni V, Banerjee S, Cash BD, Fisher L, Harrison ME, Fanelli RD, Fukami N, et al. . The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest Endosc 2010;71:1–9. doi:10.1016/j.gie.2009.09.041 [DOI] [PubMed] [Google Scholar]
- 19.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS. Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102–11. doi:10.1136/gutjnl-2012-302779 [DOI] [PubMed] [Google Scholar]
- 20.Schulz KF, Altman DG, Moher D. CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 2010;152:726–32. doi:10.7326/0003-4819-152-11-201006010-00232 [DOI] [PubMed] [Google Scholar]
- 21.Wijeysundera DN, Austin PC, Hux JE, Beattie WS, Laupacis A. Bayesian statistical inference enhances the interpretation of contemporary randomized controlled trials. J Clin Epidemiol 2009;62:13–21. doi:10.1016/j.jclinepi.2008.07.006 [DOI] [PubMed] [Google Scholar]
- 22.da Costa DW, Bouwense SA, Schepers NJ, Besselink MG, van Santvoort HC, van Brunschot S, Bakker OJ, Bollen TL, Dejong CH, van Goor H, et al. . Same-admission versus interval cholecystectomy for mild gallstone pancreatitis (PONCHO): a multicentre randomised controlled trial. Lancet 2015;386:1261–8. doi:10.1016/S0140-6736(15)00274-3 [DOI] [PubMed] [Google Scholar]
- 23.Kwong WT, Vege SS. Unrecognized necrosis at same admission cholecystectomy for pancreatitis increases organ failure and infected necrosis. Pancreatology 2017;17:41–4. doi:10.1016/j.pan.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 24.Papachristou GI, Muddana V, Yadav D, O’Connell M, Sanders MK, Slivka A, Whitcomb DC. Comparison of BISAP, Ranson’s, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol 2010;105:435–41. doi:10.1038/ajg.2009.622 [DOI] [PubMed] [Google Scholar]
- 25.Valverde-López F, Matas-Cobos AM, Alegría-Motte C, Jiménez-Rosales R, Úbeda-Muñoz M, Redondo-Cerezo E, Bisap R-ce. BISAP, RANSON, lactate and others biomarkers in prediction of severe acute pancreatitis in a European cohort. J Gastroenterol Hepatol 2017;32:1649–56. doi:10.1111/jgh.13763 [DOI] [PubMed] [Google Scholar]
- 26.Yang L, Liu J, Xing Y, Du L, Chen J, Liu X, Hao J. Comparison of BISAP, Ranson, MCTSI, and APACHE II in predicting severity and prognoses of hyperlipidemic acute pancreatitis in Chinese patients. Gastroenterol Res Pract 2016;2016:1–7. doi:10.1155/2016/1834256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Shahbaz M, Fang R, Liang B, Gao C, Gao H, Ijaz M, Peng C, Wang B, Niu Z, et al. . Comparison of the BISAP scores for predicting the severity of acute pancreatitis in Chinese patients according to the latest Atlanta classification. J Hepatobiliary Pancreat Sci 2014;21:689–94. doi:10.1002/jhbp.118 [DOI] [PubMed] [Google Scholar]
- 28.Gao W, Yang HX, Ma CE, Ce M. The value of BISAP score for predicting mortality and severity in acute pancreatitis: a systematic review and meta-analysis. PLoS One 2015;10:e0130412 doi:10.1371/journal.pone.0130412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parkin E, Stott M, Brockbank J, Galloway S, Welch I, Macdonald A. Patient-reported outcomes for acute gallstone pathology. World J Surg 2017;41:1234–8. doi:10.1007/s00268-016-3854-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pendharkar SA, Salt K, Plank LD, Windsor JA, Petrov MS. Quality of life after acute pancreatitis: a systematic review and meta-analysis. Pancreas 2014;43:1194–200. doi:10.1097/MPA.0000000000000189 [DOI] [PubMed] [Google Scholar]
- 31.Mueck KM, Cherla DV, Taylor A, Ko TC, Liang MK, Kao LS. Randomized controlled trials evaluating patient-reported outcomes after cholecystectomy: a systematic review. J Am Coll Surg 2017. doi:10.1016/j.jamcollsurg.2017.10.023 [DOI] [PubMed] [Google Scholar]
- 32.Yaghoubian A, De Virgilio C, El-Masry M, Lewis RJ, Stabile BE. Gallstone pancreatitis: a benign disease in Hispanics. Am Surg 2007;73:1071–4. [PubMed] [Google Scholar]
- 33.Nguyen GC, Tuskey A, Jagannath SB. Racial disparities in cholecystectomy rates during hospitalizations for acute gallstone pancreatitis: a national survey. Am J Gastroenterol 2008;103:2301–7. doi:10.1111/j.1572-0241.2008.01949.x [DOI] [PubMed] [Google Scholar]
- 34.Freeman J, Boomer L, Fursevich D, Feliz A. Ethnicity and insurance status affect health disparities in patients with gallstone disease. J Surg Res 2012;175:1–5. doi:10.1016/j.jss.2011.06.064 [DOI] [PubMed] [Google Scholar]
- 35.Huang RJ, Barakat MT, Girotra M, Banerjee S. Practice patterns for cholecystectomy after endoscopic retrograde cholangiopancreatography for patients with choledocholithiasis. Gastroenterology 2017;153:762–71. doi:10.1053/j.gastro.2017.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]